Abstract
Fluorescent probes with specific and rapid response to fluoride ions are important mediators for detecting fluoride ions in biological systems. In this study, a phenothiazine-based fluorescent probe, PTC, was designed and synthesized, which undergoes cleavage activation and cyclization induced by fluoride ions targeting Si–O bonds. The probe exhibits strong anti-interference properties and reaches peak fluorescence within 5 min, allowing for quantitative detection of fluoride ions content in the concentration range of 0 to 12.5μM, suitable for live cell fluorescence imaging. The research findings suggest its potential application value in biological systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoride ions, the smallest and most electronegative anions, are of paramount importance to organisms [1,2,3,4]. Adequate intake of F− plays a crucial role in health, such as maintaining skeletal structure and physiological functions [5,6,7,8]. However, excessive fluoride can have harmful effects on health, including acute and chronic fluoride poisoning, fluorosis, neurodegenerative diseases, gastric and renal issues, and even death [9,10,11,12,13,14,15,16]. Therefore, developing highly selective and sensitive detection and quantification methods for fluoride ions to visualize their distribution in organisms is crucial [17,18,19,20,21,22,23,24].
Fluorescence sensing is considered an ideal technique among commonly used methods like colorimetry [25, 26], atomic absorption spectroscopy, and ion chromatography for F− detection [27, 28], especially in bioimaging applications due to its non-invasive and real-time nature [29,30,31,32,33]. Currently, despite significant progress in the development of F− fluorescent probes, there are still some drawbacks in their application in live cells and in vivo, such as high background interference, insufficient sample penetration, and long response times. Therefore, designing fluoride ion probes that can rapidly respond and have a large Stokes shift remains a challenge that needs to be addressed [34,35,36,37].
Therefore, we have designed a new fluorescence probe, PTC, for detecting fluoride ions in cells. It utilizes a fluorescein moiety and relies on fluoride-induced Si–O bond cleavage as its recognition mechanism (Scheme 1). We aim to develop a fluorescence probe with fast response time, large Stokes shift, and excellent specificity for fluorescence imaging of fluoride ions in live cells.
Experimental
Materials and Instruments
All chemicals were sourced as analytical-grade from Energy Chemical Ltd. (Shanghai) and Sigma-Aldrich (Shanghai) Co., Ltd. NMR spectra (1H and 13C) were recorded on a Bruker (Avance) 400 MHz NMR instrument at Guizhou University’s School of Pharmacy. Absorption spectra were captured on a UV-5500PC UV–Vis spectrophotometer, and fluorescence spectra were measured using a Hitachi F4700 fluorescence spectrometer, both provided by Guizhou University’s School of Pharmacy. Mass spectra were obtained using a TSQ 8000 high-resolution mass spectrometer (Thermo Fisher Scientific Co., Ltd.). Melting points were determined with an X-4X digital melting point apparatus (uncorrected, Shanghai Microelectronics Technology Co., Ltd.). For biological imaging, an inverted fluorescence microscope (NIB600, Ningbo Novel Co., Ltd.) was utilized.
Preparation and Measurement of Probe Solutions
To prepare a 1 mM PTC stock solution, the probe PTC was dissolved in DMSO. A stock solution of tetrabutylammonium fluoride (TBAF) at a concentration of 100 mM was produced using THF. Analytes were made as 100 mM stock solutions in deionized water for storage, including common cations (Na+, Ca2+, K+, Fe2+, Fe3+, Cu2+, Ag+, Zn2+) and anions (Cl−, Br−, I−, SO4 2−, HSO3−, H2PO42−, HCO3−, SCN−, NO3−, HPO4−). The fluorescence spectra of PTC for various analytes were investigated using 4 mL of DMSO solution at room temperature. Unless otherwise noted, fluorescence spectra were typically collected after 1 h of analyte addition. All aqueous solutions were made using ultrapure water from a Milli-Q purifier.
Cell Cytotoxicity Assay and Cell Image
Human hepatocellular carcinoma cells (HepG2 cells) were procured from the Kunming Cell Bank of Chinese Academy of Sciences and maintained in our laboratory. The cytotoxicity of the probe PTC against HepG2 cells was evaluated using the MTT assay. HepG2 cells were sown in 96-well plates and cultivated for 24 h. PTC solutions at different concentrations (0, 1, 5, 10, and 20 µM) were added, incubated with cells for 24 h. The MTT assay was performed using the literature methods [38]. In cellular imaging experiments, HepG2 cells were initially incubated with PTC (3 µM, in PBS) for 30 min, then TBAF (200 µM) was added, further incubated for 30 min. After three times washes with PBS, imaging was performed using laser confocal scanning microscopy.
