Abstract
Colorectal cancer (CRC) is the leading cause of cancer-related mortality among U.S. Hispanics, with screening proven to decrease both incidence and mortality. Despite rising CRC screening rates in the U.S., Hispanic participation remains disproportionately low. Stool-based tests, particularly popular for reaching underserved populations, may enhance screening adherence. This study evaluates the performance of a 1-day versus a 3-day stool-based testing kit in improving screening completion rates and reducing the need for reminder calls in a Hispanic community along the U.S.-Mexico border. In our quasi-experimental observational study, participants aged 45–75 years who were uninsured or underinsured and overdue for CRC screening were recruited. They received colorectal cancer education and no-cost stool-based screening facilitated by promotoras. Participants were randomly assigned to receive a 1-day or 3-day Fecal Immunochemical Test (FIT) kit. The promotoras swapped FIT kit distribution roles midway through the study period to mitigate performance bias. Our analysis covered 6,660 FITs—3,067 using the 3-day kit and 3,593 with the 1-day kit. Results indicated a higher return rate for the 1-day FIT kit (61.3% vs. 58.7%, adjusted odds ratio [aOR] = 1.22, p < 0.001), fewer reminders needed (69.7% vs. 78.1%, aOR = 0.65, p < 0.001), and lower abnormal FIT results (5.3% vs. 8.1%, aOR = 0.61, p < 0.001). Conclusively, the 1-day FIT kit required fewer reminders and significantly improved return rates, suggesting it may be a more effective option for increasing CRC screening completion among hard-to-reach Hispanic populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) causes significant morbidity and is a leading cause of cancer-related deaths in the United States (US). Worldwide, CRC ranks third in cancer-related deaths, with mortality rates trending up over the last 20 years [1, 2]. There remains a disparity in CRC incidence and mortality among different racial and ethnic groups. Among Hispanics specifically, CRC ranks second in cancer-related death [3]. Many reasons are attributed to this disparity; however, these reasons have not been fully elucidated [4]. Exploring these disparities and possible interventions further is essential, especially given that Hispanics comprise 20% of the US population [5].
CRC is a slowly progressing disease, and early screening is vital to prevention and to achieving better treatment outcomes. In a longitudinal study, CRC screening reduced the CRC mortality rate from 20 to 12% between 2000 and 2018 [6]. Currently, several methods have been recommended by the United States Preventive Services Task Force (USPSTF) for CRC screening. They include the guaiac fecal occult blood testing (gFOBT), fecal immunochemical test (FIT), multitarget stool DNA (sDNA-FIT or MT-sDNA), sigmoidoscopy, computed tomographic colonography (CT colonography), and colonoscopy [7].
Despite the wide variety of CRC screening methods available, Hispanics continue to be screened at a consistently lower rate than the general population [5]. This disparity in screening may contribute to Hispanics being less likely to receive an early-stage diagnosis and more likely to be diagnosed with advanced disease than non-Hispanic whites (NHWs) [8]. Studies have tried to explain these screening disparities, with some attribution to lack of insurance, fear, lack of knowledge, financial resources, mistrust of the healthcare system, and embarrassment [9, 10]. Other studies attributed these disparities to ineffective communication with physicians due to language differences or other scheduling difficulties. A few studies went so far as to conclude that despite having a Spanish-speaking office visit, Hispanic patients were 43% less likely to receive CRC screening [11,12,13].
Stool-based testing, such as with fecal immunochemical test (FIT) kits, may reduce the CRC screening disparity between Hispanics and NHWs. The primary advantage of FIT kits’ is the relative ease of use compared to alternative CRC screening tests. For instance, patients do not need to modify their diet or undergo surgery, and they can return the tests in person or by mail. Studies have shown that, in general, Hispanics express satisfaction with the ease of use of FIT kits, increasing their likelihood of adopting FIT [14]. If the FIT is abnormal, patients must still undergo a diagnostic colonoscopy to confirm the diagnosis, helping reduce CRC mortality. Although prior studies disagree on whether or not Hispanics have higher or lower diagnostic follow-up rates than NHWs, the bottom line still stands that increased CRC screening should also improve the pool of screened Hispanics, thereby expanding the pool of Hispanics willing to undergo a diagnostic follow-up [15, 16].
