Abstract
Human papillomavirus (HPV) vaccination continues to lag behind other adolescent vaccines, especially in areas with pervasive disparities in HPV-related cancers. The purpose of this study was to examine HPV vaccine completion and dosing intervals among low-income adolescents in urban areas. The study included electronic health record data on HPV vaccination for 872 adolescents who received at least one dose of the HPV vaccine. Only 28.4 % completed the 3-dose series. For the whole sample, HPV vaccine completion was higher for non-English speakers and among adolescents seen at Newark-South and East Orange sites. Completion was higher among non-English speaking female and Hispanic adolescents, females seen in Newark-South and East Orange sites, and insured Black adolescents. Completion was also dramatically lower among non-English speaking Black adolescents seen at Newark-North, Irvington, and Orange sites (12.5 %) compared to other Black adolescents (22.0–44.4 %). The mean dosing intervals were 5.5 months (SD = 4.6) between dose 1 and 2 and 10 months (SD = 6.1) between dose 1 and 3. Longer durations between vaccine doses were found among uninsured adolescents and those seen at Newark-North, Irvington, and Orange sites. Non-English speakers had longer duration between dose 1 and 3. Further, durations between dose 1 and 3 were dramatically longer among insured adolescents seen at Newark-North, Irvington, and Orange locations for the whole sample (M = 11.70; SD = 7.12) and among Hispanic adolescents (M = 13.45; SD = 8.54). Understanding how the study predictors facilitate or impede HPV vaccination is critical to reducing disparities in cervical and other HPV-related cancer, especially among Black, Hispanic, and low-income populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
HPV Vaccination Completion and Compliance with Recommended Dosing Intervals Among Female and Male Adolescents in Low-Income Urban Areas
The human papillomavirus (HPV) infection is a known risk factor for the development of several cancers. In 2004–2008, there was an average of 33,369 HPV-associated cancers annually, including cervical, vulvar, vaginal, penile, anal, and oropharyngeal cancers in the US [1]. The Centers for Disease Control and Prevention (CDC) estimates 26,000 new HPV-associated cancers each year, 18,000 for females and 8,000 for males [1], which could be prevented through the HPV vaccine. National morbidity and mortality rates of HPV-related cancers show pervasive disparities among Black, Hispanic, and low-income individuals [2, 3]. The State of New Jersey has the 10th highest morbidity rate for cervical cancer in the US for 2006–2010 [4]. According to the New Jersey State Cancer Registry (NJSCR), cervical cancer morbidity in 2005–2009 was significantly higher in the Greater Newark area (RR: 1.86) (the study target area) than other areas in the state, as well as among women who are Black, Hispanic, and foreign-born; non-English speaking; uninsured; with lower income and education; unmarried; unemployed; and living in rented residences [5]. The HPV vaccine is an effective approach to dramatically reduce vaccine-type HPV infection [6] as well as the rates of high-grade cervical lesions [7]. Unfortunately, this trend was not evident in urban areas as well as in areas with higher concentrations of Black, Hispanic, and low-income populations [7].
Based on 2013 national data, the HPV vaccine completion rates were 37.6 and 13.9 % for female and male adolescents, respectively [8]. This reflects minimal improvement over the past few years and reaching a plateau for female vaccination at a level dramatically lower than Healthy People 2020’s goal of an 80 % completion rate. Site-based studies in the US have reported even lower rates of HPV vaccine completion, ranging between 1.9 and 26.5 % among female adolescents [9–11] and between 1.3 and 4 % among male adolescents [12, 13].
Pervasive disparities exist in HPV vaccination among Black, Hispanic, and low-income groups. National data for the US in 2010–2013 show a continuing trend of lower completion rates for Black and Hispanic adolescents as well as those below poverty [8, 14–16]. For female adolescents who initiated the HPV vaccine nationally in 2013, the 3-dose completion rates were lower for Black and Hispanic adolescents (64 and 70 % respectively) and among adolescents below poverty (66 %) compared to White adolescents and those above poverty (72 %) [16]. For male adolescents who initiated the HPV vaccine nationally in 2013, the 3-dose completion rates were lower for Black and Hispanic adolescents (45 % and 47 %, respectively) and those below poverty (44 %) compared to White adolescents (51 %) and those above poverty (50 %) [16]. Further, several site-based studies in the US have demonstrated a significant and continuing trend of lower HPV vaccination among Black and Hispanic adolescents [17–24], as well as in low-income and urban areas [21, 25–27]. In addition to race/ethnicity and poverty status, completion for females appears significantly lower among older adolescents [17, 28, 29].
