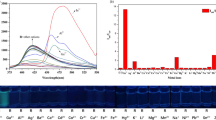
Abstract
Here, we developed a novel isoniazid based fluorescent probe (E)-N’-(thiophen-2-ylmethylene)isonicotinohydrazide (TINH) through simple condensation reaction and employed for selective detection of Pd2+ ions with a low detection limit of 4.102 × 10–11 M. Among the many existing cations, TINH bound Pd2+ ions with an association affinity of 9.794 × 105 M−1. Adding Pd2+ ions to ligand solution increased the absorption intensity in UV–Visible and quenched the emission intensity in fluorescence spectroscopic experiments. More importantly, this TINH complexed to Pd2+ ions in 1:1 stoichiometric ratio. To evaluate the stability of complexed system, pH experiments has been performed. The binding insights among the ligand and Pd2+ ions has been confirmed by IR spectroscopic and MASS spectrometric methods. Additionally, TINH also applied to real water samples for the identification and measurement of Pd2+ ions. Hence, this system could be highly applicable for detection of Pd2+ ions in environmental and industrial samples with in low detection range.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The non-covalent interactions are the key concept for the host–guest chemistry [1]. In the recent years, researchers have mainly focused on chemosensing of such analytes (cations, anions and neutral species) that may affect the biological, environmental and ecological systems [2,3,4,5]. The detection and quantification of such analytes using simple molecules have a great area of interest for researchers. However, the metal ions are crucial and overabundance or inadequacy of metal ions gives rise to serious threats to human beings [6]. Palladium (Pd) is a shiny, silvery white and rare metal in the Pt group which is highly resistant to corrosion so that it is used for making several items including jewelry, electronic items [7, 8]. Also used for dental fillings, alloys, catalytic converters for automobiles, energy producing fuel cells [9]. Pd acts as a catalyst and converts major environmental pollutants such as carbon monoxide and nitrogen oxides respectively into carbon dioxide and nitrogen gas [10]. It is also tremendously assisted to form difficult bonds in catalytic processes in coupling reactions like Suzuki Miyaura, Buchwald–Hartwig, Heck and Sonogashira reactions [11, 12]. Though even after utmost purification processes, Pd2+ ions often released as by-product along with desired product formation, these residual Pd2+ contents can cause serious threats to human life as it cause DNA degradation and adversely alters the cellular processes due to its coordination ability to sulphur containing proteins, vitamins [13, 14]. As per recommendations of WHO, the dietary intake limit of Pd is near about 15 µg per day and 5-10 ppm in drugs [15, 16]. That is why it is becoming necessary to develop highly selective and sensitive methods for precise detection and quantification of these hazardous Pd2+ ions from the ecological system.
The literature reports showed that traditional techniques such as AAS, AMS, ICP-AES, NAA, SPME-HPLC and HRF etc. has also been used for detection of Pd2+ions [14, 17]. However, most of these techniques are time-consuming and require very costly, sophisticated instrumentation, handling problems, a large amount of samples is required. Hence, there is a great need to develop such chemosensors that can detect these ions selectively and sensitively, requires small amount of sample and less time consuming.
From literature, we come to know that most organic molecules used for Pd2+ selective binding have complex synthetic routes. In many published reports, one of the reactants is Rhodamine, has a large size with a complex structure. Also, we investigated that the researchers have used Isoniazid based chemosensors for detection of Zn2+, Hg2+, Al3+ and Cu2+ ions but not a single report for the selective detection of Pd2+ ions. Here, in this article we have developed a simple and cost effective Isoniazid based Schiff base chemosensor(E)-N’-(thiophen-2-ylmethylene)isonicotinohydrazide (TINH) having sulphur and nitrogen donor atoms for selective binding of Pd2+ ions. The synthesized molecule showed a low detection range with good association affinity for sensing of Pd2+ ions.
Experimental Section
Materials and Instrumentation
The glassware used during experimental work was dried in an oven before being used. Highest purity analytical grade chemicals were used. Ordinary melting point apparatus was used for melting point measurements. For NMR spectra of chemosensor TINH, JEOL JNM ECZ400S (400 MHz) (Japan) was used by dissolving in DMSO-d6 solvent. ABB MB3000 FTIR spectrophotometer (Japan) was used for collecting the IR spectral data. For Mass spectrum, Xevo spectrometer (Waters, USA) was used. The absorption spectra in the range 600 nm-200 nm were collected on LabIndia (3200) spectrophotometer. The fluorescent spectral data were recorded on Shimadzu-5301 pc spectrofluorophotometer in acetonitrile solvent. The pH data was recorded on a simple digital pH meter with the use of a diluted NaOH and HCl buffer solution system.
