Abstract
Two coordination polymers (CPs), Cd(bpp)(H2O)L (BUC-18) and Zn(bpp)L (BUC-19), (H2L = cis-1,3-dibenzyl-2-imidazolidone-4,5-dicarboxylic acid, bpp = 1,3-bis(4-pyridyl)propane), have been synthesized under hydrothermal conditions, and characterized by single crystal X-ray analysis, Fourier transform infrared spectra (FTIR), thermogravimetric analyses (TGA), CNH element analysis and UV–Vis diffuse reflectance spectra (UV–Vis DRS). Upon the UV light irradiation, BUC-19 exhibited excellent photocatalytic performances toward methylene blue (initial concentration 10 mg L−1), methyl orange (initial concentration 10 mg L−1) and reactive red X-3B (initial concentration 50 mg L−1) with degradation efficiency of 93, 80 and 92%, respectively. The possible mechanism was proposed, which was further confirmed by trapping experiments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, coordination polymers (CPs) have attracted much attention due to their diverse synthetic strategies [1, 2] and potential applications like photocatalysis [3, 4], sensing [5, 6], drug delivery [7, 8], pollutants adsorption [9, 10], separation [11, 12], gas storage [13, 14], catalysis [15,16,17,18,19] and so on [20,21,22,23,24,25]. Generally, some CPs constructed from carboxylate ligands are highly stable, and exhibit structural diversities and immense applications [26] such as UiO-66 [27] and MIL-53(Cr) [28] linked by 1,4-dicarboxybenzene. As well, d-block transition metals (e.g. ZnII, CdII) usually play significant contribution in diverse structures and topologies as well as unique roles like photocatalysts [26]. From this point of view, 1,3-dibenzyl-2-imidazolidone-4,5-dicarboxylic acid (H2L) was utilized as a ligand assisted with 1,3-bis(4-pyridyl)propane (bpp), and ZnII/CdII were used as central metals to construct coordination polymers, and their photocatalytic performances were studied. Being compared to TiO2, CdS and other semiconductor photocatalysts, coordination polymers have some advantages in photocatalysis [29,30,31,32,33,34]: (i) the well-defined crystalline structures of CPs are beneficial to study the structure–property relationship of these solid photocatalysts; (ii) the modular nature of the CPs synthesis allows the rational design and fine tuning of these catalysts at the molecular level, making the electronic structure of the CPs catalysts to be easily tailored.
Wastewater containing organic compounds, generated in many industrial processes, can cause severe problems and damage to human being when they are discharged into aquatic environment [35, 36]. Organic pollutants, including dyes, are usually toxic, chemically stable and not amenable to direct biological treatment. Although dyes could be removed by photocatalysis [37,38,39,40,41,42], many photocatalysts are suffered from efficiency. Herein, four organic dyes including two cationic methylene blue (MB) & rhodamin B (RhB) and two anionic Methyl Orange (MO) & Reactive Red X-3B (X-3B), were selected as pollutant models to study photocatalytic activities of both BUC-18 and BUC-19.
2 Experimental
2.1 Materials and Instruments
All commercially available chemicals are reagent grade, and used as received without further purification. Thermogravimetric analyses (TGA) were performed from 80 to 800 °C in air stream at a heating rate of 10 °C min−1 on a DTU-3c thermal analyzer using α-Al2O3 as reference. UV–Vis diffuse reflectance spectra of solid samples were measured by Lambda 650S spectrophotometer, in which BaSO4 was used as the standard reference with 100% reflectance. The powder X-ray diffraction (PXRD) patterns were obtained on a DX-2700B X-ray diffractometer with powder diffraction procedure, using a Cu Kα radiation.
2.2 Synthesis of BUC-18
A mixture of Cd(NO3)2·4H2O (0.3 mmol, 92.54 mg), bpp (0.3 mmol, 59.4 mg) and H2L (0.3 mmol, 106.31 mg) was sealed in a 25 mL Teflon-lined stainless-steel Parr bomb containing deionized H2O (18 mL), heated at 160 °C for 72 h, and cooled down slowly to room temperature. Yellow block-like crystals of Cd(bpp)(H2O)L (BUC-18, yield 68% based on Cd(NO3)2·4H2O) were isolated and washed with deionized water and ethanol in turn. Anal. Calcd. for BUC-18, C32H32N4O6Cd: C, 56.4%; N, 8.2%; H, 4.7%. Found: C, 56.5%; N, 8.2%; H, 4.8%. FTIR (KBr) cm−1: 3674, 3409, 3085, 3064, 2943, 3028, 2875, 1959, 1708, 1612, 1496, 1405, 1345, 1306, 1232, 1210, 1168, 1122, 1069, 1046, 1030, 1015, 987, 959, 918, 877, 845, 808, 773, 748, 727, 704, 670, 642, 623, 611, 596, 566, 520, 508, 492, 474, 460, 403.