The recommended dosing intervals for the HPV vaccine 3-dose series are 1–2 months between dose 1 and 2 and 6 months between dose 1 and 3 [30]. Delaying completion of the series places adolescents at risk for acquiring HPV infection due to gaps in immunologic protection from the vaccine doses. National data for 2008–2009 show that only 46 % of female adolescents who completed the 3-dose series did so in a period longer than the recommended interval [31]. In a site-based study [18], completion rates of the 3-dose series were 14 % by 7 months and 28 % by 12 months. Completion within the recommended dosing intervals has been found to be significantly lower for Black adolescents and those who are uninsured or with public insurance [18, 32]. Information is lacking on dosing intervals among male adolescents.
In summary, the literature lacks information on factors associated with completion as well dosing intervals of the HPV vaccine, especially in areas and populations with the greatest disparities in cervical and other HPV-related cancers. Accordingly, the purpose of this study was to examine the correlates of HPV vaccine completion and compliance with recommended dosing intervals in a sample of predominantly Black and Hispanic adolescents in a low-income, urban area. The study target area represents a population with pervasive disparities in cervical cancer morbidity and mortality [5].
Methods
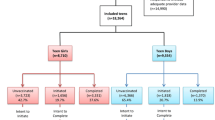
This is a descriptive correlational study of factors associated with HPV vaccine completion and compliance with recommended dosing intervals. Study data were obtained from Electronic Health Record (EHR) data from the Newark Community Health Centers (NCHC) in New Jersey. The NCHC is a federally qualified healthcare organization composed of seven sites in three communities of the Greater Newark area. Four of their sites are located in Newark, one site in Irvington, one site in East Orange, and one site in Orange. Annually, NCHC serves nearly 19,000 ethnically diverse patients mostly from low-income and uninsured families. Only five of NCHC’s sites were included in this study; the two sites excluded do not provide pediatric services.
The initial study sample included 3,180 adolescents, 10–20 years old, who had a pediatric, OB/GYN, internal medicine, or nurse visit at the study site in 2011. However, only 27.4 % received at least one dose of the HPV vaccine. Therefore, the study sample to examine completion included only the 872 adolescents who had initiated HPV vaccination. Rates and correlates of HPV vaccine initiation are reported elsewhere [33]. Table 1 provides a summary of the study sample characteristics; 61 % were female; 85 % were Black or Hispanic adolescents; 25 % were non-English speakers; 33 % were uninsured; and 67 % were seen by a pediatric healthcare provider (HCP). The outcome variables are: (1) Completion: adolescents who completed the 3-dose series versus those who initiated but not completed; and (2) Duration Vaccination Doses: duration between dose 1 and 2 and duration between dose 1 and 3. The predictor variables include gender, age, race/ethnicity, insurance status, preferred language, and specialty of HCP, and site of service. For the data analysis, the predictor variables were dichotomized as follows:
-
Age: younger (10–15 years old) versus older (16–20 years old)
-
Insurance status: uninsured versus insured (including private insurance and Medicaid).
-
Preferred language: English versus non-English (including Spanish or Creole).
-
HCP Specialty: Pediatric versus non-pediatric (including OB/GYN, internal medicine, or nurse visit).
-
Site of service for HPV vaccine: the two sites with highest completion rates (Locations 1: Newark-South and East Orange) versus the remaining three sites (Locations 2: Newark-North, Irvington, and Orange).
The EHR data were imported into SPSS statistical software for analysis. Bivariate and multivariate analyses were conducted to examine the associations between the study predictors and HPV vaccine completion and durations between vaccine doses, for the whole study sample as well as for female, male, Black, and Hispanic adolescents. For the bivariate analysis of HPV vaccine completion, we conducted Chi square tests. For the bivariate analysis of the durations between HPV vaccine doses, we conducted t tests. Multivariate analyses included logistic regression for HPV vaccine completion and calculation of adjusted odds ratios (aOR) and 95 % confidence intervals (CI). In that process, we centered the study predictors and conducted logistic regression analyses for completion with PIN: 0.050; POUT: 0.051; CUT: 0.5 and tests of collinearity. Multivariate analyses also included backwards linear regression for durations between HPV vaccine completion doses. Also using centered study predictors, we conducted linear regression analyses for durations between dose 1 and 2 and between dose 1 and 3 with PIN: 0.050; POUT: 0.051; and tests of collinearity. Tests of collinearity for study predictors were within acceptable parameters [34], with the variance inflation factor (VIF) values below two. In all regression analyses, we also examined for any interaction effects among study predictors. The study was reviewed and approved by the Institutional Review Board (IRB).