Despite inconsistencies in studies reporting CRC follow-up rates in Hispanics, studies have at least shown that sending reminders to patients to return the FIT kits has improved baseline FIT kit return rates with upwards of 17% increase in return rate, with some studies suggesting a response rate directly proportional to the number of reminders [17, 18]. While studies have shown that the number of reminders corresponds to FIT return rates, no studies thus far have examined a direct comparison between 3-day vs. 1-day FIT kit return rates and whether the 1-day FIT, because of its ease of use, would suggest the possibility of a higher return rate. Our study aims to evaluate the effect of a 1-day FIT vs. a 3-day FIT on CRC screening completion in a predominantly Hispanic population living on the US-Mexico border. In addition, our study hopes to explore the effect of navigation intensity measured by the number of outreach on participants’ FIT return.
Methods
Design
We designed a quasi-experimental, observational study to evaluate CRC screening completion rates for FITs when comparing the 1-day FIT kit to the 3-day FIT kit. Data collected was part of the routine functioning of the program. We also assessed the navigation intensity (measured by the number of reminder calls) required for the FIT kit return. This study is embedded within a CRC screening program, SuCCCeS (Southwest Coalition for Colorectal Cancer Screening). SuCCCeS is an evidence-based multicomponent CRC screening program that involves community engagement, outreach, education, navigation, and no-cost CRC screening for uninsured and underinsured individuals. This program was built on and expanded from the framework of the original program ACCION (Against Colorectal Cancer in Our Neighborhoods) [19].
Participants
Eligibility for recruiting community members to the SuCCCeS program are individuals aged 45 to 75 years who are due or overdue for CRC screening, uninsured or underinsured, and have a Texas address. This recruitment process occurred as part of a culturally tailored CRC screening program.
Setting
This study was conducted in a community on the U.S.-Mexico border, providing a unique context for understanding CRC screening behaviors in a predominantly Hispanic population. The community population is approximately 850,000, with the majority (82%) identifying as Hispanic of mostly Mexican origin [20]. The population is socioeconomically challenged, with a higher-than-average poverty rate and low rates of health coverage (21% without health insurance compared to 8% in the US) [21].
Intervention
Participants recruited into the program received a comprehensive intervention that included education, outreach, navigation, and the provision of no-cost stool-based CRC screening kits. A small percentage of participants considered high risk due to their family and personal history were navigated to primary screening colonoscopy and hence were not included in this study. There are several stool-based tests with slightly different properties. The 3-day test uses qualitative immunochemical Chromatography to detect human hemoglobin from blood in stool. In contrast, the 1-day test uses an immunological method to detect or measure hemoglobin in a clinical stool (feces) specimen.
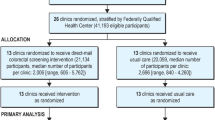
The intervention was delivered by trained community health workers known as promotoras (PM). Average-risk individuals received screening via stool-based testing. Four promotoras carried out the distribution of the FIT kits. Two PMs (PM 1 and 2) distributed the 3-day FIT kit, while two PMs (PM3 and 4) distributed the 1-day kit. A midpoint adjustment was implemented to account for the potential variations in promotor(a) ‘s performance, wherein the distribution responsibilities were switched between the two promotor(a) groups (See Fig. 1). The date of distribution for the stool-based screening kits was recorded, along with information on the type of kit provided (1-day or 3-day). Our primary exposure was 1-day FIT vs. 3-day FIT.
Data Collection
Data collection included participant demographics such as age, gender, marital status, education, ethnicity, birth country, language, years in the US, and employment status. We also collected data on participants’ screening history, including previous tests and results and specific study-related information such as perceived health and awareness of CRC and CRC screening.
Outcomes: The primary outcome was screening completion, measured by the FIT return rate. The secondary outcome was the number of participant reminders needed before the FIT return.
Statistical Analysis
We described all the study variables by groups (1-day vs. 3-day FIT testing kits) using summary statistics such as mean with standard deviation (SD) for continuous data and frequency and percentages for categorized data. The baseline data and distribution of outcome data were compared between groups using either an unpaired t-test or a chi-square test. A multivariable logistic regression model was used to determine the adjusted effect of the FIT testing group on each binary outcome separately. The model adjusted all the baseline covariates per the research objective [22]. The effect size was also summarized with a relative risk (RR) regression analysis using robust Poisson regression analysis [23]. The results were validated by additionally adjusting for the cross-over effect of promotoras using multiple logistic regression analyses. We further validated results by performing propensity scores-matched and adjusted analyses. In the propensity scores analyses, we first determined propensity scores for 1-day vs. 3-day FIT testing kits using a logistic regression model. After that, we matched the propensity scores between 1-day vs. 3-day FIT testing kits and determined the effect of this grouping on each outcome using a logistic regression analysis. The results were validated by additionally adjusting for propensity scores in the logistic regression analyses or propensity scores with adjustment for additional covariates. The results of logistic regression analyses were summarized with odds ratio (OR), 95% confidence interval (CI), and p-value. All the statistical analyses were carried out using STATA 17, and a p-value less than 5% was considered a statistically significant result.
