Abstract
Whole exome sequencing (WES) is an integral tool in the diagnosis of genetic conditions in pediatric patients, but concerns have been expressed about the complexity of the information and the possibility for secondary findings that need to be conveyed to those deciding about WES. Currently, there is no validated tool to assess parental understanding of WES. We developed and implemented a survey to assess perceived and actual understanding of WES in parents who consented to clinical WES for their child between July 2013 and May 2015. Fifty-three eligible surveys were returned (57% response rate). Areas with both low perceived and actual understanding about WES included how genes are analyzed and lack of protection against life insurance discrimination. Parents also had low actual understanding for two questions related to secondary findings – reporting of secondary findings in a parent (if tested) and whether secondary findings can be related to traits such as height and hair color. Further work to develop a validated tool to assess understanding of WES would be beneficial as WES is integrated more frequently into clinical care.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Whole exome sequencing is rapidly becoming an integral tool for the diagnosis of genetic disorders in patients, especially after a genetic cause cannot be identified by more targeted methods (Yang et al.). With approximately 85% of disease-causing mutations found in the exome, there are high hopes for WES to find new genetic origins of disease (Grody et al. 2013; Rabbani et al. 2014). WES primarily benefits patients with rare diseases with unknown etiologies, atypical clinical presentations, or more than one genetic etiology contributing to the phenotype (Johansen Taber et al. 2014; Kingsmore and Saunders 2011; Pinxten and Howard 2014). Recent data suggests WES has a diagnostic yield of 25–35% (Farwell et al. 2014; Lee et al. 2014; Valencia et al. 2015; Yang et al. 2014). Currently, pediatric patients represent the majority of patients undergoing WES (Yang et al. 2013). Therefore, parents are the primary population consenting to this test.
Until recently, genetic testing was hypothesis-driven, with health care providers ordering testing for specific genes based on phenotype; therefore, the consent discussion was limited to these specific genes and their possible implications (Bunnik et al. 2014; Rigter et al. 2014). WES generates more data and potential results, making it unrealistic to explain each potential finding and implication (Bradbury et al. 2015) and unreasonable to expect a patient to understand and retain such information (Bunnik et al. 2014; Ormond 2013). Although facilitating understanding about WES is essential, it can be difficult to find the balance between too much information that may overwhelm a family and not enough information to make an informed decision (Appelbaum et al. 2014; Ormond 2013; Pinxten and Howard 2014; Rigter et al. 2013; Rigter et al. 2014).
An additional barrier to facilitating understanding about WES is the possibility of incidental or secondary findings. Incidental findings are unanticipated results unrelated to the primary indication for testing that are found after completion of filtering and segregation analysis (Weiner 2014). Secondary findings are also unrelated to the primary indication, but they are purposely sought out during the analysis of the test results. Although both incidental and secondary findings may have health, reproductive or personal importance for the patient and his/her family, more time may be spent informing patients about the possibility of secondary findings, particularly the American College of Medical Genetics and Genomics (ACMG) 56 genes, since these possible results are more tangible (Allyse and Michie 2013; Bowdin et al. 2014; Bunnik et al. 2014; Burke et al. 2013; Green et al. 2013; Pinxten and Howard 2014).
As the use of WES expands into specialties outside of genetics, such as immunology, it is not clear if each clinic is equipped with the resources, knowledge, or personnel to provide pre-test counseling about WES (Bowdin et al. 2014; Kingsmore and Saunders 2011; Rigter et al. 2013). Additionally, patients in various specialty clinics may be in very different situations: some may be on a diagnostic odyssey and searching for answers to their condition, whereas others may be facing a life-threatening health crisis (such as cancer or a severe immunodeficiency) and may be more focused on recovering. Whether differences in clinic personnel and health status of the patient impact the understanding of those consenting to WES is yet to be explored.
In response to concerns about providing informed consent for whole genome and whole exome sequencing, Ayuso et al. (2013) reviewed consent documents and expert opinions to propose a list of necessary elements in clinical consent for these tests. However, simply including these recommended elements does not ensure that patients actually understand the information. There are five commonly accepted requirements for informed consent: 1) information disclosure, 2) competence, 3) voluntariness, 4) comprehension, and 5) consent (Beauchamp and Faden 1995). Since genetic counselors and research coordinators experienced in obtaining informed consent for genomic sequencing have previously identified participant understanding as one of the primary challenges to the informed consent process (Tomlinson et al. 2016), we chose to focus on the requirement of comprehension in our study. Comprehension itself may be viewed as two separate elements: perceived understanding (the person must feel that they comprehend the information) and actual understanding (they must have correct knowledge of the information) (Joffe et al. 2001). Assessment of patients’ perceived and actual understanding of WES and identification of any gaps in understanding, could allow providers to address these gaps.
Since there is currently no validated tool to assess understanding of exome/genome sequencing, we developed and administered a survey to measure the two essential elements of comprehension, perceived and actual understanding. These elements have been previously used to assess understanding of informed consent for other medical tests and procedures (Joffe et al. 2001; Klima et al. 2014; Miller et al. 1996; Noll et al. 2001). The primary purpose of this study was to identify gaps in areas of perceived and actual understanding among parents who consented to WES for their children. The secondary purpose was to compare understanding of WES among parents of patients seen in genetics and non-genetics specialty clinics.