2.3 Synthesis of BUC-19
Yellow block-like crystals of Zn(bpp)L (BUC-19, yield 86% based on ZnCl2·6H2O) were synthesized following the same procedure as for BUC-18, except that Cd(NO3)2·4H2O was replaced with ZnCl2. Anal. Calcd. for BUC-19, C32H30N4O5Zn: C, 62.3%; N, 9.1%; H, 4.9%. Found: C, 62.5%; N, 9.2%; H, 5.0%. FTIR(KBr) cm−1: 3439, 3062, 2923, 1691, 1634, 1494, 1447, 1397, 1362, 1307, 1273, 1228, 1148, 1071, 1032, 989, 946, 798, 752, 702, 667, 609, 582, 518, 459.
2.4 X-ray Crystallography
X-ray single-crystal data collection for BUC-18 and BUC-19 was performed with a Bruker CCD area detector diffractometer with graphite-monochromatized MoKa radiation (λ = 0.71073 Å) using the φ − ω mode at 298(2) K. The SMART software [43] was used for data collection and the SAINT software [44] for data extraction. Empirical absorption corrections were performed with the SADABS program [45]. The structures were solved by direct methods (SHELXS-97) [46] and refined by full-matrix-least squares techniques on F2 with anisotropic thermal parameters for all of the non-hydrogen atoms (SHELEL-97) [46]. All hydrogen atoms were located by Fourier difference synthesis and geometrical analysis. These hydrogen atoms were allowed to ride on their respective parent atoms. All structural calculations were carried out using the SHELX-97 program package [46]. Crystallographic data and structural refinements for the two CPs are summarized in Table 1. Selected bond lengths and angles are listed in Table 2.
2.5 Evaluation of Photocatalytic Activity
Methylene blue (MB, 10 mg L−1), rhodamin B (RhB, 10 mg L−1), methyl orange (MO, 10 mg L−1) and reactive red X-3B (X-3B, 50 mg L−1) were selected to evaluate the photocatalytic performances of BUC-18 and BUC-19 under the UV light (500 W Hg lamp, Beijing Aulight Co., Ltd) irradiation in a photocatalytic reaction system. Fifteen microgram powder photocatalysts samples were put into 200 mL above-stated solutions containing organic dyes. Before photocatalysis reaction, the suspension was magnetically stirred in dark for 30 min to ensure the adsorption–desorption equilibrium. During the photocatalytic experiment, 3 mL aliquot was extracted at every 3 min intervals for analysis. A Laspec Alpha-1860 spectrometer was used to monitor the concentration changes determined at the maximum absorbance of 664, 554, 463 and 540 nm for MB, RhB, MO and X-3B, respectively.
3 Results and Discussion
3.1 Crystallographic Structure Analyses
3.1.1 BUC-18
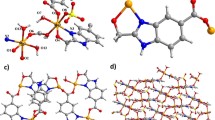
The crystal structure analysis revealed that [CdL(H2O)] binuclear centrosymmetric unit is built up of two crystallographically equivalent Cd(II) atoms and two bidentate bridging carboxylate groups from two different L2−, which is further linked into 1D neutral [Cd(bpp)(H2O)L]2n chain by flexible bpp ligands, as shown in Fig. 1a, b. The Cd(II), adopting distorted octahedron geometry Fig. 1d, are coordinated by two oxygen atoms from one completely deprotonated L2− ligand, one oxygen atom from the other L2− ligand, one oxygen atom from coordinated water molecules and two nitrogen atoms from two different bpp ligands. The completely deprotonated L2− ligand exhibits cis mode to join one Cd(II) through the bidentate-chelating µ1-carboxylato-κ2O:O group (Scheme 1a) and another Cd(II) via monodentate-bridging into [Cd2L2] binuclear unit. The Cd–O distances are in the range of 2.292(2)–2.519(2) Å, and the Cd–N distances are in the range of 2.318(2)–2.347(2) Å. The Cd–O bond lengths and Cd–N bond lengths are in good agreement with previous studies [47, 48]. Hydrogen bonding interactions can be seen in binuclear centrosymmetric unit, as shown in Fig. 1c and Table 3.