Results
This study included all the participants who received a FIT kit in the SuCCCeS program regardless of type. 6660 participants were analyzed, including 3067 in the 3-day and 3593 in the 1-day group. The average age of the participants was 57 (SD: 6.3) years, with the majority Hispanics (99.3%), birth country Mexico (86%), Spanish speaker (93%), and married (59.4%). In both groups (those receiving the 3-day FIT[3DF] and 1-day FIT [1DF]), there was no statistically significant difference in age, race, marital status, education, birth status, years spent in the US, employment status, or health status (Table 1). Most of the participants in both groups identified as White with Hispanic ethnicity. However, at baseline, the group receiving the 1DF was less likely to have a primary care provider (PCP) (26.2% vs. 29.1%, p = 0.008), have heard of CRC screening (69.5% vs. 73.1%, p = 0.001), have had a previous recommendation for CRC screening (53.8% vs. 58.6%, p < 0.001), and less likely to have had a prior FIT test done (56.1% vs. 60.5%, p < 0.001) (Table 1).
Overall, the unadjusted FIT kit return rate was higher for the 1DF than the 3DF FIT return rate (61.3% vs. 58.7%, p = 0.035). The group receiving the 1DF required fewer reminders than those receiving the 3DF (69.7% vs. 78.1%, p < 0.001). Additionally, it was found that the 1DF required fewer reminders, especially no reminders (30.3% vs. 21.9%) and less than or equal to 1 reminder (21.1% vs. 27.0%), and the 1DF was less likely to have abnormal results (5.3% vs. 8.1%, p < 0.001) (Table 2).
Following adjustment for all baseline characteristics, 1DF was associated with a higher FIT return rate (OR = 1.22; 95%CI: 1.10–1.35, p < 0.001), lower reminder (OR = 0.62; 95%CI: 0.55–0.69, p < 0.001), and fewer abnormal results (OR = 0.61, 95%CI: 0.47–0.79, p < 0.001) (Table 3). The relative effect size was also found to be significantly associated with 1DF compared to 3DF for FIT return (RR = 1.08, p < 0.001), FIT reminder (RR = 0.89, p < 0.001), and FIT abnormal result (RR = 0.63, p < 0.001) (Table 3). These associations remained unchanged even after additionally adjusted for the cross-over effect of promotoras (Table 4) or propensity scores-matched, propensity scores–matched with additionally adjusted covariates, or weighted analyses (Table 4).
Discussion
We found a significant increase in screening completion, as measured by FIT return rates, in the 1-day FIT kit compared to the 3-day FIT kit. Moreover, our analysis revealed that the 1-day FIT kit was associated with fewer reminders and a lower incidence of abnormal results than the 3-day FIT kit. As an aside, it is essential to note that we will not draw definitive conclusions regarding the clinical efficacy or diagnostic accuracy of the 1-day FIT kit over the 3-day FIT kit based solely on a difference in abnormal results between the tests: Potential implications of the observed differences in abnormal result rates require careful consideration, as they could reflect variations in sensitivity, specificity, or other factors that were not directly measured in our study. Additionally, of the two FIT tests that were used, there is no existing literature directly comparing the efficacy of the 1-day test versus the 3-day test, with the closest paper discussing the transition from guaiac-fecal occult blood testing to the FIT test in a Canadian screening program [24]. Therefore, therein lies a potential avenue to corroborate these results with colonoscopic findings to fully understand the clinical relevance of the reduced rate of abnormal results in a 1-day FIT kit. Regardless, the results remained consistent regardless of baseline differences in covariates or even when accounting for cross-over effects owing to promotora changes. Thus, our findings suggest that the 1-day kit may offer a more efficient screening option in resource-limited settings with less need for navigation efforts.
