Abstract
Cationic cobaltocenium-containing polyelectrolytes have a unique ability to form ionic complex with various anionic species. We carried out two sets of model study to compare the relative binding strength of a cobaltocenium-containing polyelectrolyte. First, the nature and relative strength of intermolecular interaction between cobaltocenium-containing polyelectrolytes and different anionic probes were investigated by spectroscopic methods. A dye-displacement method was used to monitor absorbance and fluorescence emissions. Second, the binding strength of this cobaltocenium-containing polyelectrolyte was compared with a classical quaternary ammonium polymer. Formation of polyelectrolyte complex between the cobaltocenium-containing polyelectrolyte and a common anionic polyelectrolyte at various concentrations was examined by optical absorption and light scattering.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A polyelectrolyte refers to a macromolecule with ionically charged motifs either on the side chain or on the backbone. It is often termed as a polymeric-electrolyte with dual characteristics of a polymer and an electrolyte. The most significant property of polyelectrolytes turns out to be the water solubility, which opens the door for various applications in food industry,[1, 2] ultrafiltration,[3, 4] waste water treatment,[5, 6] fuel cells,[7,8,9,10] biomaterials [11,12,13,14] and nanotechnology [15,16,17,18]. Cationic polyelectrolytes have basic groups such as phosphonium, sulfonium, ammonium, imidazolium or pyridinium in their structure, while anionic polyelectrolytes have acidic groups like sulfonate, phosphate, carboxylate, phosphonate and arsenate [19,20,21]. Some of the commonly used polyelectrolytes are polystyrene sulfonate, polyacrylic and polymethacrylic acid along with their salts, DNA and other polybases and polyacids [22]. There have been notable advancements made in designing structures with respect to the nature of ionic moieties. Cationic metallocene such as cobaltocenium is particularly interesting as it possesses intrinsic cationic metal center coupled with counterions and is a good candidate to be used as an ionic center in polyelectrolytes. However, synthetic challenges posed by the high chemical stability of cobaltocenium limits the synthesis of cobaltocenium-containing polymers [23, 24].
Most of metallocene-containing electrolytes have been exclusively based on ferrocene and widely utilized for applications ranging from electrochemical sensors to templates for advanced materials to biomedicines [25,26,27,28,29,30,31,32,33,34]. Compared with ferrocene, it is difficult to prepare derivatives from cobaltocene or cobaltocenium by electrophilic substitution due to the ease in oxidation of cobaltocene and the inertness of cobaltocenium salts [35]. Thus, the incorporation of cobaltocene/cobaltocenium into polymers has been far less explored than ferrocene. Most of the initial work on cobaltocenium-containing polymers started off with synthesis of short main-chain polymers via condensation polymerization [36,37,38]. It was partly because of the straightforward synthesis of disubstituted cobaltocenium. Manners and coworkers were able to employ ring-opening polymerization (ROP) to prepare water soluble main-chain cobaltocenium-containing polymers [39, 40]. In addition, significant amount of efforts was directed to synthesize cobaltocenium-containing dendrimers [41,42,43,44]. Solid phase peptide synthesis was utilized by Metzler-Nolte and co-workers to make cobaltocenium bioconjugates using cobaltocenium acid [45, 46]. The development of practical synthesis of monosubstituted cobaltocenium achieved by us [47] and others [48] paved the way for the efficient and scalable synthesis of side-chain cobaltocenium-containing polyelectrolytes.
A major thrust for creating novel cobaltocenium polyelectrolytes is fueled by the progress in controlled radical polymerization (CRP) techniques [49]. Recently, we reported the synthesis of side-chain cobaltocenium-containing polyelectrolytes and their applications as advanced materials [50,51,52,53]. In addition, side-chain cobaltocenium-containing polyelectrolytes were utilized as novel antimicrobial materials to kill bacteria [54]. These cobaltocenium-containing polyelectrolytes were able to rejuvenate the traditional β-lactam antibiotics by forming an ionic interaction between carboxylic anions and cationic cobaltocenium moieties to inhibit the degradation of drugs. However, the effects of different parameters of small anionic probes such as electrostatic charge, size, conformation and π-electron system on the interactions of these useful polyelectrolytes have not been investigated. Also, the relative binding strength of these polymers in comparison to other cationic polymers is still a mystery.