Analysis of Binding Ratio and Association Constant
Binding ratio of TINH and Pd2+ ion has been evaluated by using Job’s plot and Benesi-Hilderbrand plot methods. Association constant of interacting species was calculated with the help of standard Benesi-Hildebrand plot and equation follows as 1/(A − Ao) = 1/(A∞ − Ao) + 1/K (A∞ − Ao)[C], where Ao is the absorbance of ligand in the absence of linked metal ion, A is the absorbance calculated when ligand is attached to metal ion, A∞ is the maximum absorbance in presence of metal ion and K is the association constant, [C] is the concentration of metal ion. The value of the association constant (K) has been calculated from the slope of the straight line of the plot of 1/(A-Ao) against 1/[C] [18].
Determination of LOD (Limit of Detection) and LOQ (Limit of Quantification)
LOD and LOQ of probe TINH has been determined by well known equations 3σS−1 and 10σS−1 respectively; where σ is the standard deviation value of blank measurement and S represents the slope of linear calibration plot [19].
Results and Discussion
General Procedure for the Synthesis of Chemosensor (E)-N’-(thiophen-2-ylmethylene)isonicotinohydrazide (TINH)
The compound (E)-N’-(thiophen-2-ylmethylene)isonicotinohydrazide (TINH) has been synthesized by mixing the Isonicotinic acid hydrazide (0.3 g, 2.13 mmol) and Thiophene-2-carbaldehyde (0.24 g, 2.13 mmol) in ethanol and dichloromethane (DCM) solvent mixture in the presence of a catalytic amount of concentrated sulfuric acid under reflux conditions (Scheme 1) [20]. The reaction progress has been investigated using the TLC technique. After reaction completion, the solvent was evaporated from the reaction mixture and obtained a crude solid product. At last, this crude solid has been recrystallized in ethanol:acetonitrile (3:1, v/v) to obtained the final product as yellow colored solid powder.
Characterization Data
FTIR data: (Fig. S1) (cm−1) 3210 (-NH), 1658 (> C = O), 1574, 1279, 847, 748; 1H NMR data: (Figs. S2 & S3) (400 MHz, DMSO-d6) δ 12.04 (s, 1H, -NH), 8.80–8.79 (m, 2H, Ar–H), 8.69 (s, 1H, -CH), 7.83–7.81 (m, 2H, Ar–H), 7.73–7.71 (d, 1H, J = 5.2 Hz, Ar–H), 7.54–7.52 (m, 1H, Ar–H), 7.18–7.16 (dd, 1H, J = 5.2 Hz, J = 3.6 Hz, Ar–H); 13C NMR data: (Fig. S4) (101 MHz) δ 161.4, 150.3, 144.0, 140.4, 138.7, 131.5, 129.4, 127.9, 121.4; ESI–MS data: (Fig. S5) m/z, observed 232.0546 [M + 1]+.
Metal Binding Experiments; Absorption and Emission Spectral Studies
Absorption Spectral Study
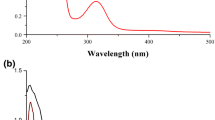
UV–Visible spectral technique has been employed to determine the metal selective characteristics of chemosensor TINH. For this, the absorption spectra of ligand TINH and ligand in presence of different cations have been recorded in acetonitrile:H2O (1:1 v/v) solvent media. The spectral investigations showed that the 20 µM solution of ligand exhibited an absorption maxima wavelength at 365 nm. This was attributed to n-π* electronic transitions in the ligand molecule (Fig. 1). In order to check the metal selective behavior of TINH towards different metal ions, the UV–Visible spectra of TINH with acetate and nitrate salts of metal ions (20 µM) including Pb2+, La3+, Y3+, Fe2+, Fe3+, Al3+, Pr3+, Pd2+, Th4+, Ca2+, Li+, Cr3+, Hg2+, Dy3+, Mn2+, K+, Cd2+, Zn2+, UO22+ and Mo2+ ions have been recorded. The outcomes of experiment revealed that ligand demonstrate a remarkable response only towards Pd2+ ion with a red shift from 365 to 372 nm (Fig. 1). However, the presence of other test ions in solution with ligand TINH didn’t exhibit any colorimetric or spectral changes.
