Abstract
In this article we present results of frequency dependent conductivity and dielectric behavior investigation of Cu doped polyindole in presence of poly(vinyl acetate). As-synthesized composites were analyzed via X-ray diffractometer, fourier transform infrared spectra spectrum, dielectric behavior and ac conductivity measurements. The Cu doped PIN/PVAc composites represent semi-crystalline nature confirmed through X-ray diffraction analysis. The dielectric behavior study of prepared composites reveals that dielectric constants and dielectric loss decreases with increase in frequency. The highest ac conductivity value of composites was estimated as 2.97 × 10−5 S/cm at frequency 1 MHz.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the field of metal doped conducting polymer composites investigations of their electrical conduction, dielectric behavior with vary in temperature and frequency is very essential. The electrical properties are apprehensive through the dielectric behavior, frequency dependence on composites resistivity. Thus, the electrical properties mainly interrelated to high-frequency applications. The two major points of view are responsible for the materials characteristics and properties. A quantitative understanding of electronic and ionic conduction through the materials properties, this is main view of physicists. For the electrical measurements, materials contain particular property values and characteristics so that engineers consider them mainly as components in electrical circuits [1, 2].
The doped conducting polymers can be represents the electrical properties of both conducting polymers and doped polymers. Moreover it may be shows the electrical properties like metals. It means the electrical conductivity changed from insulator region to semiconductor region and semiconductor region to metallic region. Thus, these composite materials are got extensive attention world-wide in the field of research and development due to their potential applications. The composite materials represent the improved properties which cannot be achieved by single materials. Therefore, the combination of inorganic materials with conducting polymers can made this composite material that reveals the properties of both materials [3–6]. The polyindole (PIN) get extensive significance because of its exceptional conduction mechanism and environment stability as compared to other conducting polymers. The innovative polymer composite materials with specific electrical properties can be produce by blending of conductive polymer and non-conducting polymer matrix. The conducting polymer gives required conducting properties and non-conducting polymer matrix improves the mechanical properties; it can be obtained by synthesizing conducting polymer composites [7, 8].
Ryu et al. investigated the physiochemical properties of polyindole/thiol composites [9]. Arslan et al. reported the dielectric and ER properties of kaolinite, PIN and PIN/kaolinite composite suspensions [10]. Tayalan et al. synthesized the PIN/PVC composite films to modify thermal, mechanical and conductivity of PIN, PVC [11]. Bhagat et al. studied the photoluminescence and optical properties of poly(vinyl acetate)/polyindole composites [12]. These researchers were reported their research work with the goal of developing conducting polymer composites.
The aim of this research work is the investigation on frequency dependent conductivity and dielectric behavior of Cu doped polyindole in presence of poly(vinyl acetate). The preparation of Cu doped polyindole/poly(vinyl acetate) (PIN/PVAc) composites was completed by the chemical polymerization of indole monomer using oxidant cupric chloride. The synthesized samples are analyzed through XRD, FTIR and frequency dependent dielectric behavior as well as ac conductivity measurements.
2 Experimental
All chemicals were analytical grade and purchased from SD Fine Chemicals, India, such as monomer indole, oxidizing agent cupric chloride (CuCl2), methanol used as organic solvent. The matrix polymer poly(vinyl acetate) (PVAc) purchased from Hi-media Chemicals, India.
Cu doped polyindole/poly(vinyl acetate) (PIN/PVAc) composites were produced by chemical polymerization technique at room temperature. The CuCl2 was used as an oxidant. A mixed solution of PVAc and methanol was prepared by taking 1:9 ratios and stirred about 1 h and kept it stable for 24 h in order to make the homogenous solution. Subsequently, monomer indole (0.5 g) was added into PVAc solution and continuously stirred to make homogeneous solution for 1 h. The oxidant cupric chloride with varying weight percentage (20, 30, 40 %) added in above mixture solution to polymerized the indole monomer respectively and continuously stirred about 2 h. The composite solutions were kept about 1 h to obtain clear (bubble free) solution. After that this composite solutions was poured on the optically plane and chemically cleaned glass plate to developed films. An isothermal evaporation of solvent method was used to dry the films. Thus, the entire assembly was put in a dust free chamber kept at constant temperature. Once the loss of solvent the films were washed with hot doubly distilled water and take out from the glass plate. Subsequently, composite films were once more dried out for 6 h at room temperature. Same method was adopted for preparation of three samples of different wt% of oxidant.