To explore the ionic binding, we designed two separate experiments. First, a qualitative study was carried out with a fluorescence dye to make a complex with polymers followed by the addition of small anionic probes to displace the dye. The released amount of dye was measured using optical absorption, which gave the relative binding affinity of anionic probes to polymers. The second aspect of this study was to conduct the comparative study of binding strength of cobaltocenium-containing polyelectrolyte with anionic polyelectrolyte. Formation of polyelectrolyte complex (PEC) in various concentrations was examined, and the optical transmittance was measured.
2 Results and Discussion
2.1 Binding of Small Anions with Cobaltocenium-Containing Polymer
In this model study, we synthesized a cobaltocenium-containing polymer, poly(cobaltocenium methacrylate) (PCoCl)with molecular weight of M n = 19,000 g/mol via RAFT polymerization (Scheme 1). The disappearance of vinyl protons from methacrylate monomers around 5.0–6.2 ppm and appearance of broad peaks around 0.5–2.0 ppm in 1H NMR spectrum suggested the successful polymerization.
A number of small organic probes were selected to systematically investigate the relative binding affinity between polyelectrolytes and anionic probes. As shown in Table 1, the anionic probes in the same category differ by one key property, so that comparison of the relative affinities provides the important structural features for intermolecular interactions. The common techniques to investigate such intermolecular interactions like nuclear magnetic resonance (NMR) and mass spectrometry (MS) are not desirable in this situation. For effective NMR, higher concentration of samples is required but those concentrations of polymer-dye complex would result in a viscous solution with slow diffusion. Therefore, an optical spectroscopy method would be ideal as it is fast and efficient even at low concentrations [55, 56]. However, neither the polyelectrolytes nor the organic probes have any significant chromophore or fluorophore to monitor their binding ability. Thus, the introduction of a reporting agent in the system is necessary.
In this approach, a trianion dye, 5(6)-carboxyfluorescein (CF), was used as a fluorescent indicator because the binding affinity of monoanion and dianion towards polyelectrolytes would be too low at a lower concentration. All experiments were conducted in a 50 mM aqueous Tris buffer solution with pH 7.4 at 25 °C. The polymer-dye complex (P-CFn) was firstly made as shown in Scheme 2, and then the nonfluorescent displacer probe was introduced to displace the dye from its polymer-dye complex to form a polymer-probe complex (P-Dm). The polymer-probe complex can be monitored through the change in spectroscopic signature of the dye from its bound state to free state by UV–visible and fluorescence emission spectroscopy. The spectroscopic signature is represented by the free dye anion.
The ratio of the concentration of polymer to the concentration of dye is critical in the displacement experiment. When the polymer is used in excess, the dye would not fully bind to the polymer. This would result in unoccupied space in the polymer and resist any signal change. On the other hand, if the polymer is not enough, the excess dye would add to the displaced dye signature, which would result in the wrong estimation. We found the polymer could bind at least 85% dye (Fig. S1). The fluorescence intensity and absorbance isotherms profiles were obtained by the titrations of displacer probes into a buffered solution of the polymer–dye complex. By comparing isotherm profiles for different displacer anions, the important structural properties for the binding to these polymers can be estimated.
2.1.1 Electrostatic Charge
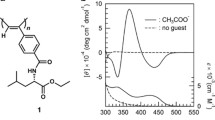
The effect of the electrostatic charge on the relative affinity for cobaltocenium polyelectrolytes was carried out by using a series of model carboxylate anions succinate, propane-1,2,3-tricarboxylate, and 1,2,3,4-butanetetracarboxylate, which respectively represents dianionic, trianionic, and tetraanionic species. The results showed that with the increase in charge in a guest anion, the binding affinity increased significantly (Fig. 1). On average, the increase of one negative charge in the probe could increase the relative binding affinity by one order of magnitude,[57] highlighting the importance of electrostatic interactions.