Synthesis and Characterization
Synthesis of Compound 2
Thiophene boronic acid ester (367 mg, 1 mmol) was added to a round-bottom flask containing Dioxane/H2O (1:1, 5 mL), followed by the addition of 4-bromo-2-methoxybenzaldehyde (215 mg, 1.1 mmol) and anhydrous Na2CO4 (212 mg, 2 mmol). Subsequently, Pd(PPh3)4 (11.55 mg, 0.01 mmol) was introduced under argon protection, and the reaction was carried out at 110 ℃ for 3 h. After cooling to room temperature, the reaction was quenched with water (5 mL), and the mixture was extracted with DCM (10 mL × 3). The combined organic phases were washed with saturated brine, dried over anhydrous Na2SO4, and concentrated under vacuum. The resulting crude product was purified by column chromatography (eluent: PE:EA = 5:1; stationary phase: 200–300 mesh silica gel) to afford compound 2 as pale yellow oil (220 mg, 58.7% yield). 1H NMR (400 MHz, CDCl3) δ 10.45 (d, J = 0.8 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.43 – 7.35 (m, 2H), 7.19 – 7.13 (m, 3H), 7.08 (d, J = 1.6 Hz, 1H), 6.95 – 6.86 (m, 3H), 3.98 (s, 3H), 3.87 – 3.83 (m, 2H), 1.89 – 1.83 (m, 2H), 1.03 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 189.4, 162.2, 147.8, 145.8, 144.7, 134.0, 129.1, 127.5, 127.4, 126.2, 125.9, 125.5, 124.1, 123.4, 122.7, 118.9, 115.6, 109.4, 55.7, 49.3, 20.1, 11.3. ESI-HRMS C23H21NO2S ([M + H]+): calcd 376.1366, found 376.1360.
Synthesis of Compound 3
AlCl3 (1.16 g, 9 mmol) was introduced into a pear-shaped flask, followed by the addition of anhydrous CH2Cl2 (10 ml). Compound 2 (1.08 g, 3 mmol) was dissolved in anhydrous CH2Cl2 (5 mL) and carefully added dropwise. The reaction proceeded at ambient temperature for 12 h. Upon completion, HCl solution (2 mol/L, 8 mL) was added dropwise and stirring continued for an additional 0.5 h. Following stirring, the organic solvent was evaporated under reduced pressure. The residual liquid was subsequently subjected to extraction with EA (20 mL × 3), and the combined organic phases were then washed with saturated brine, dried over anhydrous Na2SO4, and concentrated under vacuum. The resulting crude product underwent purification via column chromatography (eluent: PE:EA = 5:1; stationary phase: 200–300 mesh silica gel) to afford compound 3 as yellow-green solid (0.85 g, 81.2% yield). M.P: 98.3–99.4℃.1H NMR (400 MHz, DMSO) δ 11.06 – 10.60 (m, 1H), 10.22 (d, J = 1.6 Hz, 1H), 7.71 (dd, J = 8.0, 1.6 Hz, 1H), 7.53 (dt, J = 8.4, 2.0 Hz, 1H), 7.47 (d, J = 2.0 Hz, 1H), 7.26 (d, J = 8.0 Hz, 1H), 7.24 – 7.19 (m, 2H), 7.17 (d, J = 7.6 Hz, 1H), 7.10 (dd, J = 8.4, 1.6 Hz, 1H), 7.04 (d, J = 8.0 Hz, 1H), 6.96 (t, J = 7.6 Hz, 1H), 3.88 (d, J = 14.0 Hz, 2H), 1.73 (p, J = 7.2 Hz, 2H), 0.96 (td, J = 7.6, 1.6 Hz, 3H).13C NMR (100 MHz, CDCl3) δ 195.8, 162.0, 148.6, 146.1, 144.6, 134.1, 133.2, 127.5, 127.4, 126.3, 126.0, 125.5, 124.1, 122.8, 119.3, 118.1, 115.6, 115.5, 114.8, 49.3, 20.1, 11.3. ESI-HRMS C22H19NO2S ([M + H]+): calcd 362.120926, found 362.11946.