Materials and Methods
WES Consent Process
Families considering clinical WES were routinely given two documents prior to testing, a consent form and a patient brochure about WES. Information in the consent form included the purpose of WES, a description of the test process, technical and clinical limitations of the test, what information will and will not be returned to the patient, the option to receive secondary findings, how information will be kept confidential, storage and future use of the data, and the voluntary nature of the test. Information in the WES patient brochure included basic genetic information (e.g. genes, exons, and mutations), the purpose of WES, how WES is performed and interpreted, the diagnostic yield of WES, benefits and limitations of WES, the types of results that might be returned (positive, negative, uncertain, and primary or secondary), additional information that might be learned (biological relationships, risks for family members, secondary finding if desired), insurance and employment protections and concerns, storage of the sample and results, costs, and information about meeting with a genetic counselor/geneticist. These documents follow the “generic model” of consent where general information about WES and possible results are provided in pre-test counseling, and families’ specific results are discussed after the test is performed (Bunnik et al. 2014; Elias and Annas 1994; Rigter et al. 2013).
At the time of this study, most patients offered WES through the genetics clinical met with a genetic counselor for pre-test counseling and review of the consent form. Families offered WES in non-genetics specialty clinics met with a genetic counselor or other health care provider in the specialty clinic for pre-test counseling. Regardless of clinic, all families received and signed the same consent form prior to testing. The patient brochure was routinely provided to parents by genetic counselors during or prior to the pre-test counseling. Other health care providers had access to the patient brochure, but may or may not have provided it to their patients. Both the patient brochure and the consent form are publicly available on the CCHMC website (https://www.cincinnatichildrens.org/service/d/diagnostic-labs/molecular-genetics/whole-exome-sequencing).
Participants
The Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board approved the study. Only one parent or guardian who consented to clinical WES for their child at CCHMC from July 1, 2013 through May 14, 2015 was eligible to participate. We identified potential participants using the CCHMC Molecular Genetics Laboratory’s Clinical Exome Tracking System. Parents/guardians from the United States whose child, ages 0–18 years, had submitted a sample for WES in this timeframe were eligible to participate, regardless of whether or not they had received results. We included patients seen in genetics clinics and four non-genetics specialty clinics: allergy and immunology, bone marrow and immunology, cardiology, and neurology.
Recruitment and Data Collection
We mailed pre-notification letters to inform potential participants about the study and that a paper survey would be mailed to them within a few weeks. They were also given the option to opt out of the study. Three weeks later, we mailed a cover letter, a paper copy of survey and a $5 gift card to potential participants. The cover letter also included a URL and a unique study identification number to allow completion of the survey online. Follow-up reminders and surveys were both mailed and emailed (if an email was included in the electronic medical record) to non-responders three and six weeks after the initial mailing.
All participants were given the option to complete the survey by mail or online. The online version of the survey was created in REDCap (Research Electronic Data Capture), a secure, web-based application designed to support data capture for research studies (Harris et al. 2009). The responses from paper surveys were entered into REDCap by the first author, and data was managed using REDCap hosted at CCHMC.
Survey Development
We modelled our survey after the Quality of Informed Consent (QuIC) questionnaire developed by Joffe et al. (2001), which compared perceived and actual understanding of the purpose, risks, and possible outcomes related to cancer clinical trials. We (the authors) modified the questions to be applicable to a clinical population undergoing WES. The final survey consisted of four sections and a total of 63 questions. The survey was written below an 8th grade reading level (Flesch-Kincaid Grade Level: 7.9). We provided definitions in the survey instructions for whole exome sequencing, exon, and genome.
The first section included seventeen questions to assess parents’ perceived understanding of WES and secondary findings. These questions were based on the content within the ten minimum elements of informed consent proposed by Ayuso et al. (2013): scope, description, benefits, risks, voluntary, refusal, alternative test, confidentiality, future use, and secondary findings. Each of these questions addressed a specific aspect of Ayuso et al.’s (2013) elements of informed consent. For each of the perceived understanding questions, parents were asked how well they felt they understood specific information about WES. Responses options included “very well”, “mostly”, “somewhat”, “barely”, and “not at all”.
The second and third sections assessed parents’ actual understanding. The second section included twenty-one questions about WES in general and the third section included nine questions about secondary findings that could be learned from WES. Actual understanding questions were written by the authors and were based on the information about WES provided to families in the consent form and the patient brochure. When writing the questions, we used the same terminology (e.g. mutation, genetic cause, positive/negative results) as that used in the patient consent form and patient brochure. All but two questions on the survey were specifically addressed in the consent form and/or the patient brochure. The two questions not specifically addressed were: “receiving negative results from WES means that the patient will not need any genetic testing in the future”, and “a positive result for a secondary finding means the patient will develop that condition”. These questions were written to assess the parent’s comprehension of positive and negative results. The actual understanding questions were similar to true/false questions in that each had a correct answer. Response options were agree, disagree or unsure.
The fourth section of the survey focused on demographic information, including age, gender, race, education level, experience in a healthcare profession, number of children, date the parent provided consent for WES, results of WES, and information about the child (the patient). The final question was an open-ended question about what parents felt was most important to tell families about WES.
We pre-tested the perceived and actual understanding sections of the survey by administering it to four parents of patients undergoing WES at CCHMC after May 14, 2015. Pre-testing participants were instructed to answer all questions on the survey and highlight any questions or terminology they found confusing. They were then asked to explain what they found confusing and how the question could be made clearer. For example, one participant commented that a few questions were framed in the negative voice (e.g. WES does not test every exon in the patient’s DNA) which they found hard to interpret. Participants were also asked questions about questions/terminology on which we wanted specific feedback. For example, we asked participants what “positive” and “negative” results meant to them to ensure that participants consistently interpreted these terms in the same way. We modified the survey based on the feedback we received, such as reframing questions from a negative to positive voice.