2.1.2 Guest Conformation
The effect of conformation of a guest anion on the relative affinity with the polymer was investigated by using cyclohexanetricarboxylate and tricarballylate. Both anions have three negative charges, but cyclohexanetricarboxylate is much more rigid due to its chair conformation whereas tricarballylate has less conformational restrictions because of linear backbone. As shown in the isotherms in Fig. S2, the two anions showed similar binding affinity.
In order to explore the effect of guest size on the relative binding affinity, succinate and pimelate anions were studied. Both anions have two negative charges but differ in carbon chain length. The electrostatic contribution in their binding to the polymer would be equal. The result showed no difference in the binding behavior between two linear anions with different chain length (Fig. S3). Thus, the size and conformation of the guest molecules do not have significant influence on binding affinity for the cobaltocenium polymer.
2.1.3 π-System
Finally, the effect of aromaticity of the displacer probe on the relative affinity was examined. For this purpose, benzene-1,3,5-tricarboxylate (trimesate) and 1,3,5-cyclohexanetricarboxylate anions were used. Both are trianions with similar size and conformation but have different π-electron system.
As shown in Fig. 2, the presence of π-system on guest anion (benzene-1,3,5-tricarboxylate) increases the affinity for cationic cobaltocenium significantly. The difference on the relative affinity is due to an interaction mediated by the aromatic core of the trimesate anion. The positive charge in the cobaltocenium cation induces significant polarization of the C–H bonds in the cyclopentadiene. One of these C–H bonds in cyclopentadiene ring interacts with the π-electron cloud of the guest species. The overall process of interaction is promoted by the electrostatic charge on cobaltocenium in the polymer (Fig. 3).
2.2 Comparative Study of Binding Strength of Cobaltocenium-Containing Polymer
The primary goal of this part of experiment was to compare the relative binding strength of cobaltocenium containing polymer with quaternary ammonium-containing polymer. For this purpose, PEC was formed between the cationic polymers under study and an anionic polymer, poly (sodium styrene sulfonate) (PSSNa), through strong Coulomb interactions. PEC has been used for various applications ranging from industrial applications for coatings, binders to biomedical and biotechnological applications [58].
Poly((2-dimethylamino)ethyl methacrylate) (PDMAEMA) is one of the widely used cationic polymers that have found applications in various fields as a polyelectrolyte [59,60,61,62]. Cationic PDMAEMA (referred simply as to PDMAEMA hereafter) with a similar structure to PCoCl was synthesized using RAFT polymerization (Scheme 3).
The degree of polymerization of both cationic polymers was controlled at 50 by tracking the monomer conversion during the polymerization process using 1H NMR. The molecular weight of PCoCl and PDMAEMA was 19,000 and 15,000 g/mol, respectively. Throughout the experiment, equivalent moles of PCoCl and PDMAEMA were used against the same volume of PSSNa to measure the optical transmittance. To get the quantitative results, the formation of PEC between a cationic polymer and PSSNa at various concentrations were examined by a UV–vis spectrophotometer at room temperature.
The transmittance of various polyelectrolyte solutions: a 1.0 mL 0.10 mM PDMAEMA solution with addition of different volumes of 0.01 mM PSSNa; b 1.0 mL 0.10 mM PCoCl solution with addition of different volumes of 0.01 mM PSSNa; c 1.0 mL 0.20 mM PDMAEMA solution with addition of different volumes of 0.01 mM PSSNa; d 1.0 mL 0.20 mM PCoCl solution with addition of different volumes of 0.01 mM PSSNa; e Transmittance ratio (Tt/To) as a function of volumes of PSSNa (mL) added with each concentration of PCoCl and PDMAEMA solutions at 0.10 mM; f Transmittance ratio (Tt/To) as a function of volumes of PSSNa (mL) added with each concentration of PCoCl and PDMAEMA solutions at 0.20 mM
As shown in Fig. 4, the transmittance of polyelectrolyte solutions (Tt) was compared with that of deionized water (To) as described by the ratio of Tt/To as a function of volume of PSSNa added. The difference observed in the transmittance of PEC solution could provide information about the relative binding strength between cationic polyelectrolytes and the anionic polyelectrolyte. Initially, 1.0 mL of 0.10 mM solutions of PCoCl and PDMAEMA was placed in two different cuvettes, and the transmittance was measured separately for both polymer solutions. Then, 0.10 mL PSSNa solution (0.01 mM) was added to both cuvettes. The transmittance of the resulting PEC solution was measured accordingly. The volume of PSSNa was increased each time by 0.10 mL until it reached 1.0 mL. The initial solutions of both cationic polymers were transparent. With the increase amount of PSSNa, the solutions gradually turned milky and turbid, formed opaque suspension, and then finally precipitated. The transmittance ratio clearly showed a higher decrease for PCoCl than PDMAEMA. When the concentration of each cationic polymer solution was 0.10 mM, the final transmittance caused by PCoCl was decreased to 52% as opposed to 74% by PDMAEMA. The decrease in %T was even more prominent when 0.20 mM cationic polymer solutions were used. The final %T with PCoCl was about 41% while it was close to 69% for PDMAEMA.