Synthesis of Compound 4
Compound 3 (100 mg, 1 mmol) was added to a two-neck flask along with DCM (5 mL), followed by the addition of DMAP (44 mg, 0.36 mmol) and TEA (91 mg, 0.9 mmol) under argon protection, and cooled to 0 ℃. Finally, tert-butyldimethylchlorosilane (181 mg, 1.2 mmol) was dissolved in DCM (5 mL) and added dropwise. The reaction mixture was stirred at room temperature for 12 h. Upon completion, deionized water (10 mL) was added to quench the reaction, and the mixture was extracted with DCM (10 mL × 3). The combined organic phases were washed with saturated brine, dried over anhydrous Na2SO4, and concentrated under vacuum. The resulting crude product was purified by column chromatography (eluent: PE:EA = 5:1; stationary phase: 200–300 mesh silica gel) to yield compound 4 as yellow-green oil (120 mg, 84% yield). 1H NMR (400 MHz, CDCl3) δ 10.44 (d, J = 0.8 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.37 – 7.32 (m, 2H), 7.21 – 7.13 (m, 3H), 7.00 (d, J = 1.6 Hz, 1H), 6.95 – 6.87 (m, 3H), 3.87 – 3.83 (m, 2H), 1.89 – 1.83 (m, 2H), 1.04 (d, J = 5.6 Hz, 12H), 0.31 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 189.6, 159.2, 147.5, 145.7, 133.8, 128.8, 127.5, 127.4, 126.1, 125.7, 124.2, 122.8, 119.8, 117.8, 115.6, 115.6, 49.3, 27.0, 25.7, 18.4, 11.3. ESI-HRMS C28H33NO2SSi ([M + Na]+): calcd 498.189348, found 498.18874.
Synthesis of Compound PTC
Compound 4 (950.4 mg, 2 mmol) was added to a round-bottom flask containing anhydrous THF (15 mL), followed by the addition of methyl cyanoformate (99 mg, 4 mmol), and tetrahydrofuran (0.53 mg, 0.0075 mmol), under argon protection, and the reaction mixture was cooled to 0 ℃ and stired for 4 h. Upon completion, the solvent was removed under vacuum, and the crude product was purified by column chromatography (eluent: PE:EA = 5:1; stationary phase: 200–300 mesh silica gel) to yield compound PTC as orange solid (420 mg, 37.7% yield). M.P:120.1–121.2℃.IR (KBr, ν, cm−1): 2948.7, 1709.6, 1579.1, 1467.0, 1245.2, 1135.65, 966.1. 1H NMR (400 MHz, DMSO) δ 8.69 (d, J = 3.6 Hz, 1H), 8.29 (dd, J = 22.4, 8.4 Hz, 1H), 7.56 (d, J = 8.0 Hz, 2H), 7.23 (dd, J = 13.6, 8.6 Hz, 3H), 7.16 (d, J = 8.6 Hz, 1H), 7.09 (d, J = 8.0 Hz, 1H), 7.01 (t, J = 7.6 Hz, 1H), 3.91 (d, J = 10.4Hz, 5H), 1.77 (q, J = 7.2 Hz, 2H), 1.07 – 0.88 (m, 12H), 0.34 (s, 5H). 13C NMR (100 MHz, CDCl3) δ 163.6, 157.2, 149.6, 146.8, 145.8, 144.6, 133.4, 129.6, 127.5, 127.4, 126.1, 125.7, 125.6, 124.1, 122.8, 121.7, 120.1, 117.1, 116.2, 115.7, 115.6, 100.0, 53.1, 49.4, 25.7, 20.1, 18.3, 11.3. ESI-HRMS C32H36N2O3SSi ([M + Na]+): calcd 579.210811, found 597.20996.
Results and Discussion
Synthesis
The fluorescent probe PTC, with a benzothiazole moiety as the fluorophore and utilizing Si–O bonds as recognition groups, was synthesized. Starting from benzothiazole boronate, it underwent a series of reactions including Suzuki coupling (yield: 58.7%), demethylation (yield: 81.2%), nucleophilic substitution (yield: 84%), and nucleophilic addition (yield: 37.7%), as depicted in Scheme 2, resulting in an overall yield of 15.1%. The structural characterization data for all intermediates and the probe can be found in the supporting information (Figures S1-S13). Mass spectrometric analysis of the fluorescent substance generated by the reaction of probe PTC with fluoride ions (Figure S14) showed consistency with the predicted reaction product, validating the fluorescence detection mechanism of this probe.