3.2 BUC-19
In [Zn(bpp)L] (BUC-19), the Zn(II) is tetrahedrally coordinated by two nitrogen atoms from two bpp ligands and two oxygen atoms from two L ligands, as shown in Fig. 2a, b. The deprotonated L2− ligand, adopting trans-mode, as shown in Scheme 1b, acts as bis-monodentate ligand to link two Zn(II) into an infinite zigzag [ZnL] chain along the b-axis. As shown in Fig. 3c, the adjacent [ZnL] chains are further linked by flexible bpp ligands into 2D network with undulating surface viewed from c-axis. The Zn–O distances are in the range of 1.916(4)–1.918(6) Å, and Zn–N distances are 1.993(8)–2.050(6) Å. The Zn–O bond lengths and Zn–N bond lengths are in good agreement with previous studies [47].
3.3 Powder X-ray Diffraction (PXRD)
PXRD of BUC-18 and BUC-19 powder were carried out confirm their phase purities, in which the simulated PXRD patterns obtained from their corresponding single crystal structure data were utilized as references. As shown in Fig. 3, the measured PXRD patterns matched well with the simulated ones, implying the high phase purity of the both coordination polymers. The slight differences in PXRD intensities can be contributed to the preferred orientation of the crystalline powder samples [49].
3.4 FTIR
The FTIR spectrums of the coordination polymers were recorded, as depicted in Fig. 4. The band at 2923 cm−1 is ascribed to v(–CH2–) vibration, and the strong bands at 1634 and 1397 cm−1 can be assigned to the asymmetric and symmetric vibrations of carboxyl groups, respectively. The bands at 1228 and 1148 cm−1 are attributed to the v(C–N) vibrations of the phenyl rings. The weak bands at 492 and 403 cm−1 can be attributed to Zn–O vibration, while band at 518 cm−1 can be ascribed to Cd–O vibration.
3.5 Thermal Properties
To investigate the thermal stability of the two CPs, thermogravimetric analyses were carried out, and the results were demonstrated in Fig. 5. It can be seen that the weight loss processes of the two CPs were very similar, although their crystal structures were different. Taking BUC-18 as an example, the TGA curve presents three stages of decomposition. The loss of water molecules was observed from 150 to 240 °C. As the temperature was increased from 240 to 490 °C, the weight loss was considered as organic ligands. The weight of the final residue was CdO, which was affirmed by a good match between the powder X-ray diffraction (PXRD) patterns of the residue and the standard patterns of CdO from the database (PDF#01-073-2245), as illustrated in Fig. 5(b).
3.6 Optical Energy Gap
The optical properties of BUC-18 and BUC-19 were estimated by UV–Vis diffuse reflectance spectrophotometer (UV–Vis DRS). As shown in Fig. 6a, the absorption bands were at 288 nm. The band gap (E g ) values were estimated using Kubelka–Munk function, F = (1 − R)2/2R [50], where R is the reflectance of an infinitely thick layer at a given wavelength. The F versus E plots for the two CPs are shown in Fig. 6b, where steep absorption edges are displayed and the Eg values of the sample can be assessed at 4.4 eV for both BUC-18 and BUC-19, which indicate that two CPs are potential wide gap photocatalysts [51, 52].
3.7 Photocatalytic Performance Studies
Emerging research has demonstrated coordination polymers including MOFs to be a new class of photocatalysts for potential applications in environmental field, such as organic pollutants degradation [26], Cr(VI) reduction [53] and ultra-high adsorption performance toward organic pollutants and heavy metals [9, 10]. In this study, four organic dyes (MB, RhB, MO and X-3B) were selected to evaluate the activities of the two new CPs under UV light irradiation. Control experiments were conducted to compare the removal efficiencies of organic dyes in two different systems with identical conditions except for in presence of photocatalysts in dark and absence of photocatalysts under UV irradiation, respectively. The adsorptive removals of four selected organic dyes in the presence of the two CPs in dark can be negligible, as shown in Fig. 7. After UV light irradiation for 30 min, limited dyes were decomposed in absence of any photocatalysts. BUC-19 displayed excellent photocatalytic degradation performances toward MB and X-3B, evidenced by their decomposition efficiency being 93 and 92%, respectively, as illustrated in Tables 4 and 5.