A separate experiment was carried out by increasing the concentration of PSSNa to 0.02, 0.03 and 0.07 mM. Accordingly, 1.0 mL of higher concentration PSSNa was added to 1.0 mL 0.01 mM cationic polymer solutions. With the addition of 1.0 mL 0.03 mM PSSNa, the reduction in transmittance was quite noteworthy for PCoCl. It decreased to 45% as compared to 66% for PDMAEMA (Fig. S9). Higher concentrations of PSSNa provided negatively charged motifs in excess so that cationic polymers can bind instantly, leading to a sudden decrease in transmittance. The effect of higher concentration was prominent when 0.07 mM PSSNa was used. The transmittance of PDMAEMA decreased to 65%, while the final transmittance of PCoCl reached down to 35%. It can be inferred that more PECs were formed in case of PSSNa-PCoCl than PSSNa-PDMAEMA as a result of stronger binding between PSSNa and PCoCl. Any concentrations higher than 0.07 mM PSSNa resulted in instant precipitation.
We further studied the size of PEC particles resulted from the mixing of cationic and anionic polymers by dynamic light scattering (DLS). As shown in Fig. 5, the average diameter of PDMAEMA-PSSNa particles was about 4 µm, much larger than the average size of 300 nm for PCoCl-PSSNa particles. Based on these findings a more compact structure for PCoCl-PSSNa can be speculated in comparison to PDMAEMA-PSSNa.
3 Conclusions
In summary, we carried out two model studies to investigate the nature and relative strength of intermolecular interactions between cobaltocenium-containing polyelectrolytes and anionic probes using optical spectroscopy methods. We found that the electrostatic charge and the interaction with a π-electron moiety in the small molecular probes are the most influential factors in determining the binding affinity. However, the conformation and size of anionic probes do not significantly influence the binding affinity with the cationic polyelectrolyte. Furthermore, the polyelectrolyte complexes between a cationic cobaltocenium polymer and an anionic polymer were prepared to compare with a cationic quaternary ammonium polymer. The results demonstrated the cobaltocenium-containing polymer showed significantly higher binding with the anionic polymer than the quaternary ammonium counterpart. This work facilitated a better understanding on the fundamental interactions between cobaltocenium-containing polyelectrolytes and anionic probes.
4 Experimental
4.1 Characterization
1H NMR spectra (400 MHz) were recorded on a Varian Mercury 400 NMR spectrometer with tetramethylsilane (TMS) as an internal reference. UV–vis was carried out on a Shimadzu UV-2450 spectrophotometer with a 10.00 mm quartz cuvette and monochromatic light of various wavelengths over a range of 190–900 nm. Dynamic light scattering (DLS) was operated on a Nano-ZS instrument, model ZEN 3600 (Malvern Instruments. Fluorescence emission intensity was measured by using Cary Eclipse Fluorescence Spectrophotometer).