A Fourier transform infrared spectrum (FTIR) of samples was recorded on SHIMADZU FTIR-8400. X-ray diffractometer (XRD) taken on Rigaku, Miniflex-II, Japan and the XRD spectrum of samples were determined using CuKα (λ = 1.5406 Å). The ac conductivity measurements of samples were recorded on 4284 A Precision LCR meter (20 Hz–1 MHz, over 8610 selectable frequencies), Agilent Technology, Singapore.
3 Results and Discussion
3.1 FTIR Analysis
The FTIR spectrum of Cu doped PIN/PVAc composites represents in Fig. 1. The peaks at 644 cm−1 is ascribed to CH=CH bond. The peaks at 773, 2962 cm−1 is ascribed to the C–H vibration of indole units. The peak at 1131 cm−1 ascribed to C–N stretch. The presence of N–H stretch in spectrum is confirmed by peaks at 1396, 3397 cm−1. The peak at 1630 cm−1 ascribed to C=C stretch. The peak at 2477 cm−1 ascribed to amine due to doping of Cl-ions. The four major peaks at 1283, 1396, 1765, 2962 cm−1 are characteristics peaks of poly(vinyl acetate) [13–21].
3.2 XRD Analysis
The XRD patterns for the Cu doped PIN/PVAc composites are shown in Fig. 2. The XRD patterns represent the broad hump and noisy peaks that indicates amorphous nature of prepared samples. Moreover, some characteristics peaks are present in XRD patterns of all samples that attributed to semi-crystalline phase in composites. The existence of a few major peaks, broad hump and noisy peaks shows the semi-crystalline nature of synthesized composite materials. The intensity of major peaks rises with raise in wt% of oxidizing agent concentration. Also, it can be directly seen from figure that the semi-crystalline nature of composites raises with raise in wt% of oxidizing agent concentration [14]. The presence of major peaks in XRD patterns confirm the doping of Cu in prepared composites. Otherwise the XRD patterns show only the noisy peaks with broad hump which indicates materials contain amorphous nature. The major peaks present in XRD patterns agree with ICDD file number 01-078-7708 and confirms the successful synthesis of metal doped conducting polymer composites that is Cu doped PIN/PVAc composites [22].
3.3 Dielectric Behavior and AC Conductivity Measurements
Using the values of equivalent parallel capacitance (Cp), and parallel equivalent resistance (Rp) that are taken on LCR meter at selected frequency range (f), the dielectric constants (ε′ and ε″), dielectric loss (tanδ) and ac conductivity (σac) parameters have been determined. The dielectric behavior and ac conductivity measurements of samples are estimated in the frequency range 1 KHz–1 MHz and the temperature in the range 313–373K.
3.3.1 Dielectric Constants
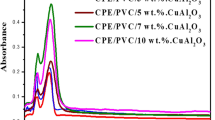
Figure 3 shows the variation of the real part of dielectric constant (ε′) with an increase in frequency for Cu doped PIN/PVAc composites for different wt% of oxidizing agent. The real part of dielectric constant regularly reduces with increase in frequency and reaches a constant value at high frequency. This may be ascribed to the tendency of dipoles in macromolecules to orient themselves in the direction of the applied field in the low frequency range. Temporarily, in high frequency range the dipoles would hardly be able to orient themselves in the direction of the applied field and hence the value of the dielectric constant is nearly constant [23].
The variation of imaginary part of the dielectric constant (ε″) with an increase in frequency for different wt% of oxidizing agent is shown in Fig. 4. The imaginary part of the dielectric constant continuously decreases with increase in frequency and reach steady value at high frequency for all composites. The maximum value of ε″ is belong to 30 wt% sample might be due to dielectric relaxations. The dielectric constants (ε′ and ε″) values increase up to 30 wt% and decrease with further increase in oxidant concentration.