In addition, the interference of other metal ion to complexed species has been conducted with help of UV–Visible experiments. For this, 5-fold of different cations were added to the solution of the TINH-Pd2+ system and spectra were recorded and analyzed (Fig. 2).The recorded spectral data showed that none of the added metal ions interfered with the binding between the TINH-Pd2+ system.
Emission Spectral Study
The emission spectra of TINH and TINH in presence of different cations were recorded in acetonitrile:H2O (1:1 v/v) solution. The 20 µM solution of the ligand TINH exhibited two emission maxima at 395 nm and 412 nm due to the photo induced electron transfer process (PET) in the ligand molecule [21]. The experimental outcomes confirms that the addition of various acetate and nitrate salts of metal ions (20 µM) including Pb2+, La3+, Y3+, Fe2+, Fe3+, Al3+, Pr3+, Th4+, Ca2+, Li+, Cr3+, Hg2+, Dy3+, Mn2+, K+, Cd2+, Zn2+, UO22+ and Mo2+ ions to ligand TINH didn’t affect the emission intensity of ligand band except for Pd2+ ions. However, the addition of Pd2+ ions to TINH, caused a quenching in emission intensity of the band for ligand molecule (Fig. 3).
Effect of pH
The effect of pH changes on the binding of TINH and Pd2+ ions were analyzed by recording the UV–Visible and Fluorescence data of complexed system at wavelength 365 nm and 412 nm respectively. For this, different solutions of TINH-Pd2+ system have been prepared with varying pH range from 2–12, using a pH meter and HCl (1 M) and NaOH (1 M) solutions. The spectral data revealed that the TINH-Pd2+ binding showed a decrease (in UV–Visible spectra) in spectral intensity at a pH range from 2 to 5. This instability in binding, may be due to the less stability of imine bond in pH range (less than 6). Also, a decrease in fluorescence intensity has been observed for complexation from a pH of 2 to 7. The intensity of complexation increases at higher pH may be due to formation of metal ion hydroxides. The experiment demonstrated that the ligand significantly bound to Pd2+ ions at physiological and higher pH range (Figs. S6 & S7) [22].
UV–Visible and Fluorescence Titrations
UV–Visible titrations have been performed to investigate the binding insights among sensor TINH with Pd2+ ions [23]. The gradual increase in the concentration of Pd2+ ions (0 µM to 100 µM) to the ligand TINH solution caused the enhancement in the absorption intensity at 395 nm (Fig. 4).
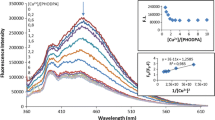
Furthermore, the effect on fluorescence intensity of TINH with the increasing concentration of Pd2+ ions has been investigated through fluorescence titration method. Emission spectral results showed that sequential increase in concentration of Pd2+ ions(0 µM to 100 µM) to TINH solution quenched the emission intensity of TINH at 412 nm and 395 nm (Fig. 5).
Binding Analytical Parameters
Job’s Plot and Benesi-Hildebrand Studies
To check the binding ratio among the complexing system, Job’s plot method has been employed [24]. For this, ten solutions of varying mole fractions have been prepared and UV–Visible spectra at a fixed wavelength have been recorded. A graph of absorbance v/s mole fraction has been plotted by using the absorption data at constant wavelength (Fig. S8). The deviation in absorbance maximum in graph at 0.5 identify a 1:1 binding stiochiometry for the binding of TINH-Pd2+ system.
The data obtained from the fluorescence titrations have been used to draw a plot called Benesi-Hildebrand plot[18]. The Benesi-Hildebrand equation has been applied to derive the association affinity among the ligand and analyte. The linearity in the graph showed 1:1 binding for the complexing system (Fig. 6). However, the association affinity for TINH- Pd2+ complex comes out to be 9.794 × 105 M−1 (Fig. 6).