It is remarkable to note that despite the initial disadvantage that the 1DF group had in that the group was less likely to have a PCP, less likely to have had a prior recommendation for a FIT screen, and less likely to have had a FIT in the past, that the 1DF group still had higher return rates than the 3DF group. The role of PCPs in bridging the gap between overall healthcare services and underserved communities is well-established in the medical literature [25]. This role holds for CRC screenings, where a study comparing the effects of having a PCP instead of simply receiving informational materials found that having a PCP increases rates of CRC screening adherence and participation threefold when compared to a control group [26]. In addition to having a PCP, a lack of awareness around CRC screening and a lack of prior FIT experiences were also correlated to the FIT participation drop-out rate [27]. Despite all of the advantages PCPs bring, it is possible that the influence of promotoras in this study also increased FIT return rates. Indeed, the role of promotoras in bridging the gap between healthcare services and underserved Hispanic communities is also documented in the medical literature [28]. Due to their advantage of being deeply embedded within the cultural fabric of the Hispanic community, it is possible that their culturally competent approach played a role in increasing overall FIT return rates. The trust and rapport that promotoras build within their communities highlight a crucial lesson from our study: in addition to access to healthcare with PCPs, it is vital to leverage community-based health workers as well as healthcare workers to reach improvement in public health outcomes, particularly in underserved or minority communities [29]. Despite the initial barriers faced by the 1DF group, such as a lower likelihood of having a PCP, being recommended for prior FIT screening, and previous FIT participation—this group still demonstrated higher return rates than the 3DF group. This finding highlights a significant opportunity emphasizing the distribution of FIT kits and integrating comprehensive physician engagement strategies to improve screening outcomes in underserved areas.
Although we did observe differences in reminders between groups, the overall reminders were much lower in 1DF compared to 3DF. This finding reflects that non-responsive participants will remain similar irrespective of the type of testing. However, the ease of the 1-day test might be associated with fewer reminders. In addition to revealing statistical significance after matching samples, our study also showed that the 1DF needed fewer reminders for test compliance than the 3DF, and the 1DF’s test results in the 1DF showed a lower likelihood of abnormal findings in contrast to the 3DF. This finding holds promising implications for reducing CRC screening disparities within the Hispanic population. The need for fewer reminders and the enhanced test integrity observed in the 1-day FIT kit group indicate a positive impact on screening participation and outcomes.
As our matched samples saw a higher FIT return rate combined with a lower abnormal test rate than the null hypothesis, our results strongly suggest that the 1-day FIT kit is promising to improve CRC screening rates in the Hispanic community. More recent literature shows that after receiving a positive FIT test result, patients are quick to follow up with a diagnostic colonoscopy and are more likely to adhere to future CRC screens [30, 31]. Moreover, previous studies show that the estimated cost of mailing FIT CRC screening intervention of a patient fell between $60.03 and $67.05 [32]. In general, patients without insurance are less likely to be screened for cancer and more likely to be diagnosed than patients with insurance [33]. Historically, uninsured rates in El Paso have been very high, with uninsured rates hovering around 25% in 2016 [34]. Seeing as mailed FIT tests are much more cost-effective annually than a colonoscopy while still maintaining a high “catch” rate for CRC, FIT tests remain highly accessible, highly affordable, and likely to improve health outcomes for Hispanics in areas such as El Paso [35].
Keeping with the results, the data suggest that adopting a 1-day FIT kit can significantly enhance return rates compared to its 3-day counterpart. In a broader context, utilizing mailed FIT tests is a promising pathway to augment CRC screening rates within Hispanic communities. Mailed FIT tests present a compelling advantage due to their cost-effectiveness and widespread use, making them more accessible and appealing than colonoscopies for a demographic where approximately a quarter of the El Paso population lacks insurance coverage. This financial aspect becomes a pivotal determinant in a patient’s decision to seek care, and the affordability of FIT tests may bridge a critical gap in preventive healthcare. Furthermore, the appeal of the 1-day FIT kit lies in its lower likelihood of returning abnormal results. This characteristic not only eases the patient process but also reduces the burden on healthcare providers, promoting a more convenient and efficient screening experience.