3.3.2 Dielectric Loss
The Fig. 5 reflects the plot of dielectric loss with an increase in frequency. The dielectric loss have been calculated from given equation [24],
Where, tanδ is the tangent loss, Cp is the parallel capacitance, Rp is the parallel resistance and f is the frequency. It is directly investigated form figure that dielectric loss decreases regularly with increase in frequency for all samples. It is more exciting that the dielectric loss is higher for 30 wt% of Cu doped PIN/PVAc composites that possibly owing to the boost in interaction leads to higher crystalinity (it correlates with XRD data). In dielectric composite samples, the change of positive and negative space charge distribution in interfaces is possibly due to the interface with a huge volume portion have a great amount of defects, such as vacancies and vacancy clusters. These space charges move when subjected to an electric field. The number of dipole moments are created after the space charges trapped by defects. These dipole moments are effortless to follow variations in the electric field at low frequency [25, 26]. Hence, at low frequency the dielectric constant (real and imaginary) and dielectric loss demonstrate a large value. In overlap potential well, small amount of holes perform inter well hopping repeal the direction of motion while the electric field direction reverses. Therefore, at low frequency for the dielectric relaxation there is a contribution by inter well hopping [26].
3.3.3 The AC Conductivity Measurements
It is fairly recognized information that the frequency dependent ac conductivity in case of the disordered materials like polymers/conducting polymers can be caused by the interfacial polarization at the contacts, grain boundaries and other in homogeneities present in the sample [27]. The ac conductivity of conductor/insulator composites has been modeled through the resistance–capacitance (RC) network, where the conducting dispersants are represented as a resistor and dielectric of the insulating matrix is represented by the capacitors [28, 29]. Figure 6 reveals the plot of ac conductivity (σac) versus frequency. It reveals that the ac conductivity increases with increase in frequency. Also, the temperature affects the conductivity that can be observed from the figure. The σac can be determined from the given expression [24, 30],
Where, σac is the ac conductivity, ε′ is the real part of dielectric constant, f is the applied frequency (Hz), tanδ is the dielectric loss [24, 30]. It is clearly seen that the ac conductivity of Cu doped PIN/PVAc composites increases for 20 and 30 wt% samples and reduces for 40 wt% samples.
The highest ac conductivity value determined as 2.97 × 10−5 S/cm at frequency 1 MHz for 30 wt% sample. This could be owing to the extensive chain length of polyindole that help the polarization of charge carriers, which can support for the extra amount of charge carriers to polarize among favorable localized sites results into a improve in ac conductivity. Further, the drop off in ac conductivity might be owing to the low polarization of charge carriers [24, 27–29]. The values of ac conductivity (σac) for Cu doped PIN/PVAc composites at a frequency in the range 1 KHz–1 MHz are given in Table 1.
4 Conclusions
The FTIR analysis concluded that Cu doped PIN/PVAc composites with different wt% were prepared by chemical polymerization of monomer indole. The XRD analysis reveals the semi-crystalline nature of Cu doped PIN/PVAc composites. The results of XRD, dielectric constant, dielectric loss and ac conductivity show strong dependence on weight percent of oxidant concentration. The ac conductivity increases because of hopping of polarons from one localized states to another localized states at higher frequency. It concluded from electrical conductivity studies that 30 wt% of Cu doped PIN/PVAc composites have highest conductivity and it was determined as 2.97 × 10−5 S/cm at frequency 1 MHz.