Photophysical Properties
Absorption and Fluorescence Spectrum of PTC
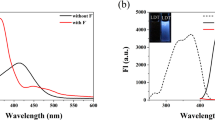
The detection capability of the probe PTC for fluoride ions was investigated using absorption spectroscopy and fluorescence spectroscopy. As shown in Fig. 1a, the first absorption band of PTC (30 μM DMSO) appears at 425 nm. Upon response to fluoride ions in DMSO solution, the absorption band shifts to around 520 nm, with an isosbestic point emerging at 475 nm. Figure 1b illustrates that under an excitation wavelength of 520 nm, the probe PTC exhibits weak fluorescence at 580 nm. Upon addition of F−, the fluorescence intensity at 580 nm significantly increases under the same excitation wavelength, with the solution color turning pink. Furthermore, spectra of the probe PTC response to F− were recorded separately at an excitation wavelength of 580 nm and 520 nm, forming a mirror relationship with the emission spectrum. The result shows that, in DMSO solution, the response of the probe PTC to fluoride ions is evident, accompanied by a change in solution color from light to pink, had the ability to detect F−.
The excitation and emission peaks intersect, termed the fluorescence resonance energy transfer (FRET) mechanism. Four common strategies construct fluorescence probes using FRET [39]. This study transforms non-fluorescent receptors into fluorescent structures. In the PTC molecule, the phenothiazine ring serves as the fluorescent donor, with the receptor as a substituent on this ring. Fluorescence at 580nm is minimal without F−. Upon reacting with F−, a new ring forms, initiating FRET and yielding strong emission fluorescence at 580 nm.
Fluorescence Spectra of in Different Solvents
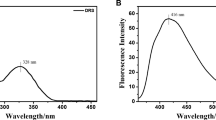
Subsequently, the fluorescence response of probe PTC to F− in various solvents was investigated (Fig. 2). The results reveal that its fluorescence response is most pronounced in THF solution. In acetone (DMK), acetonitrile (CAN), and DMSO solvents, probe PTC also demonstrates a noticeable fluorescence response to F−, with significant changes in fluorescence intensity observed pre- and post-fluoride ion addition. Considering the favorable biocompatibility of DMSO, it was selected as the foundational solvent for subsequent performance tests.
Response Time of PTC
Building on the favorable fluorescence characteristics of probe PTC in DMSO solution, we conducted a detailed examination of the fluorescence intensity variations of probe PTC in response to F− at various time intervals in DMSO solution to elucidate its response kinetics to F-. As illustrated in Fig. 3, the fluorescence intensity of the solution peaked at 3200 a.u. within 5 min post addition of fluoride ions and remained relatively constant within 45 min. This observation suggests that PTC exhibits a rapid and substantial response to fluoride ions, manifested by notable changes in fluorescence intensity.
Job’s Curve and Detection Limit of PTC
To investigate the quantitative relationship between probe PTC and F− concentrations, fluorescence titration spectroscopy was employed. Figure 4a illustrates that at an excitation wavelength of 520 nm, the solution exhibited its maximum emission fluorescence at 580 nm. As the equivalent ratio of F− to probe PTC increased, the fluorescence intensity of the solution gradually intensified. Within the range of 0 to 12.5 μM, the fluorescence intensity exhibited a linear correlation with F− concentration (y = 124.49143C + 1084.7619, R2 = 0.98773) (Fig. 4b). The detection limit (LOD) was calculated using the formula LOD = 3 σ/κ and was found to be 0.51 μM.
(a) Fluorescence titration of PTC (5 μM) upon of F− (0–47.5 μM); Insert: the fluorescence changes with F− concentration (0–47.5 μM) under 365 nm light; (b) Linear correlation between the intensity of fluorescence and F− concentration (0–12.5 μM), λex = 520 nm, λem = 580 nm; Inset: the correlation between the fluorescence intensity and F− concentration (0–47.5 μM), λex = 520 nm
Selectivity of PTC
To ensure the precise recognition of the target by probe PTC, a comprehensive array of common cations (Na+, Ca2+, K+, Fe2+, Fe3+, Cu2+, Ag+, Zn2+) and anions (Cl−, Br−, I−, SO4 2−, HSO3−, H2PO42−, HCO3−, SCN−, NO3−, HPO4−) were utilized as interferents to assess the selectivity of probe PTC towards F−. The outcomes, as illustrated in Fig. 5, reveal minimal interference from common cations and anions, thereby affirming the probe's efficacy in detecting F− within complex biological environments.