4.2 Materials and Methods
2-Cobaltocenium amidoethyl methacrylate hexafluorophosphate (CoAEMAPF6) was synthesized according to our earlier report [51]. 2-Aminoethyl methacrylate hydrochloride (90%), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC-HCl, 98%), 4-(dimethylamino) pyridine and tetrabutylammonium chloride (TBACl) were purchased from Aldrich and used as received. Tris buffer solution was prepared from tris(hydroxymethyl)aminomethane (Tris base) (Sigma–Aldrich) and concentrated hydrochloric acid (Sigma–Aldrich). Poly(sodium styrene sulfonate) (Mn = 70,000 g/mol) was purchased from Sigma as an anionic polyelectrolyte. The fluorescent probe 5(6)-carboxyfluorescein (as a mixture of isomers) was purchased from Sigma–Aldrich. Displacer anion solutions were prepared from succinic acid (Sigma), tricarballylic acid and 1,4-cyclohexanedicarboxylic acid (Acros), 1,2,3,4-butanetetracarboxylic acid and pimelic acid (Alfa Aesar), trimesic acid and 1,3,5-cyclohexanetricarboxylic acid (TCI America). Water was from Thermo Scientific with ion conductivity at 18.20 MΩ. Azobisisobutyronitrile (AIBN) was recrystallized from methanol before use. All other chemicals were from commercial sources and used as received.
4.2.1 Synthesis of Cationic (2-dimethylamino)ethyl methacrylate (DMAEMA)
Neutral DMAEMA (5.00 g, 31.90 mmol) was dissolved in 30 mL of dry DCM and chilled to 0 °C. Iodomethane (9.00 g, 63.80 mmol) was then added dropwise to the solution under stirring and reacted for 30 min at room temperature. After precipitation in ether, a white powder was obtained with a yield of 87%.
4.2.2 Synthesis of Cationic Poly((2-dimethylamino)ethyl methacrylate) (PDMAEMA)
Throughout this experiment cationic PDMAEMA will be used and will be referred as simply PDMAEMA. Cationic DMAEMA (0.50 g, 2.42 mmol), RAFT agent (6.75 × 10−3 g, 24.20 × 10−3 mmol) and AIBN (1.20 × 10−3 g, 7.31 × 10−4 mmol) were dissolved in 1.5 mL of dry MeOH in a 10 mL Schlenk flask and degassed by three cycles of freeze–pump–thaw. The reaction was heated under 70 °C until the desired conversion was reached and quenched by opening to air and cooled with ice water. The reaction was precipitated in acetone three times and vacuum-dried to afford a pink powder.
4.2.3 Synthesis of 2-Cobaltoceniumamidoethyl Methacrylate Hexafluorophosphate (MAEACoPF6)
An amidation reaction was employed to synthesize MAEACoPF6. Cobaltocenium carboxylic acid hexafluorophosphate (2.00 g, 5.29 mmol), 2-aminoethyl methacrylate hydrochloride (0.94 g, 5.68 mmol), and 4-(dimethylamino) pyridine (0.13 g, 1.06 mmol) were dissolved in 20 mL dry acetonitrile and the solution was cooled to 0 °C. Solution of EDC-HCl (1.10 g, 5.74 mmol) was then slowly added into previously cooled solution. Then, dry triethylamine (1.60 g, 15.80 mmol) was added into the reaction. The reaction was stirred for 4 h. Solution was then extracted by saturated sodium hexafluorophosphate aqueous solution three times to remove unreacted starting materials. The organic phase was collected, condensed and precipitated into diethyl ether. Yellow solid was collected and dried under vacuum overnight. Yield: 1.60 g, 58%. 1H NMR (CD3COCD3, δ, ppm): 8.30 (broad, NHCH2, 1 H), 6.42 (t, Cp, 2 H), 6.22 (m, CH2C, 1 H), 6.10 (t, Cp, 2 H), 5.92 (s, Cp, 5 H), 5.62 (m, CH2C, 1 H), 4.42 (m, OCH2CH2NH, 2 H), 3.66 (m, OCH2CH2NH, 2 H), 1.94 (m, CH3CCO, 3 H).