This study has several limitations. First, the participant demographic was predominantly Hispanic, which may limit the generalizability of the findings to broader populations. Although the results may have limited external validity, we are confident that the study’s internal validity is intact. We also believe that a more convenient testing method, such as the 1-day FIT, could be similarly effective in broader patient populations, including non-Hispanics. Additionally, the uneven distribution of participants across the four promotoras could have introduced variability that could affect the consistency of the results. However, because the promotoras followed a uniform guide when interacting with patients, we are confident in the consistency and reliability of our results. A significant strength of our study is its applicability to the border population. With a nearly 100% Hispanic origin of our sample, our study provides a substantial insight into the likelihood of adopting a 1-day FIT over a 3-day FIT in similar populations. Such specificity enhances our understanding of screening behaviors and outcomes within this underrepresented group, offering valuable perspectives for targeted health interventions.
Data Availability
Data was collected as part of program implementation and is not available in an accessible database. However, deidentified data may be provided by the corresponding author upon reasonable request.
References
Rawla, P., Sunkara, T., & Barsouk, A. (2019). Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przeglad Gastroenterologiczny, 14(2), 89–103. https://doi.org/10.5114/pg.2018.81072
Gupta, S. (2022). Screening for Colorectal Cancer. Hematology/oncology Clinics of North America, 36(3), 393–414. https://doi.org/10.1016/j.hoc.2022.02.001
Sharma, I., Kim, S., Sridhar, S., & Basha, R. (2020). Colorectal Cancer: An emphasis on factors influencing Racial/Ethnic disparities. Critical Reviews in Oncogenesis, 25(2), 151–160. https://doi.org/10.1615/CritRevOncog.2020035174
Winkler, C. S., Hardaway, J. C., Ceyhan, M. E., Espat, N. J., & Calvino, S., A (2022). Decreasing colorectal cancer screening disparities: A culturally tailored patient navigation program for hispanic patients. Cancer, 128(9), 1820–1825. https://doi.org/10.1002/cncr.34112
Miller, K. D., Ortiz, A. P., Pinheiro, P. S., Bandi, P., Minihan, A., Fuchs, H. E., Martinez Tyson, D., Tortolero-Luna, G., Fedewa, S. A., Jemal, A. M., & Siegel, R. L. (2021). Cancer statistics for the US Hispanic/Latino population, 2021. CA: A cancer Journal for Clinicians, 71(6), 466–487. https://doi.org/10.3322/caac.21695
National Cancer Institute, Surveillance Research Program (2023, April 19). SEERExplorer: An interactive website for SEER cancer statistics*. [Internet]. Updated June 8, 2023. Cited September 29, 2023. https://seer.cancer.gov/statistics-network/explorer/. Data source(s): SEER Incidence Data, November 2022 Submission (1975–2020), SEER 22 registries; U.S. Mortality Data (1969–2020), National Center for Health Statistics, CDC.
Lin, J. S., Perdue, L. A., Henrikson, N. B., Bean, S. I., & Blasi, P. R. (2021). Screening for Colorectal Cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. Journal of the American Medical Association, 325(19), 1978–1998. https://doi.org/10.1001/jama.2021.4417
Jackson, C. S., Oman, M., Patel, A. M., & Vega, K. J. (2016). Health disparities in colorectal cancer among racial and ethnic minorities in the United States. Journal of Gastrointestinal Oncology, 7(Suppl 1), S32–S43. https://doi.org/10.3978/j.issn.2078-6891.2015.039
Byrd, T. L., Calderón-Mora, J., Salaiz, R., & Shokar, N. K. (2019). Barriers and facilitators to Colorectal Cancer Screening within a hispanic Population. Hispanic Health Care International: The Official Journal of the National Association of Hispanic Nurses, 17(1), 23–29. https://doi.org/10.1177/1540415318818982
Fernandez, M. E., Wippold, R., Torres-Vigil, I., Byrd, T., Freeberg, D., Bains, Y., Guajardo, J., Coughlin, S. S., & Vernon, S. W. (2008). Colorectal cancer screening among latinos from U.S. cities along the Texas-Mexico border. Cancer Causes & Control: CCC, 19(2), 195–206. https://doi.org/10.1007/s10552-007-9085-6
Diaz, J. A., Roberts, M. B., Goldman, R. E., Weitzen, S., & Eaton, C. B. (2008). Effect of language on colorectal cancer screening among latinos and non-latinos. Cancer epidemiology, biomarkers & prevention: A publication of the American Association for Cancer Research. Cosponsored by the American Society of Preventive Oncology, 17(8), 2169–2173. https://doi.org/10.1158/1055-9965.EPI-07-2692