Results
HPV Vaccination Completion
As shown in Table 1, 28.4 % completed the 3-dose series of the HPV vaccine. According to Table 2, the rates of HPV vaccine completion among female, male, Black, and Hispanic adolescent were 28.6, 28.2, 26.6, 33.1 %, respectively. The bivariate analysis shown in Table 2 revealed that HPV vaccine completion was associated with age, language, and site for the whole sample. Among female adolescents, completion was associated with language and site. Among Black adolescents, completion was associated with age, insurance, HCP specialty, and site. Among Hispanic adolescents, completion was associated with language. None of the predictors were associated with completion among male adolescents.
Predictors of HPV vaccine completion using multivariate analysis are shown in Table 3. For the whole sample, the odds of HPV vaccine completion were 78 % higher for non-English speakers (aOR 1.78; 95 % CI 1.20, 2.64) and 93 % higher for adolescents seen at site locations 1 (aOR 1.93; 95 % CI 1.32, 2.83). Among female adolescents, the odds of HPV vaccine completion were twice as high among non-English speakers (aOR 2.00; 95 % CI 1.19, 3.36) and 79 % higher at site locations 1 (aOR 1.79; 95 % CI 1.06, 3.01). Among male adolescents, the odds of completion were twice as high at site locations 1 (aOR 2.0; 95 % CI 1.16, 3.57). Among Black adolescents, the odds of HPV vaccine completion were 66 % lower for insured adolescents (aOR 0.35; 95 % CI 0.16, 0.77). Further, completion was associated with the interaction of site and language, which was also significant in a post hoc analysis among Black adolescents (Pearson X 2 = 9.02; p = 0.029). In this analysis, HPV vaccine completion was much lower for non-English speakers seen at site locations 2 (12.5 %) compared to English speakers seen at site locations 1 and locations 2 (29.2 and 22.0 %, respectively) and Non-English speakers seen at site locations 1 (44.4 %). Among Hispanic adolescents, the odds of completion were almost three times higher among non-English speakers (aOR 2.83; 95 % CI 1.33, 6.03).
Durations Between Vaccine Dose 1 and 2
The mean duration between dose 1 and 2 was 5.5 months (SD = 4.6). The bivariate analysis in Table 4 shows that the duration between dose 1 and 2 was associated with age and insurance status for the whole sample and among female adolescents. Among Hispanic adolescents, duration between dose 1 and 2 was associated with insurance status and site. None of the study predictors were associated with the duration between dose 1 and 2 among male and Black adolescents.
As shown in Table 5, using a backward linear regression analysis, the duration between dose 1 and 2 was associated with insurance status for the whole sample (t = −3.74; p < 0.001; B = −2.28; 95 % CI −3.49, −1.08) as well as among female (t = −3.65; p < 0.001; B = −3.17; 95 % CI −4.88, −1.45) and Black adolescents (t = −2.01; p = 0.046; B = −1.89; 95 % CI −3.75, −0.04). Also, duration between dose 1 and 2 was associated with site for the whole sample (t = −2.75; p = 0.006; B = −1.58; 95 % CI −2.71, −0.45) and among Hispanic adolescents (t = −2.64; p = 0.010; B = −2.93; 95 % CI −5.13, −0.73). None of the study predictors were associated with duration between dose 1 and 2 among male adolescents. The interaction between site and language among Black adolescents was associated with duration between dose 1 and 2. However, a post hoc analysis of this interaction using ANOVA did not reveal any significant difference in duration between dose 1 and 2 (F [4, 175] = 0.04; p = 0.990).
Durations Between Vaccine Dose 1 and 3
The mean duration between dose 1 and 3 was 10 months (SD = 6.1). Only 42.3 % completed all three doses within 6 months. The bivariate analysis in Table 4 shows that the duration between dose 1 and 3 was associated with insurance status for the whole sample and among female and Black adolescents. Further, the duration between dose 1 and 3 was associated with language among males and with site among Hispanic adolescents.