4.2.4 Synthesis of Poly(2-(methacrylolyamide) ethyl cobaltoceniumcarboxylate hexafluorophosphate) via RAFT polymerization. (PCoPF6)
MAECoPF6 (0.30 g, 0.61 mmol), cumyl dithiobenzoate (CDB) (1.67 × 10−3 g, 6.10 × 10−3 mmol) and AIBN (0.20 × 10−3 g, 1.23 × 10−3 mmol) were dissolved by 0.40 mL DMF in a 10 mL Schlenk flask and then were purged with N2 for 30 min. The polymerization was started by heating the mixture at 90 °C. Samples were taken out periodically to monitor the monomer conversion. Once the desired conversion was reached, polymerization reaction was quenched by exposure to air. The reaction mixture was then precipitated in cold DCM three times and vacuum dried overnight. Yield: 150 × 10−3 g, 80%. 1H NMR (CD3CN, δ): 7.4 (broad NHCH2), 6.2, 5.9, 5.8 (m, m, s, Cp), 4.2(broad, CH2OO) 3.6 (broad, NHCH2), 1.8 (broad, CH2C), 0.6–1.0 (broad, CCH3).
4.2.5 Titration Conditions
All experiments were carried out in aqueous solutions buffered to pH 7.4 with Tris buffer solution at a temperature of 25 °C. The pH of the working solutions was adjusted prior to use by addition of NaOH or HCl solution and checked during a titration to make sure that it stayed at the desired value of 7.4. The concentration of polyelectrolytes in all experiments was maintained at 1.00 × 10−6 M.
4.2.6 Binding Isotherm
Stock solutions of dye, polymer, and displacer anions in a buffered solution were used as starting points for all experiments. All solutions used in this study were made by dilution of aliquots of these stock solutions. Binding isotherm experiment was conducted using constant polyelectrolytes (1.00 × 10−6 M) and different fluorophore concentration. Absorbance and fluorescence emission readings were blanked by subtracting the corresponding reading for the buffer. The resulting data were plotted as a function of the [polyelectrolytes]/[fluorophore] ratio to produce binding isotherms.
4.2.7 General Displacement Titration Protocol
Displacement experiments were carried out using two separate working solutions: a “titrant” and a “cuvette” solution. Titrant and cuvette solutions were made fresh for each experiment. A cuvette solution contained both the dye ([CF] = 2.00 × 10−6 M) and the polyelectrolyte ([P] = 1.00 × 10−6 M) at an appropriate ratio to form the desired bound dye complex (P-CFn). The titrant solution contained a displacer anion at an appropriate concentration to carry out the titration. Titrations on benchtop instruments were carried out by addition of different aliquots of titrant solution to a constant volume of cuvette solution. The total volume of mixture was kept constant by addition of buffer solution. Using this method, the concentration of dye and polyelectrolyte remained constant but the concentration of displacer anion varied throughout the titration. The resulting data from fluorescence and UV–vis spectroscopy were plotted as a function of the [displacer anion]/[fluorophore] ratio to produce fluorescence intensity and absorbance isotherms.
4.2.8 Measurement of Transmittance
A typical experiment is described as an example. 1.00 mL of 0.10 mM PCoCl and PDMAEMA was placed in two different glass cuvettes followed by the measurement of transmittance. Different volumes of PSSNa (concentration = 0.10 mM) from 0.10 to 1.00 mL was added to both cuvettes. The addition of PSSNa was increased each time by 0.10 mL and subsequent transmittance of the resulting PEC solution was measured.