Scoring and Data Analysis
To assess possible differences between clinics where patients were seen, we compared perceived and actual understanding scores between genetics and non-genetics specialty clinics. Parents’ answers to the perceived understanding questions were converted to a score from 1 to 5 (1 = “Not at all”, 2 = “Barely”, 3 = “Somewhat”, 4 = “Mostly”, 5 = “Very well”) and median scores were compared between clinics using the Wilcoxon rank-sum test. The proportion of correct, incorrect, and “unsure” answers to the actual understanding questions were compared between clinics using Fisher’s exact test. We used p ≤ 0.05 to indicate the statistical significance due to the exploratory nature of the study.
Results
Study Population
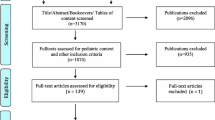
Information about our cohort is provided in Fig. 1. We received 53 eligible completed surveys, giving a response rate of 57%. Demographic information of the parents/guardians is presented in Table 1. All participants were parents of the patient except one, who was the legal guardian. We refer to participants as “parents” from this point forward. The majority of the parents were Caucasian (96%), female (91%), and had some post-secondary education (83%). Demographics did not show differences between parents consenting to WES in the genetics clinic versus a non-genetics speciality clinic. We had limited information on non-responders but were able to obtain ethnicities for 24 of the 42 non-responders: 88% identified as Caucasian or Caucasian plus another ethnicity, 8% identify as African American, and 4% identify as “other” (Nepalese).
We received responses from parents seen for WES in genetics clinic (n = 41) and two non-genetics specialty clinics (bone marrow and immunology (n = 11), and allergy and immunology (n = 1)). All parents consenting to WES through the genetics clinic received pre- and post-test counseling from a genetic counselor and/or geneticist. Based on documentation provided to the molecular laboratory when WES was ordered, nine of the twelve parents (75%) consenting to WES through a non-genetics specialty clinic received pre-test counseling from a genetic counselor. Of these nine parents, seven also received post-test counseling from a genetic counselor. One parent received pre-test counseling from a specialist but was referred to genetics for post-test counseling. The remaining two parents received pre- and post-test counseling from their specialist without a genetic counselor or geneticist involved.
Perceived Understanding
General Information about WES
For each of the 13 perceived understanding questions, more than 50% of parents felt that they had a good understanding of the information (responses of “Mostly” or “Very well”; Table 2). Parents reported the highest perceived understanding for “…it is [their] choice to have WES” (100% good understanding), “Who will be tested” (98% good understanding), and “The benefits of WES” (93% good understanding). Parents reported the least perceived understanding of “The possible discrimination based on WES results” (53% good understanding) and “How genes are analyzed by WES” (55% good understanding).
Secondary Findings
Regarding perceived understanding about secondary findings, parents reported highest perceived understanding for “…it is [their] choice to learn about secondary findings” (93% good understanding) and least perceived understanding of “The implications of secondary findings” (74% good understanding; Table 2).
Actual Understanding
General Information about WES
Eighteen of the 21 questions evaluating actual understanding of WES were answered correctly by more than half of the parents. The questions with the highest proportion of correct answers were “WES may find a genetic cause for the patient’s condition, even when other genetic tests could not” (98%), “Results from WES may help guide the patient’s medical care” (98%), and “WES might find genetic changes (mutations) that we do not fully understand yet” (98%). Less than half of parents correctly answered “WES analyzes genes one at a time” (21%), “Life insurance companies can use the results of WES to discriminate against who to cover” (36%), and “WES tests every exon in a patient’s DNA” (40%) (Table 3).
Secondary Findings
Seven of the nine questions about actual understanding of secondary findings were answered correctly by more than half of the parents. The questions with the highest proportion of correct answers were “Secondary findings may reveal that other family members are at risk for a genetic condition” (89%), “A positive result for secondary findings means the patient will develop that condition” (87%), and “Secondary findings are genetic changes that may impact the patient’s health” (83%). There were two questions that less than half of the parents answered correctly: “If a parent was also tested, secondary findings in the parent will only be reported if they were also found in the patient” (43%), and “Secondary findings can be related to personal traits, such as height and hair color” (48%) (Table 3).
Comparison of Understanding Based on Clinic of Referral
To examine whether understanding varied by clinic, we compared the understanding scores (perceived understanding) and the proportion of correct/incorrect/unsure responses (actual understanding) between parents who consented to WES through a genetics clinic and a non-genetics specialty clinic. The only significant difference was in perceived understanding about “re-analysis of the results in the future, if new information is found.” Parents from a genetics clinic perceived their understanding to be higher than parents from a specialty clinic (p = 0.045; Table 2). There were no significant differences detected in actual understanding between parents from the two different clinic settings (Table 3).
Secondary Findings
Overall, 70% of parents (genetics: n = 30; specialty: n = 7) reported that they asked to receive secondary findings and 15% (genetics: n = 6; specialty: n = 2) reported that they had received positive results for secondary findings. There was no significant difference in the proportion of participants requesting secondary findings in genetics compared to specialty clinics (p = 0.652). Thirteen percent of parents (genetics: n = 5; specialty: n = 2) reported they did not know if they had asked to receive secondary findings. One parent from a specialty clinic did not indicate whether they had requested secondary findings or not.