As shown in Table 5, using a backward linear regression analysis, the duration between dose 1 and 3 was associated with insurance status for the whole sample (t = −2.46; p = 0.015; B = −2.81; 95 % CI −5.08, −0.55) and among females (t = −2.65; p = 0.010; B = −4.30; 95 % CI −7.53, −1.07). Also, duration between dose 1 and 3 was associated with site for the whole sample (t = −2.69; p = 0.008; B = −2.72; 95 % CI −4.72, −0.72) as well as among females (t = −2.09; p = 0.040; B = −3.18; 95 % CI −6.21, −0.15) and Hispanic adolescents (t = −2.69; p = 0.009; B = −5.16; 95 % CI −9.00, −1.32). Language was associated with the duration between dose 1 and 3 only among male adolescents (t = 2.11; p = 0.039; B = 2.90; 95 % CI 0.15, 5.65). None of the study predictors were associated with the duration between dose 1 and 3 among Black adolescents. Lastly, the interaction of site and insurance was associated with the duration between dose 1 and 3 for the whole sample (t = 2.17; p = 0.032) and among Hispanic adolescents (t = 2.34; p = 0.023).
A post hoc analysis of this interaction using ANOVA revealed an association with the duration between dose 1 and 3 for the whole sample (F [3, 44] = 4.64; p = 0.004) and among Hispanic adolescents (F [3, 80] = 5.10; p = 0.003). For the whole sample, the duration between dose 1 and 3 was dramatically higher among insured adolescents seen at site locations 2 (M = 11.7; SD = 7.1) compared to uninsured adolescents seen at site locations 1 (M = 8.5; SD = 4.9) and locations 2 (M = 8.3; SD = 3.9) and insured seen at locations 1 (M = 9.5; SD = 5.5). Among Hispanic adolescents, the duration between dose 1 and 3 was also dramatically higher among insured adolescents seen at site locations 2 (M = 13.5; SD = 8.5) compared to uninsured adolescents seen at site locations 1 (M = 8.9; SD = 5.5) and locations 2 (M = 8.7; SD = 4.5) and insured seen at locations 1 (M = 6.5; SD = 1.4).
Further post hoc analysis was conducted using t tests which revealed a significantly higher duration between dose 1 and 3 among insured adolescents seen at locations 2 within the whole sample (t = 3.555; p < 0.001) and among Hispanic adolescents (t = 3.750; p < 0.001). The mean difference in duration between dose 1 and 3 among insured adolescents seen at locations 2 compared to other adolescents was 2.72 months for the wholes sample (95 % CI 1.21, 4.23) and 5.26 months among Hispanic adolescents (95 % CI 2.47, 8.06).
Discussion
The study findings provide insight on correlates of HPV vaccine completion in a population with the greatest disparities of HPV-related cancers, particularly cervical cancer. The study addresses several gaps in the literature about HPV vaccination, including vaccination of male adolescents, underserved populations, low-income groups, and Black and Hispanic adolescents. The rate of HPV vaccine completion in this study is dramatically lower for females than the rate reported in NIS-Teen data for the same year (2011) nationally (24 % in our study vs. 71 % nationally) [15]. However, HPV vaccine completion rates for the whole sample and among female, Black, and Hispanic adolescents are closer to those reported in studies in underserved and low-income areas [12, 13, 17, 20, 21, 23, 25, 35]. Further, the rate of HPV vaccine completion among males in this study (28 %) is similar to the rate reported in NIS-Teen data nationally [15].
Language was a significant predictor in our study in which English speakers had lower HPV vaccine completion in the whole sample and among female and Hispanic adolescents. This may be due to cultural norms among non-English speakers that lead them to comply with doctor’s recommendations and not question medical authority. However, some studies found lower HPV vaccine knowledge and uptake among Spanish speakers [25, 36, 37] while others reported no difference by language [25, 38]. The site at which the services were obtained was a significant predictor for HPV vaccine completion in our study. Further, the site had a significant interaction effect on HPV vaccine completion with language among Black adolescents, in which non-English speakers (mostly Creole speaking) seen at site locations 2 had a dramatically lower HPV vaccine completion (12.5 %) than other adolescents (between 22 and 44 %). This finding may be attributable to variation by site in resources, hours of operation, use of appointment reminders, and cultural competency of staff.
