Abstract
Our previous report demonstrated the fabrication and functionalization of the luminous dendrimer based on phenylazomethine-BiCl3 complexes (Kambe et al. in Angew Chem Int Ed 55:13151, 2016). It is now revealed that the bismuth assembled phenylazomethine dendrimers have different luminous efficiencies depending on the layers of the DPAG4 by photoemission and absorption spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metallodendrimers [1,2,3] are attractive materials because they enable a multifunctional property based on metal complexes and nano-sized dendrimer structures. Thus far, various kinds of metallodendrimers have been investigated, and their applications are expected for optical materials [4,5,6,7,8,9,10,11], catalysis [12,13,14,15,16,17,18,19,20,21,22,23], sensors [24,25,26,27], precursors for nanoparticles, etc [28,29,30,31,32,33].
Dendritic polyphenylazomethines (DPAs) are imine-based dendrimers that can provide tunable metallodendrimers by assembly of metal units on the branches [34,35,36,37]. In addition to the metal coordination sites, the branches have potential gradient derived from the π-conjugated skeleton. It generates an electronic donation toward the inner sites, resulting in stronger and weaker basic imines at the inner and outer positions, respectively. Due to the gradual change in the Lewis basicity of the imine sites, stepwise complexation of the Lewis acidic units is available. We have already reported the fine-controlled assembly of organic or inorganic species including metal chlorides in the 4th generation DPA (DPAG4; Fig. 1a) [38,39,40,41,42,43,44,45,46,47,48,49].
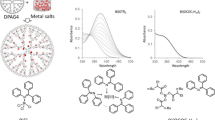
a Chemical structure of the dendritic polyphenylazomethines (DPAG4). b Schematic illustration of the metal assembly into the DPAG4 in a radial stepwise fashion. c Photoluminescence spectra induced by the assembly of BiCl3. The graphs corresponds to the cases of 4, 12, 28 and 60 equivalents of BiCl3 in the DPAG4. The excitation wavelength is 410 nm
The number of metal units in the DPAG4 is controlled by the number of imine sites. The DPAG4 has four branches connected to the tetraphenylmethane core. The four branches provide a layered structure composed of 4, 8, 16 and 32 imine sites from the central positions, and the potential gradient enhances basicity of the inner imines. By utilizing their feature, we assembled 4, 12, 28 and 60 chemical units in the DPAG4. Such an assembly of the metal units was used for the fabrication of size regulated metal clusters. For example, Pt clusters prepared from the corresponding metallodendrimers demonstrated a high catalytic activity for the reduction of oxygen and organic compounds [39, 50]. Furthermore, this layered structure was also used for the combination of metal units up to four kinds in the nano-sized molecule.
We recently reported luminous dendrimers based on the controlled assembly of bismuth chloride in the DPAG4 [45]. We investigated tuning of the photoluminescence intensity. In addition, the shell-effect of the DPAG4 provided a solid-state emission and optical on–off switching by chemical or electrochemical stimulus. On the other hand, this phosphor has a radial layered structure based on the potential gradient. It is also a characteristic point, and leads to luminous features depending on the layers. Based on these points, we researched the effect of the layers on their photoluminescence in this study.
2 Experimental Section
2.1 Materials
BiCl3 (ultra dry) was purchased from Alfa Aesar, A Johnson Matthey Company. Dehydrated acetonitrile and chloroform were obtained from Kanto Chemicals and Wako Pure Chemical Industries, Ltd., respectively. The DPAG4 was synthesized according to a literature [37].
2.2 Characterization
The UV–Vis spectra were measured by Shimadzu UV-3600 and UV-3100PC spectrometers at 20 °C. A quartz cell having a 1 cm optical length was used. The photoluminescence spectra were recorded at room temperature by a Jasco FP-8300.
2.3 Complexation Between Bismuth Salts and DPAG4
In a nitrogen-filled glove box, BiCl3 and the DPAG4 were dissolved in acetonitrile (2.73 × 10−3 M) and an acetonitrile/chloroform (1:1) mixed solution (1.53 × 10−6 M), respectively. The appropriate amount of the bismuth salt (4, 12, 28 or 60 equivalents) was added to the DPAG4 solution under room temperature, and the solution was stirred vigorously for complexation.
3 Results and Discussion
The luminous dendrimers based on the BiCl3-imine complexes in the DPAG4 were prepared by the addition of BiCl3 to the solution. The complexation ratio between BiCl3 and the imine part was 1:1 fashion, and the photoluminescence intensity could be tuned by the amount of BiCl3 (Fig. 1b). The photoluminescence was observed from 500 to 800 nm, and it was quenched by dissociation of the coordination bond of the bismuth units. Such unique features of this dendritic phosphor is due to the stepwise assembly process from the inner imine sites based on the different basicity. It was determined by the four-step shift of the isosbestic point observed in the UV–Vis spectra. This shift matched the number of imine sites in each layer. It indicated the stepwise assembly of BiCl3 from the inner imines in the DPAG4. These results were reported in the previous literature [45].