Determination of LOD (Limit of Detection) and LOQ (Limit of Quantification)
Linear correlation curve against emission intensity v/s increasing concentration of Pd2+ ions was plotted using fluorescence titration data of TINH with Pd2+ ions (Fig. 7) [19]. From the curve, LOD and LOQ values were determined using the equations 3σ/S and 10σ/S respectively. The LOD value was found to be 4.102 × 10–11 M and LOQ was 1.367 × 10–10 M. Further, these values were found relevant and acceptable in comparison with the literature (Table 1).
IR and Mass Spectral Study of Complex
To further check the binding insights of ligand, the IR spectra of ligand TINH and TINH-Pd2+ complex have been recorded in solid-state and compared. The shifting in IR bands (in cm−1) of TINH from 3210, 1658,1574, 1279, 847, 748 to 3043, 1676, 1589, 1282, 831 and 730 were observed (Fig. 8). The shifting in the bands may be due to interactions among the lone pairs of nitrogen atom of imine and sulphur atom of thiophene to Pd2+ ions[37]. Also, the Fig. S5 showed the presence of an intense molecular ion peak at m/z 232.0546 corresponding to [TINH + 1]+ in the mass spectrum of ligand confirmed the formation of a predictable outcome. However, the Fig. S9 demonstrated that the formation of TINH-Pd2+ species displayed a molecular ion peak at m/z 338.1665 corresponding to [TINH + Pd2+ + 1]+, which supported the expected 1:1 stoichiometric ratio of ligand TINH and Pd2+ ions.
Applicability of Chemosensor TINH to Real Water Samples
Fluorescence techniques have been employed for investigating the practical utility of chemosensor TINH through real sample analysis, which was accomplished with ground and tap water samples. For this, different water samples with known concentration of Pd2+ ions have been analyzed with TINH (Table S1). From the results, it has been observed that the chemosensor TINH could be applicable for quantitative analysis of Pd2+ ions in real water samples [38].
Conclusion
In conclusion, the presented work explored the metal sensing properties of a simple isoniazid based Schiff base sensor (E)-N’-(thiophen-2-ylmethylene)isonicotinohydrazide (TINH).The synthesized molecule selectively bound the Pd2+ ions upto a detection limit of 4.102 × 10–11 M and with a LOQ of 1.367 × 10–10 M. The binding modes 1:1 complexation stoichiometry has been confirmed by Job's plot method. The stability and sensitivity of the TINH and TINH-Pd2+ system has been investigated using pH tests. Moreover, binding analytical parameters (LOD, LOQ) were compared with literature and found acceptable. TINH selectively identify the Pd2+ metal ion using the standard addition method has been validated in real water sample, highlighting its potential field advantage. Thus, the sensor TINH could be applied as a potential tool for detecting Pd2+ ions in bio-medical and environmental applications.
Availability of Data and Materials
Data and materials are available on demand.
References
Maharramov AM, Mahmudov KT, Kopylovich MN, Pombeiro AJ (2016) Non-covalent interactions in the synthesis and design of new compounds. John Wiley & Sons
Kumar A, Saini M, Mohan B, Kamboj M (2022) Colorimetric and fluorescent Schiff base sensors for trace detection of pollutants and biologically significant cations: A review (2010–2021). Microchem J 107798
Qiu J, Jiang S, Lin B, Guo H, Yang F (2019) An unusual AIE fluorescent sensor for sequentially detecting Co2+-Hg2+-Cu2+ based on diphenylacrylonitrile Schiff-base derivative. Dyes Pigm 170:107590
Bregović VB, Basarić N (2015) Anion binding with urea and thiourea derivatives. Coord Chem Rev 295:80–124
Frost MC, Meyerhoff ME (2015) Real-time monitoring of critical care analytes in the bloodstream with chemical sensors: progress and challenges. Annu Rev Anal Chem 8:171–192