Insurance in our study was a predictor of HPV vaccination among only Black adolescents. The impact of insurance status has been reported in other studies [19, 39, 40], more specifically with regards to longer duration on enrollment in health insurance [23], which was not examined in our study. Completion of the 3-dose series may be impacted by mothers’ concern for cost of subsequent visits when uninsured. Age and HCP specialty were not significant predictors of HPV vaccine completion in our study, even though studies have shown that vaccine initiation is higher among adolescents seen by pediatric providers [19, 41, 33].
The durations between HPV vaccine doses in our study 5.5 months between dose 1 and 2 and 10 months between dose 1 and 3, compared to the 2–3 and 6 month, are dramatically longer than the CDC recommended intervals [30]. This is consistent with the findings of other studies [18, 31, 32]. Delaying completion of the series places adolescents at risk for acquiring HPV infection due to gaps in immunologic protection from the vaccine doses. Insurance was a recurring predictor for the duration between vaccine dose 1 and 3, however, in a way that is inconsistent with the findings of other studies [18, 32]. Our study shows longer durations between vaccine doses among insured adolescents, especially when combined with site. Insured adolescents seen at Newark-North, Irvington, and Orange locations had higher durations between dose 1 and 3 for the whole sample and among Hispanic adolescents. Along with this finding, site was another recurring predictor of duration between doses. The impact of insurance and site on duration between doses could be related to specific site resources (e.g., flexible scheduling, use of reminders for appointments, etc.), which were not examined in this study.
The study findings provide several implications for practice and research. Public health practitioners and HCPs should discuss HPV vaccination with mothers and address any concerns they may have about the vaccine efficacy and safety, vaccination recommendations, cost, and insurance coverage. The findings also emphasize the need to increase efforts to improve timely completion of the HPV vaccine among mothers of uninsured adolescents. Completion of the 3-dose series may be impacted by mother’s perception of cost issues or lack insurance coverage associated with HPV vaccination. Mothers may not be aware that the vaccine is available for free regardless of vaccination status or may worry about additional cost related to have to return for the second and third HPV vaccine doses. Lastly, the study findings indicate the need for more studies to examine sociocultural correlates as well as barriers and facilitators for HPV vaccine completion in populations with the greatest disparities in cervical and other HPV-related cancers, particularly Black and Hispanic adolescents in low-income areas.
Notwithstanding these implications, our findings should be considered in the context of a few limitations. Given that the study examined 2011 data, HPV vaccine uptake may have changed over the past 2 years and the results may not accurately reflect the current situation. While awareness of the vaccine may have increased over the years [42, 43], research is still needed to examine more current data on HPV vaccine uptake and awareness about the vaccine within the target population. Hence, this study is still pertinent in underserved populations whose awareness of the HPV vaccine and accessibility to HCPs remains low.
In conclusion, improving HPV vaccination is a national priority to reduce cancer burden and eliminate future cancer disparities in the US. Healthy People 2020 objective IID-11.4 is to “Increase the vaccination coverage level of 3 doses of human papillomavirus (HPV) vaccine for females by age 13–15 years” to 80 %, nationally [44] the President’s Cancer Panel (PCP) work on “Accelerating Progress in Cancer Prevention: The HPV Vaccine Example” [45] and the CDC Director’s announcement on setting priority to increasing uptake of the HPV vaccine in 2014 [46]. Improving HPV vaccination is critical not only for cancer prevention but also for elimination of disparities in cervical and other HPV-related cancers among Black and Hispanic populations in low-income areas.
References
Centers for Disease Control and Prevention. (2012). Human papillomavirus-associated cancers: United States, 2004–2008. MMWR. Morbidity and Mortality Weekly Report, 61, 258–261.
US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and mortality web-based report. Available at: www.cdc.gov/uscs.
Benard, V. B., Johnson, C. J., Thompson, T. D., et al. (2008). Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer, 113, 2910–2918.
US Cancer Statistics Working Group. United States Cancer Statistics: 2006–2010, cervical cancer ranked by state. Available at: http://apps.nccd.cdc.gov/uscs/cancersrankedbystate.aspx#text.
Roche, L. M., Niu, X., & Henry, K. A. (2014). Invasive cervical cancer disparities in New Jersey. Presented at the 2014 Annual Retreat on Cancer Research in New Jersey, Piscataway, NJ (May 21, 2014).
Markowitz, L. E., Hariri, S., Lin, C., et al. (2013). Reduction in Human Papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. Journal of Infectious Diseases, 208, 385–393.