Parents Report about What Is most Important to Tell Families about WES
Forty-six parents answered the open-ended question about what they felt was most important to tell families about WES (16 received positive WES or secondary findings, 25 received negative WES or secondary findings, and 5 had not yet received results). The most common response (n = 15) provided by participants regardless of their test status, was that it was important for providers to tell families that WES may not find a diagnosis for their child. Parents receiving positive results also commented on the importance of discussing the implications of secondary findings, that WES could end a diagnostic odyssey, and that the results may change medical management. Of those who received negative results, many indicated that WES did not meet their expectations. One parent commented that negative results can negatively impact the care their child receives. “The quality of care that my child now receives from a negative result is a lot lower now. Health concerns and complaints aren’t being listened to… Medical issues that were being addressed before the test were being treated. After the test they are being ignored.” (Parent 2015-BIM-020).
Discussion
Until the wide-spread use of next-generation sequencing, genetic testing targeted one gene at a time. The pre-test consent process for single-gene testing was simpler and more specific: explain the rationale behind testing, which gene was being analyzed, and the possible results and implications. However, to apply this same model of consent to WES could take as many as eight hours (Bick and Dimmock 2011). Therefore, when obtaining consent for WES, the “specific model” of informed consent has widely been replaced with the “generic model” in which broad information about WES is discussed during pre-test counseling and more specific information is discussed once results have been received (Bunnik et al. 2014; Elias and Annas 1994; Rigter et al. 2013). Whether the generic model of consent for WES is effective at sufficiently informing parents about WES has been largely unstudied. Our findings suggest that the generic model of informed consent for WES is effective with regards to parental comprehension, as parents scored high on both perceived and actual understanding.
While both perceived and actual understanding were generally high in this sample, we identified several areas with lower understanding. Parents reported lower actual understanding about whether the purpose of WES is to find every genetic change in a patient. A limitation of WES is that the test is not able to detect every type of mutation. For example, WES cannot detect trinucleotide repeat expansions, intronic mutations, mitochondrial mutations, and large insertions/deletions (O’Daniel and Lee 2012). It is not clear if perhaps parents understood the limitations of WES, but still agreed that the purpose of WES is to identify every mutation because of the genome-wide approach aimed at finding disease-causing mutations. However, the lower perceived understanding may reflect parents’ higher expectations to find a diagnosis through WES than is possible with this technology. In fact, in the final question of our survey, multiple parents whose children did not receive a diagnosis from WES said that they felt that the test did not meet their expectations while no parents who had received a diagnosis felt this way. Genetics professionals with experience obtaining informed consent for WES have previously raised concerns about patients/families having unrealistic expectations about the results of exome/genome sequencing and have highlighted the importance of helping patients develop realistic expectations (Bernhardt et al. 2015; Tomlinson et al. 2016).
Lower scores for the actual understanding questions “WES analyzes genes one at a time” and “WES tests every exon in a patient’s DNA” suggest that parents do not consistently understand how the results of WES are generated. In fact, parents seemed to recognize their lack of familiarity with this subject, as only 55% of parents felt they had a good understanding of how genes are analyzed by WES. It is possible that less time is spent during the consent process describing the technical aspects of WES. Alternatively, the technical aspects may be less salient to parents and therefore not retained. Among all the information presented about WES, one could argue that the method of analysis may be less important than the implications of the results. Indeed, genetic counselors and research coordinators experienced in obtaining informed consent for genome/exome sequencing report focusing less on technological aspects of sequencing and more on the types and implications of possible results (Bernhardt et al. 2015). Additionally, the ACMG Board of Directors recommend that counseling for genome and exome sequencing focus on expected outcomes of testing, types of results that may be generated and will be returned, the benefits and risks, limitations of testing, implications for family members, and alternatives to testing; however, they do not mention emphasizing the technical aspects of the sequencing procedure in the consent discussion (ACMG Board of Directors 2013).
Both perceived and actual understanding of the possibility of discrimination based on WES results were low. Parents had the best understanding of their protection against discrimination by employers, followed by protection against discrimination by health insurance companies. However, only about one third of parents correctly reported that life insurance companies could use these results to discriminate against who to cover. The low proportion of correct responses to insurance questions may be related to lack of familiarity with protections against insurance discrimination. Indeed, research suggests that less than 1 in 5 American adults are familiar with laws protecting their genetic information (Parkman et al. 2015). In the United States, the Genetic Information Non-discrimination Act of 2008 protects against health and employment discrimination based on genetic information, but life insurance is not afforded the same protections. Insurance discussions during the consent process for WES may not be extensive enough to familiarize every parent with the differences in health and life insurance protections.
Alternatively, information about life insurance may not be a salient issue for parents of young children, particularly if the child has chronic health issues that could prevent them from accessing life insurance in the future regardless of genetic testing results, or if parents prioritize the child’s current health issues over potential life insurance discrimination in the future. Although it is not clear how salient parents in our study found insurance discrimination, previous research suggests that insurance and employment discrimination due to the results of whole exome or whole genome sequencing are a significant concern in both parents of children with and without health concerns (Bergner et al. 2014; Goldenberg et al. 2014; Joseph et al. 2016; Oberg et al. 2015). In practice, however, while there are reports of specific cases in which genetic information is used by life insurance companies to make policy decisions, such as application outcomes and premium costs (Barlow-Stewart et al. 2009; Joly et al. 2013), there is limited evidence of routine genetic discrimination by these companies (Armstrong et al. 2003; Joly et al. 2013). Still, concerns about insurance discrimination may be particularly heightened when considering secondary findings since they provide information on predisposition to disease in the future as opposed to a diagnosis for an existing condition.
Although nearly all parents felt they had good understanding about it being a choice to learn secondary findings, fewer (64%) correctly responded to the question “Secondary findings must be looked for in everyone who undergoes WES.” The differences in responses to these similar questions could be due to the wording of the two questions: parents may believe that the laboratory looks for secondary findings in everybody but that the parents then have the choice whether they want to learn about the results or not. Further work to validate this questionnaire in an independent population would be useful to help clarify parents’ interpretation of these questions.