3.8 Recyclability of BUC-19
The recyclability of BUC-19 was tested by circulating runs in the photocatalytic degradation of MB over BUC-19/UV light system. The results in Fig. 8 showed that the photocatalytic degradation performance of BUC-19 remained almost unchanged after 5 runs for MB degradation, indicating that BUC-19 was stable and could be used for the repeated of MB decomposition.
To investigate the roles of the reactive species (·O2−, ·OH and h+) in the photocatalytic process, 0.2 mmol L−1 of benzoquinone (BQ), isopropyl alcohol (IPA) and ethylene diamine tetraacetic acid disodium salt (EDTA-2Na) were added into MB solution with BUC-19 as scavengers of ·O2−, ·OH and h+, respectively. As shown in Fig. 9, the photocatalytic degradation of MB was inhibited in the order of BQ > IPA > EDTA-2Na. Three reactive species were important to degrade MB molecules, while ·O2− played a dominant role for the MB degradation in the BUC-19 system.
Generally, photocatalytic mechanism of coordination polymers, including metal–organic frameworks (except MOF-5), is explained based on frontier molecular orbital theory [26]. Based on the above-stated results and analyses, a possible mechanism for the photocatalysis with BUC-19 was proposed, as illustrated in Fig. 10. Typically, the photocatalysis process involves three steps. Firstly, dye molecules are adsorbed onto the surface of BUC-19. Next, upon UV light irradiation, BUC-19 absorbs photons and excites electrons (e−) from highest occupied molecular orbital (HOMO) and leaves holes (h+) in lowest unoccupied molecular orbital (LUMO). Finally, active species are generated and decompose dyes. Briefly, the photo-induced electron in the LUMO losses and transfers to O2 generating ·O2−, which decompose dye molecules. The holes can oxide dye directly. Besides, the HOMO strongly demands electrons to return to its stable state, therefore, one electron is captured from water molecules and product ·OH active species. The ·OH radicals can further destroy organic dyes efficiently.
4 Conclusion
In summary, the syntheses of two new coordination polymers, BUC-18 and BUC-19, have been accomplished by hydrothermal method and characterized using X-ray single crystal diffraction analysis, TGA, CNH element analysis and UV–Vis DRS. The results show that BUC-18 possesses 1D infinite chain structure, while BUC-19 displays 2D layer structures, which can be assigned to the influence of L2− ligand and flexible bpp ligands. BUC-19 exhibited excellent photocatalytic degradation performances toward organic dyes. The trapping experiences proved that ·O2−, ·OH and h+ played important roles in decomposing dyes.