The emission spectra from the DPAG4 with 4, 12, 28 and 60 BiCl3 are shown in Fig. 1. The observed spectra were assignable to two peaks centered at 1.70 × 104 and 1.46 × 104 cm−1 corresponding to fluorescence and phosphorescence, respectively. Whereas the emission intensity increases according to the equivalents of the assembled BiCl3, the emission wavenumber (wavelength) remained about the same (Fig. 1c).
The 60BiCl3-DPAG4 has four kinds of luminous BiCl3-imine complexes according to the layers (Fig. 2a). Since the BiCl3 assembles from the inner sites, the 28BiCl3-DPAG4, 12BiCl3-DPAG4 and 4BiCl3-DPAG4 have three, two and one kind of luminous complexes, respectively. Regarding these complexes, a different luminescent efficiency is expected based on the flexibility of the branches and accessibility of the solvent that trigger deactivation.
Figure 2b shows the photoluminescence intensity standardized by the absorbance at the excitation wavelength (λ ex = 410 nm). This vertical axis corresponds to the quantum yield, because the concentration and solvent are same [51]. We found that the value decreases as the BiCl3 increases. This lower shift in the intensity value showed the deactivation of the photoluminescence in the outer position of the dendrimer.
The observed values of the F/A values are summarized in Fig. 3 (F: peak area of photoluminescence, A: absorbance at the excitation wavelength). We found that the observed values decreased with an increase in the added BiCl3, and the plotted curve changed at 4, 12, and 28 equivalents, corresponding to the imine sites of the layers when the BiCl3 was assembled into the DPAG4 from the inner sites.
From these data demonstrating the higher luminescent efficiency of the complexes positioned at the inner layer, we considered that the dendritic branches effectively protect the emissive units from the solvent molecules [42].
In summary, the photoluminescence spectra of the BiCl3 assembled DPAG4 were analyzed based on the different layers in the dendrimer. The luminous intensity was standardized by the absorbance at the excitation wavelength and the value (F/A) decreased with an increase in the assembled number of BiCl3. These data show different optical properties according to the layers, suggesting protection of metal complexes in the dendrimer from external solvent molecules.
References
G.R. Newkome, E. He, C.N. Moorefield, Chem. Rev. 99, 1689 (1999)
D. Astruc, C. Ornelas, J. Ruiz, Acc. Chem. Res. 41, 841 (2008)
C. Gorman, Adv. Mater. 10, 295 (1998)
M. Kawa, J.M.J. Fréchet, Chem. Mater. 10, 286 (1998)
S.-H. Hwang, C.N. Moorefield, G.R. Newkome, Chem. Soc. Rev. 37, 2543 (2008)
V. Vicinelli, P. Ceroni, M. Maestri, V. Balzani, M. Gorka, F. Vögtle, J. Am. Chem. Soc. 124, 6461 (2002)
N. McClenaghan, R. Passalacqua, F. Loiseau, S. Campagna, B. Verheyde, A. Hameurlaine, W. Dehaen, J. Am. Chem. Soc. 125, 5356 (2003)
H.B. Baudin, J. Davidsson, S. Serroni, A. Juris, V. Balzani, S. Campagna, L. Hammarström, J. Phys. Chem. A 106, 4312 (2002)
S. Campagna, C.D. Pietro, F. Loiseau, B. Maubert, N. McClenaghan, R. Passalacqua, F. Puntoriero, V. Ricevuto, S. Serroni, Coord. Chem. Rev. 229, 67–74 (2002)
R.G. Ispasoiu, L. Balogh, O.P. Varnavski, D.A. tomalia, T. Goodson III, J. Am. Chem. Soc. 122, 11005 (2000)
K.A. Green, M.P. Cifuentes, M. Samoc, M.G. Humphrey, Coord. Chem. Rev. 255, 2025 (2011)
P. Bhyrappa, J.K. Young, J.S. Moore, K.S. Suslick, J. Am. Chem. Soc. 118, 5708 (1996)
M. Kimura, T. Shiba, M. Yamazaki, K. Hanabusa, H. Shirai, N. Kobayashi, J. Am. Chem. Soc. 121, 5636 (1999)