There also appeared to be some confusion about the types of information that secondary findings can reveal. Less than half of parents correctly identified that secondary findings are not related to personal traits, such as height and hair color, and only two thirds of parents correctly identified that secondary findings are not related to the patient’s current symptoms. More parents understood that secondary findings could be related to cancer and heart disease, perhaps because discussions during the consent process focused on what secondary findings could reveal as opposed to what they could not reveal.
There was only one difference in perceived understanding (re-analysis of results) and no differences in actual understanding between parents whose children were offered WES in genetics clinics vs. non-genetics speciality clinics. However, nearly all parents who responded to our survey received pre-test counseling from a genetic counselor, including 75% of the parents who were seen in a non-genetics speciality clinic. Specialists in other medical centers may not have the same access to genetic counselors and/or geneticists when ordering WES. Additional research is needed to determine whether access and previous exposure to genetic specialists impacts understanding of WES, as well as whether reasons that patients present to different clinics impacts understanding. For example, research suggests that health status of the patient may impact parental understanding of WES because parents of patients on a diagnostic odyssey, frequently presenting to genetics clinics, may be more familiar with genetic concepts due to previous attempts to find a diagnosis through genetic testing, more motivated to independently research testing options for their child, and less concerned about the implications for secondary findings than parents of patients in a health crisis (Bergner et al. 2014; Levenseller et al. 2014; Oberg et al. 2015; Sapp et al. 2014; Shahmirzadi et al. 2014). Parents of patients facing an acute health crisis, frequently presenting to non-genetics speciality clinics, have expressed that they were too overwhelmed at the time of their child’s health crisis to be able to concentrate on the information given to them about WES (Oberg et al. 2015).
We were also interested in determining whether parents differed in their desire to receive secondary findings depending on the clinic in which they were treated. While there was no significant difference in the proportion of parents requesting secondary findings in the genetics versus specialty clinics, our sample size was small and further investigation into these populations’ desire for secondary findings is warranted. Parents of children on a diagnostic odyssey in a genetics clinic may have been searching for a cause for their child’s condition for years and may view any information available as useful; therefore, they may be more likely to request secondary findings (Bergner et al. 2014; Sapp et al. 2014). Additionally, many children with chronic health issues presenting for WES in the genetics clinic had a developmental or intellectual disability and their parents may feel that they will be caring for them into their child’s adult years (Bigby 1996; Essex et al. 1999; Seltzer et al. 2011). Parents in this situation may want to know about future health issues so they know what types of health concerns they should be watching for as their child ages (Sapp et al. 2014). Conversely, parents of children in an immediate health crisis report being primarily focused on helping their child recover from the immediate situation, feeling overwhelmed by the diagnosis, and wanting to feel in control of their child’s health (Kai 1996; Scollon et al. 2014). They may not be considering future health problems or may not feel mentally prepared to receive such information if it will not help guide the immediate care of their child (McCullough et al. 2016). These children are also more likely to have typical intelligence and therefore are more likely to take control of their own health as an adult; there is current debate surrounding whether testing minors for secondary findings infringes upon their autonomy to decide for themselves as adults (Burke et al. 2013; Johnson and Gehlert 2014; Levenseller et al. 2014; van El et al. 2013; Yu et al. 2014).
Study Limitations
Our study was limited by the lack of a validated tool to measure informed consent for WES. The validated tool we used as a model when designing our survey was originally developed to assess informed consent among parents enrolled in cancer research trials (Joffe et al. 2001); therefore, many of the questions were not applicable to the goals of our investigation. Further work to create a validated tool to measure understanding of WES would benefit future research and may help inform the consent process for WES.
Our study was also limited by the number of parents whose children had undergone WES at the time of the survey and the demographic homogeneity of the respondents. Based on educational attainment, the health literacy of our study population is likely higher than the health literacy of the general population, which may have an impact on understanding and recall of information about WES. It would be beneficial to assess the validity of this survey in independent populations with varied demographic factors.
Practice Implications
Although understanding of the possibility of insurance discrimination was limited in our study, the length of time available to discuss WES in the genetic counseling session may preclude lengthy discussions about the legal implications of WES results. Insurance information could be provided as supplemental material, perhaps included in the informed consent document or in a patient brochure, for the parents to review at a later time.
Our findings also highlight the importance of providers helping parents manage their expectations of WES. It may be beneficial to emphasize the low probability of a diagnosis and to provide anticipatory guidance that they may feel disappointed if they do not receive a diagnosis. Indeed, parents, regardless of whether they had received positive results, negative results, or were still waiting for the results, felt that one of the most important things to communicate to parents during the consent is that WES may not find a diagnosis for their child. Explaining the probability of a diagnosis through WES (tailored to the specific indication, if possible), the fact that WES does not test all genes and cannot find all mutations, the method in which genes are selected for interrogation, and the process in which negative results or variants of uncertain significance may be re-examined in the future may also help foster more realistic expectations.
Research Recommendations
The majority of our parents had pre- and/or post-test counseling with a genetic counselor. However, as the use of WES continues to expand into non-genetics speciality clinics, genetic counselors or geneticists may not always be involved in discussing the test with the patients. Therefore, further research is needed to determine whether there is a difference in understanding of WES when a genetic specialist is not involved in the consent process in order to guide recommendations for the informed consent process for WES in specialty clinics. Further investigation into whether parents’ choice to receive or decline secondary findings is influenced by the clinic in which they consented to WES is also warranted. Finally, future research should assess the validity of this survey in independent populations with varied demographic factors.