Cell Imaging of PTC
Initially, the cytotoxicity of probe PTC towards HepG2 cells was assessed using the MTT colorimetric method. HepG2 cells were treated with probe PTC at concentrations ranging from 0 to 20 μM. The results (Fig. 6) indicate minimal toxicity of PTC, with the cell viability remaining at 90% even at a compound concentration of 20 μM. Subsequently, selecting a concentration of 5 μM for probe PTC in cellular imaging experiments is feasible.
PBS was selected as the solvent for incubating the probe with cells. Initially, HepG2 cells were treated with PTC for 30 min, followed by the addition of F− for another 30 min, and the fluorescence changes were observed. The probe itself exhibited weak fluorescence in the green channel, but upon the addition of F−, fluorescence intensity notably increased in this channel (Fig. 7), indicating the applicability of probe PTC for fluorescent imaging of F− within living cells.
Conclusion
In summary, utilizing thiophene boronic acid ester as a starting material, the probe PTC was designed and synthesized, which exhibits specific recognition towards F−. This probe demonstrates good photophysical properties in DMSO solution, showing a good linear fluorescence response in the F− concentration range of 0 to 12.5 μM (R2 = 0.98773). It possesses a low detection limit (0.51 μM), rapid response rate (reaching peak within 5 min), and significant color change of the solution before and after response (colorless to pink), which is discernible to the naked eye. Furthermore, it exhibits strong interference resistance, low cytotoxicity, and is suitable for live cell imaging, holding promise for further development as a rapid detection reagent for F− in biological systems.
Data Availability
The date that support the findings of this study are available from the corresponding author upon reasonable request.
References
Han J, Kiss L, Mei H, Remete AM, Ponikvar-Svet M, Sedgwick DM, Roman R, Fustero S, Moriwaki H, Soloshonok VA (2021) Chemical aspects of human and environmental overload with fluorine. Chem Rev 121:4678–4742. https://doi.org/10.1021/acs.chemrev.0c01263
Karunanidhi D, Aravinthasamy P, Subramani T, Roy PD, Srinivasamoorthy K (2020) Risk of fluoride-rich groundwater on human health: remediation through managed aquifer recharge in a hard rock terrain, South India. Nat Resour Res 29:2369–2395. https://doi.org/10.1007/s11053-019-09592-4
Singh J, Singh P, Singh A (2016) Fluoride ions vs removal technologies: A study. Arab J Chem 9:815–824. https://doi.org/10.1016/j.arabjc.2014.06.005
Torra M, Rodamilans M, Corbella J (1998) Serum and urine fluoride concentration: Relationships to age, sex and renal function in a non-fluoridated population. Sci Total Environ 220:81–85. https://doi.org/10.1016/S0048-9697(98)00248-4
Moreno EC, Kresak M, Zahradnik RT (2009) Physicochemical aspects of fluoride-apatite systems relevant to the study of dental caries. Caries Res 11:142–171. https://doi.org/10.1159/000260299
Zhu ZL, Yu HY, Zeng Q, He HW (2008) Characterization and biocompatibility of fluoridated biphasic calcium phosphate ceramics. Appl Surf Sci 255:552–554. https://doi.org/10.1016/j.apsusc.2008.06.055
Eggert F, Neubert R (1999) In vitro investigation of the liberation of fluoride ions from toothpaste compounds in a permeation model. Eur J Pharm Biopharm 47:169–173. https://doi.org/10.1016/S0939-6411(98)00060-5
Winter GB (1983) Fluorides in the prevention of caries. Arch Dis Child 58:485–487. https://doi.org/10.1136/adc.58.7.485
Usuda K, Kono K, Dote T, Nishiura K, Miyata K, Nishiura H, Shimahara M, Sugimoto K (1997) Urinary biomarkers monitoring for experimental fluoride nephrotoxicity. Arch Toxicol 72:104–109. https://doi.org/10.1007/s002040050475