Niccolai, L. M., Julian, P. J., Meek, J. I., et al. (2013). Declining rates of high-grade cervical lesions in young women in Connecticut, 2008–2011. Cancer Epidemiology, Biomarkers and Prevention, 22, 1446–1450.
Elam-Evans, L. D., Yankey, D., Jeyarajah, J., et al. (2014). National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2013. MMWR. Morbidity and Mortality Weekly Report, 63, 625–633.
Bastani, R., Glenn, B. A., Tsui, J., et al. (2011). Understanding suboptimal Human Papillomavirus vaccine uptake among ethnic minority girls. Cancer Epidemiology Biomarkers & Prevention, 20, 1463–1472.
Manhart, L. E., Burgess-Hull, A. J., Fleming, C. B., et al. (2011). HPV vaccination among a community sample of young adult women. Vaccine, 29, 5238–5244.
Rubin, R., Kuttab, H.-M., Rihani, R., et al. (2012). Patient adherence to three dose completion of the Quadrivalent Human Papillomavirus (HPV) vaccine in a private practice. Journal of Community Health, 37, 1145–1150.
Reiter, P. L., McRee, A. L., Pepper, J. K., et al. (2013). Longitudinal predictors of human papillomavirus vaccination among a national sample of adolescent males. American Journal of Public Health, 103, 1419–1427.
Hirth, J. M., Tan, A., Wilkinson, G. S., et al. (2013). Completion of the human papillomavirus (HPV) vaccine series among males with private insurance between 2006 and 2009. Vaccine, 31, 1138–1140.
Centers for Disease Control and Prevention. (2011). National and state vaccination coverage among adolescents aged 13 through 17 years–United States, 2010. MMWR. Morbidity and Mortality Weekly Report, 60, 1117–1123.
Centers for Disease Control and Prevention. (2012). National and state vaccination coverage among adolescents aged 13–17 years–United States, 2011. MMWR. Morbidity and Mortality Weekly Report, 61, 671–677.
Centers for Disease Control and Prevention. (2013). National and state vaccination coverage among adolescents aged 13–17 years–United States, 2012. MMWR. Morbidity and Mortality Weekly Report, 62, 685–693.
Cook, R. L., Zhang, J., Mullins, J., et al. (2010). Factors associated with unitiation and completion of Human Papillomavirus vaccine series among young women enrolled in Medicaid. The Journal of Adolescent Health, 47, 596–599.
Widdice, L. E., Bernstein, D. I., Leonard, A. C., et al. (2011). Adherence to the HPV vaccine dosing intervals and factors associated with completion of 3 doses. Pediatrics, 127, 77–84.
Chao, C., Velicer, C., Slezak, J. M., et al. (2010). Correlates for Human Papillomavirus vaccination of adolescent girls and young women in a managed care organization. American Journal of Epidemiology, 171, 357–367.
Keenan, K., Hipwell, A., & Stepp, S. (2012). Race and sexual behavior predict uptake of the human papillomavirus vaccine. Health Psychology, 31, 31–34.
Perkins, R. B., Brogly, S. B., Adams, W. G., et al. (2012). Correlates of human papillomavirus vaccination rates in low-income, minority adolescents: A multicenter study. Journal of Women’s Health (Larchmt), 21, 813–820.
Gilkey, M. B., Moss, J. L., McRee, A.-L., et al. (2012). Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine, 30, 5928–5934.
Staras, S. A. S., Vadaparampil, S. T., Haderxhanaj, L. T., et al. (2010). Disparities in Human Papillomavirus vaccine series initiation among adolescent girls enrolled in Florida Medicaid programs, 2006–2008. The Journal of Adolescent Health, 47, 381–388.
Sadigh, G., Dempsey, A. F., Ruffin, Mt, et al. (2012). National patterns in human papillomavirus vaccination: An analysis of the National Survey of Family Growth. Human Vaccines & Immunotherapeutics, 8, 234–242.
Chando, S., Tiro, J. A., Harris, T. R., et al. (2013). Effects of socioeconomic status and health care access on low levels of human papillomavirus vaccination among Spanish-speaking Hispanics in California. American Journal of Public Health, 103, 270–272.
Tiro, J. A., Tsui, J., Bauer, H. M., et al. (2012). Human papillomavirus vaccine use among adolescent girls and young adult women: An analysis of the 2007 California Health Interview Survey. Journal of Women’s Health (Larchmt), 21, 656–665.