References
J. Weiss, P. Takhistov, D.J. McClements, J. Food Sci. 71, R107 (2006)
D. Guzey, D.J. McClements, Adv. Colloid Interface Sci. 128, 227 (2006)
I. Korus, K. Loska, Desalination 247, 390 (2009)
C.-W. Li, C.-H. Cheng, K.-H. Choo, W.-S. Yen, Chemosphere 72, 630 (2008)
B. Bolto, J. Gregory, Water Res. 41, 2301 (2007)
L.A. Chen, R.G. Carbonell, G.A. Serad, Water Res. 34, 510 (2000)
B.R. Einsla, Y.S. Kim, M.A. Hickner, Y.-T. Hong, M.L. Hill, B.S. Pivovar, J.E. McGrath, J. Membr. Sci. 255, 141 (2005)
S.P. Jiang, Z. Liu, Z.Q. Tian, Adv. Mater. 18, 1068 (2006)
B. Smitha, S. Sridhar, A.A. Khan, Macromolecules 37, 2233 (2004)
Y. Wan, B. Peppley, K.A.M. Creber, V.T. Bui, E. Halliop, J. Power Sources 185, 183 (2008)
C. Boura, P. Menu, E. Payan, C. Picart, J.C. Voegel, S. Muller, J.F. Stoltz, Biomaterials 24, 3521 (2003)
P.-H. Chua, K.-G. Neoh, E.-T. Kang, W. Wang, Biomaterials 29, 1412 (2008)
G. Kumar, Y.C. Wang, C. Co, C.-C. Ho, Langmuir 19, 10550 (2003)
C. Vodouhê, E.L. Guen, J.M. Garza, G. Francius, C. Déjugnat, J. Ogier, P. Schaaf, J.-C. Voegel, P. Lavalle, Biomaterials 27, 4149 (2006)
J. García-Serrano, U. Pal, A.M. Herrera, P. Salas, C. Ángeles-Chávez, Chem. Mater. 20, 5146 (2008)
T.K. Sau, C.J. Murphy, J. Am. Chem. Soc. 126, 8648 (2004)
T.K. Sau, A.L. Rogach, Adv. Mater. 22, 1781 (2010)
A. Rabiee, J. Vinyl Add. Technol. 16, 111 (2010)
A.M. Herrera González, M. Caldera Villalobos, J. García-Serrano and A.A. Peláez Cid, Des. Monomers Polym. 19, 330 (2016)
A. Laschewsky, Curr. Opin. Colloid Interface Sci. 17, 56 (2012)
F. Zhang, Y. Zhou, Y. Chen, Z. Shi, Y. Tang, T. Lu, J. Colloid Interface Sci. 351, 421 (2010)
A.V. Dobrynin, M. Rubinstein, Prog. Polym. Sci. 30, 1049 (2005)
L. Ren, C.G. Hardy, C. Tang, J. Am. Chem. Soc. 132, 8874 (2010)
Y. Yan, P. Pageni, M.P. Kabir, C. Tang, Synlett 27, 984 (2016)
C.G. Hardy, L. Ren, T.C. Tamboue and C. Tang, J. Polym. Sci. Part A 49, 1409 (2011)
W. Kaminsky, J. Polym. Sci. Part A 42, 3911 (2004)
A.S. Abd-El-Aziz, I. Manners, Frontiers in Transition Metal-Containing Polymers, (Wiley, Hoboken, 2007)
G.R. Whittell, M.D. Hager, U.S. Schubert, I. Manners, Nat. Mater. 10, 176 (2011)
D. Astruc, Eur. J. Inorg. Chem. 2017, 6 (2017)
D.A. Foucher, B.Z. Tang, I. Manners, J. Am. Chem. Soc. 114, 6246 (1992)
D. Astruc, Nat. Chem. 4, 255 (2012)
E.W. Neuse, J. Inorg. Organomet. Polym. Mater. 15, 3 (2005)
A.I. Mufula, B. Aderibigbe, E.W. Neuse, H.E. Mukaya, J. Inorg. Organomet. Polym. Mater. 22, 423 (2012)
E.W. Neuse, M.G. Meirim, D.D. N” Da and G. Caldwell. J. Inorg. Organomet. Polym. 9, 221 (1999)
J.E. Sheats, M.D. Rausch, J. Org. Chem. 35, 3245 (1970)
C. Pittman Jr., O. Ayers, S. McManus, J. Sheats, C. Whitten, Macromolecules 4, 360 (1971)
C.E. Carraher, G.F. Peterson, J.E. Sheats, T. Kirsch, Macromol. Chem. Phys. 175, 3089 (1974)