Conclusions
We found that parents’ perceived and actual understanding of WES was high, with some notable areas for improvement. We identified several areas in which understanding of WES could be improved, including how and which genes are analyzed, insurance implications, and information about secondary findings. The findings also suggest that providers of WES could be more active in helping parents manage their expectations of the test, especially since WES currently results in a clinical diagnosis in less than 30% of patients. Parents who received negative results expressed frustration that WES did not meet their expectations; therefore, providing anticipatory guidance that parents may be disappointed with the results of WES along with the probability of a diagnosis may be useful to manage expectations. Finally, although the clinic in which parents were seen for clinical care and offered WES (i.e. genetics versus non-genetics specialty) did not significantly impact understanding, a genetic counselor was involved in almost all of the parents’ pre-test counseling sessions. Therefore, there is an ongoing need to determine whether understanding of WES differs among those who consent to WES when a genetics professional is and is not involved in pretest counseling. In conclusion, this study represents a novel exploration of parental understanding of WES. Development of a validated tool to assess understanding of elements of informed consent for WES would be beneficial for future research.
References
ACMG Board of Directors (2013). Points to consider for informed consent for genome/exome sequencing. Genetics in Medicine, 15(9), 748–749. doi:10.1038/gim.2013.94.
Allyse, M., & Michie, M. (2013). Not-so-incidental findings: the ACMG recommendations on the reporting of incidental findings in clinical whole genome and whole exome sequencing. Trends in Biotechnology, 31(8), 439–441. doi:10.1016/j.tibtech.2013.04.006.
Appelbaum, P. S., Waldman, C. R., Fyer, A., Klitzman, R., Parens, E., Martinez, J., et al. (2014). Informed consent for return of incidental findings in genomic research. Genetics in Medicine, 16(5), 367–373. doi:10.1038/gim.2013.145.
Armstrong, K., Weber, B., FitzGerald, G., Hershey, J. C., Pauly, M. V., Lemaire, J., et al. (2003). Life insurance and breast cancer risk assessment: adverse selection, genetic testing decisions, and discrimination. American Journal of Medical Genetics. Part A, 120a(3), 359–364. doi:10.1002/ajmg.a.20025.
Ayuso, C., Millan, J. M., Mancheno, M., & Dal-Re, R. (2013). Informed consent for whole-genome sequencing studies in the clinical setting. Proposed recommendations on essential content and process. European Journal of Human Genetics, 21(10), 1054–1059. doi:10.1038/ejhg.2012.297.
Barlow-Stewart, K., Taylor, S. D., Treloar, S. A., Stranger, M., & Otlowski, M. (2009). Verification of consumers’ experiences and perceptions of genetic discrimination and its impact on utilization of genetic testing. Genetics in Medicine, 11(3), 193–201. doi:10.1097/GIM.0b013e318194ee75.
Beauchamp, T. L., & Faden, R. R. (1995). Informed consent: II. Meaning and elements of informed consent. In R. WT (Ed.), Encyclopaedia of bioethics (Vol. 14, pp. 1240–1245). New York: Simon & Schuster Macmillan.Revised
Bergner, A. L., Bollinger, J., Raraigh, K. S., Tichnell, C., Murray, B., Blout, C. L., et al. (2014). Informed consent for exome sequencing research in families with genetic disease: the emerging issue of incidental findings. American Journal of Medical Genetics. Part A, 164(11), 2745–2752. doi:10.1002/ajmg.a.36706.
Bernhardt, B. A., Roche, M. I., Perry, D. L., Scollon, S. R., Tomlinson, A. N., & Skinner, D. (2015). Experiences with obtaining informed consent for genomic sequencing. American Journal of Medical Genetics. Part A, 167a(11), 2635–2646. doi:10.1002/ajmg.a.37256.
Bick, D., & Dimmock, D. (2011). Whole exome and whole genome sequencing. Current Opinion in Pediatrics, 23(6), 594–600. doi:10.1097/MOP.0b013e32834b20ec.
Bigby, C. (1996). Transferring responsibility: the nature and effectiveness of parental planning for the future of adults with intellectual disability who remain at home until mid-life. Journal of Intellectual and Developmental Disability, 21(4), 295–312. doi:10.1080/13668259600033211.
Bowdin, S., Ray, P. N., Cohn, R. D., & Meyn, M. S. (2014). The genome clinic: a multidisciplinary approach to assessing the opportunities and challenges of integrating genomic analysis into clinical care. Human Mutation, 35(5), 513–519. doi:10.1002/humu.22536.
Bradbury, A. R., Patrick-Miller, L., & Domchek, S. (2015). Multiplex genetic testing: reconsidering utility and informed consent in the era of next-generation sequencing. Genetics in Medicine, 17(2), 97–98. doi:10.1038/gim.2014.85.
Bunnik, E. M., Janssens, A. C., & Schermer, M. H. (2014). Informed consent in direct-to-consumer personal genome testing: the outline of a model between specific and generic consent. Bioethics, 28(7), 343–351. doi:10.1111/bioe.12004.
Burke, W., Matheny Antommaria, A. H., Bennett, R., Botkin, J., Clayton, E. W., Henderson, G. E., et al. (2013). Recommendations for returning genomic incidental findings? We need to talk! Genetics in Medicine, 15(11), 854–859. doi:10.1038/gim.2013.113.
Elias, S., & Annas, G. J. (1994). Generic consent for genetic screening. The New England Journal of Medicine, 330(22), 1611–1613.