Lau, M., Lin, H., & Flores, G. (2012). Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children’s Health. Vaccine, 30, 3112–3118.
Hirth, J. M., Tan, A., Wilkinson, G. S., et al. (2012). Completion of the human papillomavirus vaccine series among insured females between 2006 and 2009. Cancer, 118, 5623–5629.
Gold, R., Naleway, A., & Riedlinger, K. (2013). Factors predicting completion of the human papillomavirus vaccine series. The Journal of Adolescent Health, 52, 427–432.
Centers for Disease Control and Prevention. (2014). HPV vaccine. Available at http://www.cdc.gov/hpv/vaccine.html.
Dorell, C. G., Stokley, S., Yankey, D., et al. (2012). Compliance with recommended dosing intervals for HPV vaccination among females, 13–17 years, National Immunization Survey-Teen, 2008–2009. Vaccine, 30, 503–505.
Tan, W., Viera, A. J., Rowe-West, B., et al. (2011). The HPV vaccine: Are dosing recommendations being followed? Vaccine, 29, 2548–2554.
Wilson, R. M., Brown, D. R., & Carmody, D. P. (2014). Prevalence and correlates of HPV vaccine initiation among low-income adolescents. Poster presentation at NIH/NIMH’s 2014 International Symposium on Minority Health and Health Disparities, Washington, DC (Dec 1, 2014).
Agresti, A., & Finlay, B. (2009). Statistical methods for the social sciences (4th ed.). Upper Saddle River, NJ: Prentice Hall.
Reiter, P. L., McRee, A. L., Kadis, J. A., et al. (2011). HPV vaccine and adolescent males. Vaccine, 29, 5595–5602.
Han, C. S., Ferris, D. G., Waller, J., et al. (2012). Comparison of knowledge and attitudes toward human papillomavirus, HPV vaccine, pap tests, and cervical cancer between US and Peruvian women. Journal of Lower Genital Tract Disease, 16, 121–126.
Cui, Y., Baldwin, S. B., Wiley, D. J., et al. (2010). Human Papillomavirus vaccine among adult women: Disparities in awareness and acceptance. American Journal of Preventive Medicine, 39, 559–563.
Marlow, L. A. V., Wardle, J., Forster, A. S., et al. (2009). Ethnic differences in human papillomavirus awareness and vaccine acceptability. Journal of Epidemiology and Community Health, 63, 1010–1015.
Schluterman, N. H., Terplan, M., Lydecker, A. D., et al. (2011). Human papillomavirus (HPV) vaccine uptake and completion at an urban hospital. Vaccine, 29, 3767–3772.
Pourat, N., & Jones, J. M. (2012). Role of insurance, income, and affordability in human papillomavirus vaccination. The American Journal of Managed Care, 18, 320–330.
Moss, J. L., Gilkey, M. B., Reiter, P. L., et al. (2012). Trends in HPV vaccine initiation among adolescent females in North Carolina, 2008–2010. Cancer Epidemiology, Biomarkers and Prevention, 21, 1913–1922.
Darden, P. M., Thompson, D. M., Roberts, J. R., et al. (2013). Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008–2010. Pediatrics.
Schmidt, S., & Parsons, H. M. (2014). Vaccination interest and trends in Human Papillomavirus vaccine uptake in young adult women aged 18–26 years in the United States: An analysis using the 2008–2012 National Health Interview Survey. American Journal of Public Health pp. e1–e8.
US Department of Health and Human Services. Healthy people 2020. Washington, DC: US Department of Health and Human Services; 2012. Available at http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23; 2012.
President’s Cancer Panel (PCP). Accelerating HPV vaccine uptake: Urgency for Action to Prevent Cancer. A Report to the President of the United States from the President’s Cancer Panel.
Frieden, T. Five Things I’ll Track Closely in 2014: The vaccine too few are getting: We’ve got to increase uptake of the HPV vaccine—it’s an anti-cancer vaccine. Available at http://www.huffingtonpost.com/tom-frieden-md-mph/cdcs-top-ten-five-for-201_b_4465682.html.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilson, R.M., Brown, D.R., Carmody, D.P. et al. HPV Vaccination Completion and Compliance with Recommended Dosing Intervals Among Female and Male Adolescents in an Inner-City Community Health Center. J Community Health 40, 395–403 (2015). https://doi.org/10.1007/s10900-014-9950-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10900-014-9950-7