C. Pittman, O. Ayers, B. Suryanarayanan, S. McManus and J. Sheats, Die Makromol. Chem. 175, 1427 (1974)
U.F. Mayer, J.B. Gilroy, D. O’Hare, I. Manners, J. Am. Chem. Soc. 131, 10382 (2009)
J.B. Gilroy, S.K. Patra, J.M. Mitchels, M.A. Winnik, I. Manners, Angew. Chem. Int. Ed. 50, 5851 (2011)
D. Astruc, C. Ornelas, J. Ruiz, Acc. Chem. Res. 41, 841 (2008)
C.M. Casado, B. González, I. Cuadrado, B. Alonso, M. Morán, J. Losada, Angew. Chem. 112, 2219 (2000)
C. Ornelas, J. Ruiz, D. Astruc, Organometallics 28, 2716 (2009)
K. Takada, D.J. Díaz, H.D. Abruña, I. Cuadrado, B. González, C.M. Casado, B. Alonso, M. Morán and J. Losada, Chemistry 7, 1109 (2001)
A. Maurer, H.B. Kraatz, N. Metzler-Nolte, Eur. J. Inorg. Chem. 2005, 3207 (2005)
A. Gross, D. Habig, N. Metzler-Nolte, ChemBioChem 14, 2472 (2013)
Y. Yan, J. Zhang, Y. Qiao and C. Tang, Macromol. Rapid Commun. 35, 254 (2014)
S. Vanicek, H. Kopacka, K. Wurst, T. Müller, H. Schottenberger, B. Bildstein, Organometallics 33, 1152 (2014)
K. Matyjaszewski, B.S. Sumerlin, N.V. Tsarevsky (eds.), Progress in Controlled Radical Polymerization: Mechanisms and Techniques, (American Chemical Society, Washington, 2012)
J. Zhang, L. Ren, C.G. Hardy, C. Tang, Macromolecules 45, 6857 (2012)
J. Zhang, J. Yan, P. Pageni, Y. Yan, A. Wirth, Y.-P. Chen, Y. Qiao, Q. Wang, A.W. Decho, C. Tang, Sci. Rep. 5, 11914 (2015)
J. Zhang, Y. Yan, M.W. Chance, J. Chen, J. Hayat, S. Ma, C. Tang, Angew. Chem. Int. Ed. 52, 13387 (2013)
J. Zhang, Y. Yan, J. Chen, W.M. Chance, J. Hayat, Z. Gai, C. Tang, Chem. Mater. 26, 3185 (2014)
J. Zhang, Y.P. Chen, K.P. Miller, M.S. Ganewatta, M. Bam, Y. Yan, M. Nagarkatti, A.W. Decho, C. Tang, J. Am. Chem. Soc. 136, 4873 (2014)
A. M. Jolly and M. Bonizzoni, Supramol. Chem. 27, 151 (2015)
A.M. Mallet, A.B. Davis, D.R. Davis, J. Panella, K.J. Wallace, M. Bonizzoni, Chem. Commun. 51, 16948 (2015)
A.M. Jolly, M. Bonizzoni, Macromolecules 47, 6281 (2014)
S. Chen, M. Liu, S. Jin and Y. Chen, Polym. Int. 56, 1305 (2007)
S. Agarwal, Y. Zhang, S. Maji and A. Greiner, Mater. Today 15, 388 (2012)
J. Niskanen, C. Wu, M. Ostrowski, G.G. Fuller, S. Hietala, H. Tenhu, Macromolecules 46, 2331 (2013)
M. Thomas, M. Gajda, C. Amiri Naini, S. Franzka, M. Ulbricht, N. Hartmann, Langmuir 31, 13426 (2015)
W. Xu, I. Choi, F.A. Plamper, C.V. Synatschke, A.H.E. Müller, Y.B. Melnichenko, V.V. Tsukruk, Macromolecules 47, 2112 (2014)
Acknowledgements
The support from National Institutes of Health (R01AI120987) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pageni, P., Kabir, M.P., Yang, P. et al. Binding of Cobaltocenium-Containing Polyelectrolytes with Anionic Probes. J Inorg Organomet Polym 27, 1100–1109 (2017). https://doi.org/10.1007/s10904-017-0561-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-017-0561-5