Garcia-Dominic, O., Lengerich, E. J., Wray, L. A., Parrott, R., Aumiller, B., Kluhsman, B., Renderos, C., & Dignan, M. (2012). Barriers to CRC screening among latino adults in Pennsylvania: ACCN results. American Journal of Health Behavior, 36(2), 153–167. https://doi.org/10.5993/AJHB.36.2.2
Natale-Pereira, A., Marks, J., Vega, M., Mouzon, D., Hudson, S. V., & Salas-Lopez, D. (2008). Barriers and facilitators for colorectal cancer screening practices in the latino community: Perspectives from community leaders. Cancer Control: Journal of the Moffitt Cancer Center, 15(2), 157–165. https://doi.org/10.1177/107327480801500208
Aguado Loi, C. X., Tyson, M., Chavarria, D., Gutierrez, E. A., Klasko, L., Davis, L., Lopez, S., Johns, D., Meade, T., C. D., & Gwede, C. K. (2020). Simple and easy:’ providers’ and latinos’ perceptions of the fecal immunochemical test (FIT) for colorectal cancer screening. Ethnicity & Health, 25(2), 206–221. https://doi.org/10.1080/13557858.2017.1418298
Oluloro, A., Petrik, A. F., Turner, A., Kapka, T., Rivelli, J., Carney, P. A., Saha, S., & Coronado, G. D. (2016). Timeliness of Colonoscopy after abnormal fecal test results in a Safety Net Practice. Journal of Community Health, 41(4), 864–870. https://doi.org/10.1007/s10900-016-0165-y
O’Connor, E. A., Nielson, C. M., Petrik, A. F., Green, B. B., & Coronado, G. D. (2020). Prospective cohort study of predictors of Follow-Up Diagnostic Colonoscopy from a pragmatic trial of FIT screening. Scientific Reports, 10(1), 2441. https://doi.org/10.1038/s41598-020-59032-0
Prakash, S., Merza, N., Hosseini, O., Ward, H., Mansi, T., Balducci, M., Trammell, D., Hernandez, B., & Obokhare, I. (2022). Increasing Fecal Immunochemical Test Return Rates by implementing effective reminder to complete kit communication with participants: A Quality Improvement Study. Cureus, 14(5), e25169. https://doi.org/10.7759/cureus.25169
Brenner, A. T., Rhode, J., Yang, J. Y., Baker, D., Drechsel, R., Plescia, M., Reuland, D. S., Wroth, T., & Wheeler, S. B. (2018). Comparative effectiveness of mailed reminders with and without fecal immunochemical tests for Medicaid beneficiaries at a large county health department: A randomized controlled trial. Cancer, 124(16), 3346–3354. https://doi.org/10.1002/cncr.31566
Shokar, N. K., Byrd, T., Salaiz, R., Flores, S., Chaparro, M., Calderon-Mora, J., Reininger, B., & Dwivedi, A. (2016). Against colorectal cancer in our neighborhoods (ACCION): A comprehensive community-wide colorectal cancer screening intervention for the uninsured in a predominantly hispanic community. Preventive Medicine, 91, 273–280. https://doi.org/10.1016/j.ypmed.2016.08.039
https://data.census.gov/profile/El_Paso_County,_Texas?g=050XX00US48141
Dwivedi, A. K. (2022). How to write statistical analysis section in medical research. Journal of Investigative Medicine: The Official Publication of the American Federation for Clinical Research, 70(8), 1759–1770. https://doi.org/10.1136/jim-2022-002479
Dwivedi, A. K., Mallawaarachchi, I., Lee, S., & Tarwater, P. (2014). Methods for estimating relative risk in studies of common binary outcomes. Journal of Applied Statistics, 41(3), 484–500. https://doi.org/10.1080/02664763.2013.840772
Sultanian, R., Du, L., Moysey, B., Morse, A., van Veldhuyzen, S., & Montano-Loza, A. J. (2020). The impact of transitioning from Guaiac-Fecal Occult Blood Testing to Fecal Immunochemical Testing in a Canadian Colon Cancer Screening Program. Journal of the Canadian Association of Gastroenterology, 3(4), 177–184. https://doi.org/10.1093/jcag/gwz009
Chou, A. F., Duncan, A. R., Hallford, G., Kelley, D. M., & Dean, L. W. (2021). Barriers and strategies to integrate medical genetics and primary care in underserved populations: A scoping review. Journal of Community Genetics, 12(3), 291–309. https://doi.org/10.1007/s12687-021-00508-5
Boguradzka, A., Wiszniewski, M., Kaminski, M. F., Kraszewska, E., Mazurczak-Pluta, T., Rzewuska, D., Ptasinski, A., & Regula, J. (2014). The effect of primary care physician counseling on participation rate and use of sedation in colonoscopy-based colorectal cancer screening program–a randomized controlled study. Scandinavian Journal of Gastroenterology, 49(7), 878–884. https://doi.org/10.3109/00365521.2014.913191
Osborne, J. M., Wilson, C., Duncan, A., Cole, S. R., Flight, I., Turnbull, D., Hughes, D. L., & Young, G. P. (2017). Patterns of participation over four rounds of annual fecal immunochemical test-based screening for colorectal cancer: What predicts rescreening? BMC Public Health, 18(1), 81. https://doi.org/10.1186/s12889-017-4634-8
Rao, S. P., Moralez, E., Livaudais-Toman, J., Lozano, V., & Thompson, B. (2013). Evaluation of a Promotora-led intervention on Colorectal Cancer among hispanics: Findings related to perceptions and communications. Californian Journal of Health Promotion, 11(2), 21–31.