References
T. W. Kim, D. F. Zeigler, O. Acton, H. L. Yip, H. Ma, K. Y. Alex, J. Adv. Mat. 24, 828 (2012)
S. Roy, K.R. Anilkumar, M.V.N.A. Prasad, J. Appl. Polym. Sci. 121, 675 (2011)
H. Bodugoz-Senturk, O. Guven, Radiat. Phys. Chem. 80, 153 (2011)
D. V. Brezoi, R. M. Ion, Sens. Acutators B 109, 171 (2005).
M. Babazadeh, F.R. Gohari, A.J. Olad, J. Appl. Polym. Sci. 123, 1922 (2012)
M.G. Hosseini, I.R. Bagheri, I.R. Najjar, J. Appl. Polym. Sci. 121, 3159 (2011)
D.J. Bhagat, G.R. Dhokane, Mater. Lett. 136, 251 (2014)
D.J. Bhagat, G.R. Dhokane, Appl. Surf. Sci. 337, 230 (2015)
K.S. Ryu, N.G. Park, K.M. Kim, Y.G. Lee, Y.J. Park, S.J. Lee, C.K. Jeong, J. Joo, S.H. Chang, Synth. Met. 135–136, 397 (2003)
Y. Arslan, H.I. Unal, H. Yilmaz, B. Sari, J. Appl. Polym. Sci. 104, 3484 (2007)
N. B. Tayalan, B. Sari, H. I. Unal, J. Polym. Sci. Part B Polym. Phys. 48, 1290 (2010).
D.J. Bhagat, G.R. Dhokane, Appl. Surf. Sci. 351, 1440 (2015)
O. Eraldmir, B. Sari, A. Gok, H.I. Unal, J. Macromol. Sci. Part A Pure Appl. Chem. 45, 205 (2008)
D.J. Bhagat, G.R. Dhokane, Electron. Mater. Lett. 11, 346 (2015)
S. H. Goh, H. S. Chan, C. H. Ong, Polymer 37, 2675 (1996).
S. Goel, N.A. Mazumdar, A. Gupta, Appl. Surf. Sci. 256, 4426 (2010)
D. J. Bhagat, G. R. Dhokane, Macromol. Symposia 362, 145 (2016).
S. An, T. Abdiryim, Y. Ding, I. Nurulla, Mater. Lett. 62, 935 (2008)
C. Zhijiang, Y. Guang, Synth. Met. 160, 1902 (2010)
K. Girirbabu, R. Manigandan, R. Suresh, L. Vijayalaxmi, A. Stephen, V. Narayan, Chem. Sci. Trans. 2, S13 (2013).
D.J. Bhagat, G.R. Dhokane, Chem. Phys. Lett. 619, 27 (2015)
T. Malcherek, J. Schluter, Acta Cryst. 65, 334 (2009).
C. Huang, Q.M. Zhang, G. deBottom, K. Bhattacharya, Appl. Phys. Lett. 84, 4391 (2004)
S.G. Vidhale, N.G. Belsare, A.S. Wadatkar, Int. J. Sci. Eng. Res. 4, 1755 (2013)
G.M. Tsangaris, G.C. Psarras, J. Mater. Sci. 34, 2151 (1999)
C. Mo, L. Zhang, G. Wang, Nanostruct. Mater. 6, 823 (1995)
N.F. Mott, E.A. Davis, Electronic Processes in Non-crystalline Materials. (Clarendon Press, Oxford, 1979)
Z.H. Wang, A. Ray, A.G. MacDiarmid, A.J. Epstein, Phys. Rev. B 43, 4373 (1991)
B. Abeles, P. Sheng, M.D. Coutts, Y. Arie, Adv. Phys. 31, 407 (1973)
D. J. Bhagat, G. R. Dhokane, J. Mater. Sci. Mater. Electron. doi:10.1007/s10854-016-5319-2.
Acknowledgments
One of the authors Mr. D. J. Bhagat is dedicated this article to his parents. All authors are very much thankful to Principal, Arts Science and Commerce College, Chikhaldara for providing necessary facilities. Also, all authors are very much thankful to Head Department of Physics, Sant Gadge Baba Amravati University, Amravati for providing the X-ray Diffraction (XRD) facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhagat, D., Dhokane, G. Frequency Dependent Conductivity and Dielectric Behavior Studies of Cu Doped Polyindole in Presence of Poly(Vinyl Acetate). J Inorg Organomet Polym 27, 46–52 (2017). https://doi.org/10.1007/s10904-016-0441-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-016-0441-4