Essex, E. L., Seltzer, M. M., & Krauss, M. W. (1999). Differences in coping effectiveness and well-being among aging mothers and fathers of adults with mental retardation. American Journal of Mental Retardation, 104(6), 545–563. doi:10.1352/0895-8017(1999)104<0545:diceaw>2.0.co;2.
Farwell, K. D., Shahmirzadi, L., El-Khechen, D., Powis, Z., & Chao, E. C. (2014). Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genetics in Medicine. doi:10.1038/gim.2014.154.
Goldenberg, A. J., Dodson, D. S., Davis, M. M., & Tarini, B. A. (2014). Parents’ interest in whole-genome sequencing of newborns. Genetics in Medicine, 16(1), 78–84. doi:10.1038/gim.2013.76.
Green, R. C., Berg, J. S., Grody, W. W., Kalia, S. S., Korf, B. R., Martin, C. L., et al. (2013). ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in Medicine, 15(7), 565–574. doi:10.1038/gim.2013.73.
Grody, W. W., Thompson, B. H., & Hudgins, L. (2013). Whole-exome/genome sequencing and genomics. Pediatrics, 132(Suppl 3), S211–S215. doi:10.1542/peds.2013-1032E.
Harris, P., Taylor, R., Thielke, R., Payne, J., Gonzalez, N., & Conde, J. (2009). Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381.
Joffe, S., Cook, E. F., Cleary, P. D., Clark, J. W., & Weeks, J. C. (2001). Quality of informed consent: a new measure of understanding among research subjects. Journal of the National Cancer Institute, 93(2), 139–147.
Johansen Taber, K. A., Dickinson, B. D., & Wilson, M. (2014). The promise and challenges of next-generation genome sequencing for clinical care. JAMA Internal Medicine, 174(2), 275–280. doi:10.1001/jamainternmed.2013.12048.
Johnson, K. J., & Gehlert, S. (2014). Return of results from genomic sequencing: a policy discussion of secondary findings for cancer predisposition. Journal of Cancer Policy, 2(3), 75–80. doi:10.1016/j.jcpo.2014.05.001.
Joly, Y., Ngueng Feze, I., & Simard, J. (2013). Genetic discrimination and life insurance: a systematic review of the evidence. BMC Medicine, 11, 25. doi:10.1186/1741-7015-11-25.
Joseph, G., Chen, F., Harris-Wai, J., Puck, J. M., Young, C., & Koenig, B. A. (2016). Parental views on expanded newborn screening using whole-genome sequencing. Pediatrics, 137(Suppl 1), S36–S46. doi:10.1542/peds.2015-3731H.
Kai, J. (1996). Parents’ difficulties and information needs in coping with acute illness in preschool children: a qualitative study. BMJ, 313(7063), 987–990.
Kingsmore, S. F., & Saunders, C. J. (2011). Deep sequencing of patient genomes for disease diagnosis: when will it become routine? Science Translational Medicine, 3(87), 87ps23. doi:10.1126/scitranslmed.3002695.
Klima, J., Fitzgerald-Butt, S. M., Kelleher, K. J., Chisolm, D. J., Comstock, R. D., Ferketich, A. K., & McBride, K. L. (2014). Understanding of informed consent by parents of children enrolled in a genetic biobank. Genetics in Medicine, 16(2), 141–148. doi:10.1038/gim.2013.86.
Lee, H., Deignan, J. L., Dorrani, N., Strom, S. P., Kantarci, S., Quintero-Rivera, F., et al. (2014). Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA, 312(18), 1880–1887. doi:10.1001/jama.2014.14604.
Levenseller, B. L., Soucier, D. J., Miller, V. A., Harris, D., Conway, L., & Bernhardt, B. A. (2014). Stakeholders’ opinions on the implementation of pediatric whole exome sequencing: implications for informed consent. Journal of Genetic Counseling, 23(4), 552–565. doi:10.1007/s10897-013-9626-y.
McCullough, L. B., Slashinski, M. J., McGuire, A. L., Street Jr., R. L., Eng, C. M., Gibbs, R. A., et al. (2016). Is whole-exome sequencing an ethically disruptive technology? Perspectives of pediatric oncologists and parents of pediatric patients with solid tumors. Pediatric Blood & Cancer, 63(3), 511–515. doi:10.1002/pbc.25815.
Miller, C. K., O’Donnell, D. C., Searight, H. R., & Barbarash, R. A. (1996). The deaconess informed consent comprehension test: an assessment tool for clinical research subjects. Pharmacotherapy, 16(5), 872–878.
Noll, S., Spitz, L., & Pierro, A. (2001). Additional medical information: prevalence, source, and benefit to parents. Journal of Pediatric Surgery, 36(5), 791–794. doi:10.1053/jpsu.2001.22962.
O’Daniel, J. M., & Lee, K. (2012). Whole-genome and whole-exome sequencing in hereditary cancer: impact on genetic testing and counseling. Cancer Journal, 18(4), 287–292. doi:10.1097/PPO.0b013e318262467e.
Oberg, J. A., Glade Bender, J. L., Cohn, E. G., Morris, M., Ruiz, J., Chung, W. K., et al. (2015). Overcoming challenges to meaningful informed consent for whole genome sequencing in pediatric cancer research. Pediatric Blood & Cancer, 62(8), 1374–1380. doi:10.1002/pbc.25520.
Ormond, K. E. (2013). From genetic counseling to "genomic counseling". Molecular Genetics & Genomic Medicine, 1(4), 189–193. doi:10.1002/mgg3.45.