M. Uyemura, T. Aida, J. Am. Chem. Soc. 124, 11392 (2002)
M. Uyemura, T. Aida, Chem. Eur. J. 9, 3492 (2003)
H.J. Brunner, Organomet. Chem. 500, 39 (1995)
H.-F. Chow, C.C. Mak, J. Org. Chem. 62, 5116 (1997)
P.B. Rheiner, D. Seebach, Chem. Eur. J. 5, 3221 (1999)
M. Kimura, Y. Sugihara, T. Muto, K. Hanabusa, H. Shirai, N. Kobayashi, Chem. Eur. J. 5, 3495 (1999)
A. Sen, K.S. Suslick, J. Am. Chem. Soc. 122, 11565 (2000)
S. Hecht, J.M.J. Fréchet, J. Am. Chem. Soc. 123, 6959 (2001)
D. Astruc, F. Chardac, Chem. Rev. 101, 2991 (2001)
L.J. Twyman, A.S.H. King, I.K. Martin, Chem. Soc. Rev. 31, 69–82 (2002)
C. Valério, E. Alonso, J. Ruiz, J.-C. Blais, D. Astruc, Angew. Chem. Int. Ed. 38, 1747 (1999)
M.-C. Daniel, J. Ruiz, J.-C. Blais, N. Daro, D. Astruc, Chem. Eur. J. 9, 4371 (2003)
C. Valério, J.-L. Fillaut, J. Ruiz, J. Guittard, J.-C. Blais, D. Astruc, J. Am. Chem. Soc. 119, 2588 (1997)
M.-C. Daniel, J. Ruiz, S. Nlate, J.-C. Blais, D. Astruc, J. Am. Chem. Soc. 125, 2617 (2003)
R.M. Crooks, M. Zhao, L. Sun, V. Chechik, L.K. Yeung, Acc. Chem. Res. 34, 181–190 (2001)
M. Zhao, R.M. Crooks, Adv. Mater. 11, 217 (1999)
M. Zhao, R.M. Crooks, Angew. Chem. Int. Ed. 38, 364 (1999)
M. Zhao, L. Sun, R.M. Crooks, J. Am. Chem. Soc. 120, 4877 (1998)
V. Chechik, R.M. Crooks, J. Am. Chem. Soc. 122, 1243 (2000)
L. Balogh, D.A. Tomalia, J. Am. Chem. Soc. 120, 7355 (1998)
M. Higuchi, S. Shiki, K. Ariga, K. Yamamoto, J. Am. Chem. Soc. 122, 4414 (2001)
K. Yamamoto, M. Higuchi, S. Shiki, M. Tsuruta, H. Chiba, Nature 415, 509 (2002)
K. Takanashi, H. Chiba, M. Higuchi, K. Yamamoto, Org. Lett. 6, 1709 (2004)
O. Enoki, H. Katoh, K. Yamamoto, Org. Lett. 5, 569 (2006)
K. Yamamoto, T. Imaoka, Acc. Chem. Res. 47, 1127 (2014)
K. Yamamoto, T. Imaoka, W.-J. Chun, O. Enoki, H. Katoh, M. Takenaga, A. Sonoi, Nature Chem. 1, 397 (2009)
R. Nakajima, M. Tsuruta, M. Higuchi, K. Yamamoto, J. Am. Chem. Soc. 126, 1630 (2004)
K. Yamamoto, M. Higuchi, A. Kimoto, T. Imaoka, K. Masachika, Bull. Chem. Soc. Jpn. 78, 349 (2005)
T. Imaoka, R. Tanaka, S. Arimoto, M. Sakai, M. Fujii, K. Yamamoto, J. Am. Chem. Soc. 127, 13896 (2005)
T. Imaoka, R. Tanaka, K. Yamamoto, Chem. Eur. J. 12, 7328 (2006)
K. Takanashi, A. Fujii, R. Nakajima, H. Chiba, M. Higuchi, Y. Einaga, K. Yamamoto, Bull. Chem. Soc. Jpn. 80, 1563 (2007)
T. Kambe, A. Watanabe, T. Imaoka, K. Yamamoto, Angew. Chem. Int. Ed. 55, 13151 (2016)
T. Kambe, T. Imaoka, K. Yamamoto, Chem. Eur. J. 22, 16406 (2016)
K. Albrecht, Y. Hirabayashi, M. Otake, S. Mendori, Y. Tobari, Y. Azuma, Y. Majima, K. Yamamoto, Sci. Adv. 2, e1601414 (2016)
K. Albrecht, Y. Kasai, Y. Kuramoto, K. Yamamoto, Chem. Commun. 49, 865 (2013)
N. Satoh, T. Nakashima, K. Kamikura, K. Yamamoto, Nat. Nanotechnol. 3(2), 106–111 (2008)
T. Imaoka, H. Kitazawa, W.-J. Chun, K. Yamamoto, Angew. Chem. Int. Ed. 54, 9810 (2015)
G.A. Crosby, J.N. Demas, J. Phys. Chem. 75, 991 (1971)
Acknowledgements
This study was supported by JSPS KAKENHI Grand Numbers 17K05804 and 15H05757, and JST ERATO Grand Number JPMJER1503.
Author information
Authors and Affiliations
Contributions
The manuscript was written based on contributions by the all authors. All the authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Kambe, T., Imaoka, T. & Yamamoto, K. Insight into the Effect of Dendrimer Structure on Photoluminescence from Assembled Bismuth Complexes. J Inorg Organomet Polym 28, 463–466 (2018). https://doi.org/10.1007/s10904-017-0705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0705-7