Volesky B (1990) Removal and recovery of heavy metals. Biosorption Heavy Met 7–43
Melentiev R, Yudhanto A, Tao R, Vuchkov T, Lubineau G (2022) Metallization of polymers and composites: State-of-the-art approaches. Mater Des 110958
Pawlak J, Łodyga-Chruścińska E, Chrustowicz J (2014) Fate of platinum metals in the environment. J Trace Elem Med Biol 28:247–254
Kielhorn J, Melber C, Keller D, Mangelsdorf I (2002) Palladium–a review of exposure and effects to human health. Int J Hyg Environ Health 205:417–432
Reşitoğlu İA, Altinişik K, Keskin A (2015) The pollutant emissions from diesel-engine vehicles and exhaust after-treatment systems. Clean Technol Environ Policy 17:15–27
Beletskaya IP, Alonso F, Tyurin V (2019) The Suzuki-Miyaura reaction after the Nobel prize. Coord Chem Rev 385:137–173
Fleckenstein CA, Plenio H (2007) 9-Fluorenylphosphines for the Pd-catalyzed sonogashira, suzuki, and buchwald–hartwig coupling reactions in organic solvents and water. Chem Eur J 13:2701–2716
Mahata S, Bhattacharya A, Kumar JP, Mandal BB, Manivannan V (2020) Naked-eye detection of Pd2+ ion using a highly selective fluorescent heterocyclic probe by “turn-off” response and in-vitro live cell imaging. J Photochem Photobiol, A 394:112441
Kumar A, Mohan B, Solovev AA, Saini M, Sharma HK (2022) Development of 2-hydroxy-naphthaldehyde functionalized Schiff base chemosensor for spectroscopic and colorimetric detection of Cu2+ and Pd2+ ions. Microchem J 180:107561
Song F, Garner AL, Koide K (2007) A highly sensitive fluorescent sensor for palladium based on the allylic oxidative insertion mechanism. J Am Chem Soc 129:12354–12355
Liang G, Cai Q, Zhu W, Xu Y, Qian X (2015) A highly selective heterogeneous fluorescent sensor for palladium ions. Anal Methods 7:4877–4880
Alqarni SA (2022) A review on conducting polymers for colorimetric and fluorescent detection of noble metal ions (Ag+, Pd2+, Pt2+/4+, and Au3+). Crit Rev Anal Chem 1–12
Wang R, Yu Z (2007) Validity and reliability of Benesi-Hildebrand method. Acta Phys Chim Sin 23:1353–1359
Zorn ME, Gibbons RD, Sonzogni WC (1999) Evaluation of approximate methods for calculating the limit of detection and limit of quantification. Environ Sci Technol 33:2291–2295
Prakash O, Hussain K, Aneja DK, Sharma C, Aneja KR (2011) A facile iodine (III)-mediated synthesis of 3-(3-aryl-1-phenyl-1 H-pyrazol-4-yl)-[1, 2, 4] triazolo [4, 3-a] pyridines via oxidation of 2-((3-aryl-1-phenyl-1 H-pyrazol-4-yl) methylene)-1-(pyridin-2-yl) hydrazines and their antimicrobial evaluations. Org Med Chem Lett 1:1–9
Chen S-Y, Li Z, Li K, Yu X-Q (2021) Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord Chem Rev 429:213691
Yu C, Chen L, Zhang J, Li J, Liu P, Wang W, Yan B (2011) “Off-On” based fluorescent chemosensor for Cu2+ in aqueous media and living cells. Talanta 85(3):1627–1633
Aggarwal R, Kumar S, Kumar A, Mohan B, Sharma D, Kumar V (2022) Development of heterocyclic 2, 7-diamino-3-phenylazo-6-phenylpyrazolo [1, 5-a] pyrimidine as antimicrobial agent and selective probe for UV–visible and colorimetric detection of Hg2+ ions. Microchem J 183:107991
Renny JS, Tomasevich LL, Tallmadge EH, Collum DB (2013) Method of continuous variations: applications of job plots to the study of molecular associations in organometallic chemistry. Angew Chem Int Ed 52:11998–12013
Adak AK, Purkait R, Manna SK, Ghosh BC, Pathak S, Sinha C (2019) Fluorescence sensing and intracellular imaging of Pd2+ ions by a novel coumarinyl-rhodamine Schiff base. New J Chem 43:3899–3906
Mahapatra AK, Manna SK, Maiti K, Mondal S, Maji R, Mandal D, Mandal S, Uddin MR, Goswami S, Quah CK (2015) An azodye–rhodamine-based fluorescent and colorimetric probe specific for the detection of Pd2+ in aqueous ethanolic solution: synthesis, XRD characterization, computational studies and imaging in live cells. Analyst 140:1229–1236