Wei Y, Zeng B, Zhang H, Chen C, Wu Y, Wang N, Wu Y, Shen L (2016) iTRAQ-based proteomics analysis of serum proteins in wistar rats treated with sodium fluoride: insight into the potential mechanism and candidate biomarkers of fluorosis. Int J Mol Sci 17:1644
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487. https://doi.org/10.1080/10643380600678112
Xie K, Wang S, Yuan M, Zhang H, Deng H, Zhang Y, Wang J, Zhuang Y (2022) Tailored defect-rich cerium metal organic frameworks for efficient fluoride removal from wastewater. Sep Purif Technol 302:122152. https://doi.org/10.1016/j.seppur.2022.122152
Marquis RE, Clock SA, Mota-Meira M (2003) Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev 26:493–510. https://doi.org/10.1111/j.1574-6976.2003.tb00627.x
Spira L (1962) Fluorine-induced endocrine disturbances in mental illness. Psychiatry Clin Neurosci 16:4–14. https://doi.org/10.1111/j.1440-1819.1962.tb01929.x
Liu Y, Jiang A, Jia Q, Zhai X, Liu L, Ma L, Zhou J (2018) Rationally designed upconversion nanoprobe for simultaneous highly sensitive ratiometric detection of fluoride ions and fluorosis theranostics. Chem Sci 9:5242–5251. https://doi.org/10.1039/C8SC00670A
Cittanova M-L, Lelongt B, Verpont M-C, Geniteau-Legendre M, Wahbe F, Prie D, Coriat P, Ronco PM (1996) Fluoride ion toxicity in human kidney collecting duct cells. Anesthesiology 84:428–435. https://doi.org/10.1097/00000542-199602000-00022
Yan L, Li D, Le Y, Dong P, Liu L (2022) Phenothiazine-based fluorescent probe for fluoride ions and its applications in rapid detection of endemic disease. Dyes Pigm 201:110200. https://doi.org/10.1016/j.dyepig.2022.110200
Samanta T, Das N, Shunmugam R (2021) Intramolecular charge transfer-based rapid colorimetric in-field fluoride ion sensors. ACS Sustain Chem Eng 9:10176–10183. https://doi.org/10.1021/acssuschemeng.1c02344
Mu M, Ke X, Cheng W, Li J, Ji C, Yin M (2022) Perylenemonoimide-based colorimetric probe with high contrast for naked-eye detection of fluoride ions. Anal Chem 94:11470–11475. https://doi.org/10.1021/acs.analchem.2c00766
Li D, Tu S, Le Y, Zhou Y, Yang L, Ding Y, Huang L, Liu L (2023) Development of carbazole-based fluorescent probe for highly sensitive application in fluoride ion detection. Spectrochim Acta Part A Mol Biomol Spectrosc 285:121816. https://doi.org/10.1016/j.saa.2022.121816
Zhang Y, Qu Y, Zhang Y, Gao Y, Wang L (2022) Development of a fluorescent strategy for quantification of fluoride ions in foods and toothpaste. Chem Eng J 448:137631. https://doi.org/10.1016/j.cej.2022.137631
Ahmadijokani F, Molavi H, Rezakazemi M, Aminabhavi TM, Arjmand M (2021) Simultaneous detection and removal of fluoride from water using smart metal-organic framework-based adsorbents. Coord Chem Rev 445:214037. https://doi.org/10.1016/j.ccr.2021.214037
Mandal TK, Hou Y, Gao Z, Ning H, Yang W, Gao M (2016) Graphene oxide-based sensor for ultrasensitive visual detection of fluoride. Advanced Science 3:1600217. https://doi.org/10.1002/advs.201600217
Wang Q, Li D, Rao N, Zhang Y, Le Y, Liu L, Huang L, Yan L (2021) Development of indole-based fluorescent probe for detection of fluoride and cell imaging of HepG2. Dyes Pigm 188:109166. https://doi.org/10.1016/j.dyepig.2021.109166
Kovalchuk Y, Podurets A, Osmolovskaya O, Nugbienyo L, Bulatov A (2024) Layered double hydroxide nanoparticles for a smartphone digital image colorimetry-based determination of fluoride ions in water, milk and dental products. Food Chem 438:137999. https://doi.org/10.1016/j.foodchem.2023.137999
Zhu C-Q, Chen J-L, Zheng H, Wu Y-Q, Xu J-G (2005) A colorimetric method for fluoride determination in aqueous samples based on the hydroxyl deprotection reaction of a cyanine dye. Anal Chim Acta 539:311–316. https://doi.org/10.1016/j.aca.2005.03.002
Zhou H, Chua MH, Tan HR, Lin TT, Tang BZ, Xu J (2019) Ionofluorochromic nanoparticles derived from octapyrene-modified polyhedral oligomeric silsesquioxane organic frameworks for fluoride-ion detection. ACS Applied Nano Materials 2:470–478. https://doi.org/10.1021/acsanm.8b01958