Moralez, E. A., Rao, S. P., Livaudais, J. C., & Thompson, B. (2012). Improving knowledge and screening for colorectal cancer among hispanics: Overcoming barriers through a PROMOTORA-led home-based educational intervention. Journal of cancer Education: The Official Journal of the American Association for Cancer Education, 27(3), 533–539. https://doi.org/10.1007/s13187-012-0357-9
Goshgarian, G., Sorourdi, C., May, F. P., Vangala, S., Meshkat, S., Roh, L., Han, M. A., & Croymans, D. M. (2022). Effect of patient portal messaging before mailing Fecal Immunochemical Test Kit on Colorectal Cancer Screening Rates: A Randomized Clinical Trial. JAMA Network open, 5(2), e2146863. https://doi.org/10.1001/jamanetworkopen.2021.46863
Lee, B., Keyes, E., Rachocki, C., Grimes, B., Chen, E., Vittinghoff, E., Ladabaum, U., & Somsouk, M. (2022). Increased Colorectal Cancer Screening sustained with mailed fecal immunochemical test Outreach. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association, 20(6), 1326–1333e4. https://doi.org/10.1016/j.cgh.2021.07.022
Pignone, M., Lanier, B., Kluz, N., Valencia, V., Chang, P., & Olmstead, T. (2021). Effectiveness and cost-effectiveness of Mailed FIT in a Safety Net Clinic Population. Journal of General Internal Medicine, 36(11), 3441–3447. https://doi.org/10.1007/s11606-021-06691-y
Joseph, D. A., King, J. B., Miller, J. W., Richardson, L. C., & Centers for Disease Control and Prevention (CDC). (2012). Prevalence of colorectal cancer screening among adults – behavioral risk factor Surveillance System, United States, 2010. MMWR Supplements, 61(2), 51–56.
U.S. Census Bureau (2018). 2008–2016 Small Area Health Insurance Estimates (SAHIE) using the American Community Survey (ACS). Retrieved from https://www.census.gov/data/datasets/time-series/demo/sahie/estimates-acs.html
Quintero, E., Castells, A., Bujanda, L., Cubiella, J., Salas, D., Lanas, Á., Andreu,M., Carballo, F., Morillas, J. D., Hernández, C., Jover, R., Montalvo, I., Arenas,J., Laredo, E., Hernández, V., Iglesias, F., Cid, E., Zubizarreta, R., Sala, T., Ponce,M., … COLONPREV Study Investigators (2012). Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. The New England journal of medicine, 366(8), 697–706. https://doi.org/10.1056/NEJMoa1108895.
Acknowledgements
This work was supported by a grant from the Cancer Prevention and Research Institute of Texas [PP210005].
Funding
Authors have final responsibility for the decision to submit for publication. The Institutional Review Board (IRB) of Texas Tech University Health Sciences Center El Paso deemed the study exempt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wing, J.D., Matharasi, P., Dwivedi, A. et al. Enhancing CRC Screening in a Predominantly Hispanic Community: Effectiveness of 1-Day vs. 3-Day Stool-Based Testing Kits. J Community Health (2024). https://doi.org/10.1007/s10900-024-01394-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s10900-024-01394-x