Bhanja AK, Mishra S, Saha KD, Sinha C (2017) A fluorescence ‘turn-on’chemodosimeter for the specific detection of Pd2+ by a rhodamine appended Schiff base and its application in live cell imaging. Dalton Trans 46:9245–9252
Liu F, Du J, Xu M, Sun G (2016) A highly sensitive fluorescent sensor for palladium and direct imaging of its ecotoxicity in living model organisms. Chem Asian J 11:43–48
Huang Q, Zhou Y, Zhang Q, Wang E, Min Y, Qiao H, Zhang J, Ma T (2015) A new “off–on” fluorescent probe for Pd2+ in aqueous solution and live-cell based on spirolactam ring-opening reaction. Sens Actuators, B Chem 208:22–29
Chen Y, Chen B, Han Y (2016) A novel rhodamine-based fluorescent probe for the fluorogenic and chromogenic detection of Pd2+ ions and its application in live-cell imaging. Sens Actuators B Chem 237:1–7
Yang M, Bai Y, Meng W, Cheng Z, Su N, Yang B (2014) A novel selective fluorescent and colorimetric chemosensor for the visual detection of Pd2+ and application of imaging in living cells. Inorg Chem Commun 46:310–314
Li H, Fan J, Song F, Zhu H, Du J, Sun S, Peng X (2010) Fluorescent probes for Pd2+ detection by allylidene–hydrazone ligands with excellent selectivity and large fluorescence enhancement. Chem Eur J 16:12349–12356
Wu H, Lin L, Zheng L, Guo H, Yang F (2022) Dual-response fluorescence sensor for detecting Cu2+ and Pd2+ based on bis-tetraphenylimidazole Schiff-base. J Photochem Photobiol A 432:114076
Kang M, Jiang S, Liu Y, Wei K, Liu P, Yang X, Pei M, Zhang G (2023) A new “off-on-off” Schiff base from quinoline and thiophene as a fluorescent sensor for sequential monitoring Ga3+ and Pd2+. J Photochem Photobiol A 438:114510
Zhou W, Gao Q, Liu D, Li C, Liu S, Xia K, Han B, Zhou C (2020) A single molecular sensor for selective and differential colorimetric/ratiometric detection of Cu2+ and Pd2+ in 100% aqueous solution. Spectrochim Acta Part A Mol Biomol Spectrosc 237:118365
Chen H, Jin X, Lu H, Shen W (2018) A new rhodamine B-based ‘off-on’colorimetric chemosensor for Pd2+ and its imaging in living cells. Inorg Chim Acta 482:122–129
Mohan B, Kumar S, Modi K, Deshmukh AH, Kumar A (2021) 2-((E)-1-((E)-(2-methoxybenzylidene) hydrazono) ethyl) phenol based cost-effective sensor for the selective detection of Eu3+ ions. Polyhedron 209:115460
Chakraborty P, Rana A, Mukherjee S, Biswas S (2022) Metal–organic-framework-based chemosensor for ultrafast and ultrasensitive detection of Pd2+ ions in water, real specimens, and test strips. Inorg Chem 62(2):802–809
Acknowledgements
The authors are thankful to the Department of chemistry, Baba Mastnath University, Asthal Bohar, Rohtak and the Department of chemistry, Kurukshetra University, Kurukshetra (India), for providing spectral facilities, including NMR spectroscopy.
Funding
There is no funding for this work.
Author information
Authors and Affiliations
Contributions
Jasbir Singh: Conceptualization, Investigation, Experimental, Resources, Formal analysis; Shubham Saini: Experimental, Formal analysis & Editing; Ravish K. Chauhan: Resources, Formal analysis; Writing- Original draft preparation; Pallavi Bhardwaj: Conceptualization, Investigation, Resources, Formal analysis; Ashwani Kumar: Writing- Original draft preparation, Formal analysis; Virender: Experimental, Writing- Original draft preparation, Formal analysis.
Corresponding authors
Ethics declarations
Ethical Approval
No such data applies to human and/ or animal studies. This work is a novel and has not been published in any journal or thesis work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, J., Saini, S., Chauhan, R.K. et al. An Isoniazid Based Schiff Base Sensor for Selective Detection of Pd2+ Ions. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03491-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03491-x