Hu KK, Huang WX, Su YH, Hu RZ (2009) Simultaneous determination of fluorine and iodine in urine by ion chromatography with electrochemical pretreatment. Chin Chem Lett 20:1483–1486. https://doi.org/10.1016/j.cclet.2009.05.030
Zhang X, Li S, Ma H, Wang H, Zhang R, Zhang X-D (2022) Activatable NIR-II organic fluorescent probes for bioimaging. Theranostics 12:3345–3371. https://doi.org/10.7150/thno.71359
Tian M, Wu R, Xiang C, Niu G, Guan W (2024) Recent advances in fluorescent probes for cancer biomarker detection. Molecules 29:1168
Tu S, Le Y, Yang L, Yi Q, Feng T, Yang J, Yang T, Wu T, Zhu W, Liu L (2024) Unraveling hydrogen sulfide detection and lysosome-mitochondria fusion in mitophagy using dual phenothiazine-based fluorescence probes. Sens Actuators, B Chem 406:135408. https://doi.org/10.1016/j.snb.2024.135408
Mengji R, Acharya C, Vangala V, Jana A (2019) A lysosome-specific near-infrared fluorescent probe for in vitro cancer cell detection and non-invasive in vivo imaging. Chem Commun 55:14182–14185. https://doi.org/10.1039/C9CC07322A
Luo Z, Huang Z, Li K, Sun Y, Lin J, Ye D, Chen H-Y (2018) Targeted delivery of a γ-glutamyl transpeptidase activatable near-infrared-fluorescent probe for selective cancer imaging. Anal Chem 90:2875–2883. https://doi.org/10.1021/acs.analchem.7b05022
Lin S, Ye C, Lin Z, Huang L, Li D (2024) Recent progress of near-infrared fluorescent probes in the determination of reactive oxygen species for disease diagnosis. Talanta 268:125264. https://doi.org/10.1016/j.talanta.2023.125264
Jiang G, Liu H, Liu H, Ke G, Ren T-B, Xiong B, Zhang X-B, Yuan L (2024) Chemical approaches to optimize the properties of organic fluorophores for imaging and sensing. Angew Chem Int Ed 63:e202315217. https://doi.org/10.1002/anie.202315217
He L, Xiong H, Wang B, Zhang Y, Wang J, Zhang H, Li H, Yang Z, Song X (2020) Rational design of a two-photon ratiometric fluorescent probe for hypochlorous acid with a large stokes shift. Anal Chem 92:11029–11034. https://doi.org/10.1021/acs.analchem.0c00030
Tian Y, Liu S, Cao W, Wu P, Chen Z, Xiong H (2022) H2O2-activated nir-II fluorescent probe with a large stokes shift for high-contrast imaging in drug-induced liver injury mice. Anal Chem 94:11321–11328. https://doi.org/10.1021/acs.analchem.2c02052
Tang B, Yu F, Li P, Tong L, Duan X, Xie T, Wang X (2009) A Near-infrared Neutral pH fluorescent probe for monitoring minor pH Changes: Imaging in living HepG2 and HL-7702 Cells. J Am Chem Soc 131:3016–3023. https://doi.org/10.1021/ja809149g
Wu L, Huang C, Emery BP, Sedgwick AC, Bull SD, He X-P, Tian H, Yoon J, Sessler JL, James TD (2020) Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem Soc Rev 49:5110–5139. https://doi.org/10.1039/c9cs00318e
Acknowledgements
The authors are grateful for the financial support from the Guizhou University SRT Program (2023SRT587).
Funding
Guizhou University SRT Program (2023SRT587).
Author information
Authors and Affiliations
Contributions
Ying Zhang: synthesis, test of probe properties and writing–original draft. Tingting Feng: NMR spectroscopy analysis and cell bioimaging. Taozhu Hu and Yi Wang: material preparation and instrument maintenance. Yi Le: software, validation, resources, writing–review & editing, supervision. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not available.
Competing Interests
The authors have no competing interests with the work presented in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Feng, T., Hu, T. et al. A New Phenothiazine-Based Fluorescent Probe for Rapid and Specific Detection of Fluoride. J Fluoresc (2024). https://doi.org/10.1007/s10895-024-03856-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-024-03856-w