Parkman, A. A., Foland, J., Anderson, B., Duquette, D., Sobotka, H., Lynn, M., et al. (2015). Public awareness of genetic nondiscrimination laws in four states and perceived importance of life insurance protections. Journal of Genetic Counseling, 24(3), 512–521. doi:10.1007/s10897-014-9771-y.
Pinxten, W., & Howard, H. C. (2014). Ethical issues raised by whole genome sequencing. Best Practice & Research. Clinical Gastroenterology, 28(2), 269–279. doi:10.1016/j.bpg.2014.02.004.
Rabbani, B., Tekin, M., & Mahdieh, N. (2014). The promise of whole-exome sequencing in medical genetics. Journal of Human Genetics, 59(1), 5–15. doi:10.1038/jhg.2013.114.
Rigter, T., Henneman, L., Kristoffersson, U., Hall, A., Yntema, H. G., Borry, P., et al. (2013). Reflecting on earlier experiences with unsolicited findings: points to consider for next-generation sequencing and informed consent in diagnostics. Human Mutation, 34(10), 1322–1328. doi:10.1002/humu.22370.
Rigter, T., van Aart, C. J., Elting, M. W., Waisfisz, Q., Cornel, M. C., & Henneman, L. (2014). Informed consent for exome sequencing in diagnostics: exploring first experiences and views of professionals and patients. Clinical Genetics, 85(5), 417–422. doi:10.1111/cge.12299.
Sapp, J. C., Dong, D., Stark, C., Ivey, L. E., Hooker, G., Biesecker, L. G., & Biesecker, B. B. (2014). Parental attitudes, values, and beliefs toward the return of results from exome sequencing in children. Clinical Genetics, 85(2), 120–126. doi:10.1111/cge.12254.
Scollon, S., Bergstrom, K., Kerstein, R. A., Wang, T., Hilsenbeck, S. G., Ramamurthy, U., et al. (2014). Obtaining informed consent for clinical tumor and germline exome sequencing of newly diagnosed childhood cancer patients. Genome Medicine, 6(9), 69. doi:10.1186/s13073-014-0069-3.
Seltzer, M. M., Floyd, F., Song, J., Greenberg, J., & Hong, J. (2011). Midlife and aging parents of adults with intellectual and developmental disabilities: impacts of lifelong parenting. American Journal on Intellectual and Developmental Disabilities, 116(6), 479–499. doi:10.1352/1944-7558-116.6.479.
Shahmirzadi, L., Chao, E. C., Palmaer, E., Parra, M. C., Tang, S., & Gonzalez, K. D. (2014). Patient decisions for disclosure of secondary findings among the first 200 individuals undergoing clinical diagnostic exome sequencing. Genetics in Medicine, 16(5), 395–399. doi:10.1038/gim.2013.153.
Tomlinson, A. N., Skinner, D., Perry, D. L., Scollon, S. R., Roche, M. I., & Bernhardt, B. A. (2016). "not tied up neatly with a bow": professionals’ challenging cases in informed consent for genomic sequencing. Journal of Genetic Counseling, 25(1), 62–72. doi:10.1007/s10897-015-9842-8.
Valencia, C. A., Husami, A., Holle, J., Johnson, J. A., Qian, Y., Mathur, A., et al. (2015). Clinical impact and cost-effectiveness of whole exome sequencing as a diagnostic tool: a pediatric Center’s experience. Front Pediatr, 3, 67. doi:10.3389/fped.2015.00067.
van El, C. G., Cornel, M. C., Borry, P., Hastings, R. J., Fellmann, F., Hodgson, S. V., et al. (2013). Whole-genome sequencing in health care: recommendations of the European Society of Human Genetics. European Journal of Human Genetics, 21(6), 580–584. doi:10.1038/ejhg.2013.46.
Weiner, C. (2014). Anticipate and communicate: ethical management of incidental and secondary findings in the clinical, research, and direct-to-consumer contexts (December 2013 report of the presidential Commission for the Study of bioethical issues). American Journal of Epidemiology, 180(6), 562–564. doi:10.1093/aje/kwu217.
Yang, Y., Muzny, D. M., Reid, J. G., Bainbridge, M. N., Willis, A., Ward, P. A., et al. (2013). Clinical whole-exome sequencing for the diagnosis of mendelian disorders. The New England Journal of Medicine, 369(16), 1502–1511. doi:10.1056/NEJMoa1306555.
Yang, Y., Muzny, D. M., Xia, F., Niu, Z., Person, R., Ding, Y., et al. (2014). Molecular findings among patients referred for clinical whole-exome sequencing. JAMA, 312(18), 1870–1879. doi:10.1001/jama.2014.14601.
Yu, J. H., Harrell, T. M., Jamal, S. M., Tabor, H. K., & Bamshad, M. J. (2014). Attitudes of genetics professionals toward the return of incidental results from exome and whole-genome sequencing. American Journal of Human Genetics, 95(1), 77–84. doi:10.1016/j.ajhg.2014.06.004.
Acknowledgments
This publication was supported by a Research Award to the first author from the Division of Human Genetics at Cincinnati Children’s Hospital Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Leandra K. Tolusso, Kathleen Collins, Xue Zhang, Jennifer R. Holle, C. Alexander Valencia, and Melanie F. Myers declare that they have no conflict of interest.
Human Studies and Informed Consent
All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all participants for being included in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Rights and permissions
About this article
Cite this article
Tolusso, L.K., Collins, K., Zhang, X. et al. Pediatric Whole Exome Sequencing: an Assessment of Parents’ Perceived and Actual Understanding.. J Genet Counsel 26, 792–805 (2017). https://doi.org/10.1007/s10897-016-0052-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10897-016-0052-9