References
N. Stock, S. Biswas, Chem. Rev. 112, 933–969 (2012)
L. Shen, G. Wang, X. Zheng, Y. Cao, Y. Guo, K. Lin, L. Jiang, Chin. J. Catal. 38, 1373–1381 (2017)
B.F. Meng, W.S. You, X.F. Sun, F. Zhang, M.Y. Liu, Inorg. Chem. Comm. 14, 35–37 (2011)
L.F. Gao, Z.Y. Zhu, W.S. Feng, Q. Wang, H.L. Zhang, J. Phys. Chem. C. 120, 28456–28462 (2017)
K. Hirai, J. Mater. Chem. C. 2, 3336–3344 (2014)
Y.Y. Liu, J. Zhang, F. Xu, L.X. Sun, T. Zhang, W.S. You, Y. Zhao, J. Zeng, Z. Cao, D. Yang, Cryst. Growth Des. 8, 3127–3129 (2008)
W.J. Rieter, K.M. Pott, K.M. Taylor, W. Lin, J. Am. Chem. Soc. 130, 11584 (2008)
I. Imaz, M. Rubiomartínez, L. Garcíafernández, F. García, D. Ruizmolina, J. Hernando, V. Puntes, D. Maspoch, Chem. Comm. 46, 4737–4739 (2010)
J.-J. Li, C.-C. Wang, H. Fu, J. Cui, P. Xu, J. Guo, J.-R. Li, Dalton T. 46, 10197–10201 (2017)
X.D. Du, C.C. Wang, J. Zhong, J.G. Liu, Y.X. Li, P. Wang, J. Environ. Chem. Eng. 5, 1866–1873 (2017)
S.I. Noro, R. Ochi, Y. Inubushi, K. Kubo, T. Nakamura, Microporous Mesoporous Mater. 216, 92–96 (2015)
Y.S. Bae, A.M. Spokoyny, O.K. Farha, R.Q. Snurr, J.T. Hupp, C.A. Mirkin, Chem. Commun. 46, 3478–3480 (2010)
O.K. Farha, A.M. Spokoyny, K.L. Mulfort, S. Galli, J.T. Hupp, C.A. Mirkin, Small 5, 1727–1731 (2009)
Y.M. Jeon, G.S. Armatas, J. Heo, M.G. Kanatzidis, C.A. Mirkin, Adv. Mater. 20, 2105–2110 (2010)
M.L. Hu, V. Safarifard, E. Doustkhah, S. Rostamnia, A. Morsali, N. Nouruzi, S. Behesht, K. Akhbari, Microporous Mesoporous Mater. 256, 111–127 (2017)
S. Rostamnia, A. Morsali, RSC Adv. 4, 10514–10518 (2014)
S. Rostamnia, Z. Karimi, Inorg. Chim. Acta. 428, 133–137 (2015)
S. Rostamnia, H. Alamgholiloo, X. Liu, J. Colloid Interf. Sci. 469, 310–317 (2016)
S. Rostamnia, H. Xin, Appl. Organomet. Chem. 28, 359–363 (2014)
M.R. Maurya, A. Kumar, P. Manikandan, S. Chand, Appl. Catal. A 277, 45–53 (2004)
S.K. Ghosh, W. Kaneko, D. Kiriya, M. Ohba, S. Kitagawa, Angew. Chem. 47, 8843–8847 (2008)
H.S. Choi, M.P. Suh, Angew. Chem. 48, 6865–6869 (2009)
R.W. Flaig, T.M. Osborn Popp, A.M. Fracaroli, E.A. Kapustin, M.J. Kalmutzki, R.M. Altamimi, F. Fathieh, J.A. Reimer, O.M. Yaghi, J. Am. Chem. Soc. 139, 12125–12128 (2017)
H. Kim, S. Yang, S.R. Rao, S. Narayanan, E.A. Kapustin, H. Furukawa, A.S. Umans, O.M. Yaghi, E.N. Wang, Science. 356, 430–434 (2017)
G. Song, Z. Wang, L. Wang, G. Li, M. Huang, F. Yin, Chin. J. Catal. 35, 185–195 (2014)
C.C. Wang, J.R. Li, X.L. Lv, Y.Q. Zhang, G. Guo, Energy Environ. Sci. 7, 2831–2867 (2014)
J.H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K.P. Lillerud, J. Am. Chem. Soc. 130, 13850–13851 (2008)
C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, A.D. Louër, G. Férey, J. Am. Chem. Soc. 124, 13519–13526 (2002)
Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li, H.-C. Zhou, Chem. Soc. Rev. 45, 2327–2367 (2016)
Y. Zhao, L. Guo, F. Gándara, Y. Ma, Z. Liu, C. Zhu, H. Lyu, C.A. Trickett, E.A. Kapustin, O. Terasaki, J. Am. Chem. Soc. 139, 13166–13172 (2017)
J. Yang, Y.B. Zhang, Q. Liu, C.A. Trickett, E. Gutierrez-Puebla, M. Monge, H. Cong, A. Aldossary, H. Deng, O.M. Yaghi, J. Am. Chem. Soc. 139, 6448–6455 (2017)
K. Choi, D. Kim, B. Rungtaweevoranit, C.A. Trickett, J.T.D. Barmanbek, P. Yang, O.M. Yaghi, J. Am. Chem. Soc. 139, 356–362 (2016)
C.C. Wang, Y.Q. Zhang, T. Zhu, X.Y. Zhang, P. Wang, S.J. Gao, Polyhedron 90, 58–68 (2015)
L. Shen, R. Liang, L. Wu, Chin. J. Catal. 36, 2071–2088 (2015)
D. Shen, J. Fan, W. Zhou, B. Gao, Q. Yue, Q. Kang, J. Hazard. Mater. 172, 99–107 (2009)
W.K. Jo, R.J. Tayade, Chin. J. Catal. 35, 1781–1792 (2014)
G. Sharma, A. Kumar, N. Mu, A. Kumar, A.A.H. Al-Muhtaseb, P. Dhiman, A.A. Ghfar, F.J. Stadler, M.R. Khan, J. Clean. Prod. 172, 2919–2930 (2017)
A. Kumar, G. Sharma, S. Kalia, C. Guo, N. Mu, J. Photochem. Photobiol. A 337, 118–131 (2017)
G. Sharma, V.K. Gupta, S. Agarwal, A. Kumar, S. Thakur, D. Pathania, J. Mol. Liq. 219, 1137–1143 (2016)
A. Kumar, M. Naushad, A. Rana, G. Sharma, Inamuddin, Preeti, G. Sharma, A.A. Ghfar, F.J. Stadler, M.R. Khan, Int. J. Biol. Macromol. 104, 1172–1184 (2017)
G. Sharma, S. Bhogal, M. Naushad, Inamuddin, A. Kumar, F.J. Stadler, J. Photochem. Photobiol. A 347, 235–243 (2017)
G. Sharma, M. Naushad, A. Kumar, S. Devi, M.R. Khan, Iran. Polym. J. 24, 1003–1013 (2015)
A.X.S. Bruker, SMART, Version 5.611 (Bruker AXS, Madison, 2000)
A.X.S. Bruker, SAINT, Version 6.28 (Bruker AXS, Madison, 2003)
A.X.S. Bruker, SADABS, Version 2.03 (Bruker AXS, Madison, 2000)
G.M. Sheldrick, SHELX-97 (Göttingen University, Germany, 1997)
X.L. Chen, B. Zhang, H.M. Hu, F. Fu, X.L. Wu, T. Qin, M.L. Yang, G.L. Xue, J.W. Wang, Cryst. Growth Des. 8, 3706–3712 (2008)
X. Shi, G. Zhu, X. Wang, G. Li, Q. Fang, G. Wu, G. Tian, M. Xue, X. Zhao, Cryst. Growth Des. 5, 207–213 (2004)
J. Hao, B. Yu, K.V. Hecke, G. Cui, CrystEngComm. 17, 2279–2293 (2015)
C.G. Silva, A. Corma, H. García, J. Mater. Chem. 20, 3141–3156 (2010)
J.L. Wang, C. Wang, W. Lin, ACS Catal. 2, 2630–2640 (2012)
J. Guo, J.F. Ma, B. Liu, W.Q. Kan, J. Yang, Cryst. Growth Des. 11, 3609–3621 (2011)
C.C. Wang, X.D. Du, J. Li, X.X. Guo, P. Wang, J. Zhang, Appl. Catal. B 193, 198–216 (2016)
H.P. Jing, C.C. Wang, Y.W. Zhang, P. Wang, R. Li, RSC Adv. 4, 54454–54462 (2014)
C.C. Wang, D.X. Xu, H.P. Jing, X.X. Guo, P. Wang, S.J. Gao, J. Inorg. Organomet. Polym. Mater. 26, 276–284 (2016)
Y. Wang, Y. He, T. Li, J. Cai, M. Luo, L. Zhao, Chem. Eng. J. 189–190, 473–481 (2012)
M.J. Height, S.E. Pratsinis, O. Mekasuwandumrong, P. Praserthdam, Appl. Catal. B 63, 305–312 (2006)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51578034), Great Wall Scholars Training Program Project of Beijing Municipality Universities (CIT&TCD20180323), Project of Construction of Innovation Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20170508) and Beijing Talent Project (2017A38).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, FX., Chen, X., Wang, P. et al. New Zn/Cd Coordination Polymers Constructed from Mixed Ligands: Crystal Structures and Photocatalytic Performances Toward Organic Dyes Degradation. J Inorg Organomet Polym 28, 1565–1573 (2018). https://doi.org/10.1007/s10904-018-0804-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-018-0804-0