Abstract
A simple and low-cost green preparation method was used for BSA capped silver nanoclusters (BSA-Ag NCs) as turn on fluorescent probe for glucose. Non-enzymatic fast glucose detection assay with a widest concentration range was proposed which requires neither nanoclusters (NCs) modification nor complicated enzyme immobilization. The DLS analysis, HRTEM patterns, fluorescence and UV-visible measurement well supported the synthesis product. The advantages of the fabricated glucose sensor based on fluorescence increasing of probe compared to other established optical techniques was inspected and summarized as well. The glucose sensor exhibited a high sensitivity, fast response time (in seconds), satisfactory selectivity, well stability (at least two months), low detection limit (31 µmol L− 1) and a wide concentration response (three orders of magnitudes) to glucose between 0.1 and 92 mmol L− 1 as calibration plot. A theoretical model of the sensing mechanism based on the binding interaction of glucose to BSA-Ag NCs is proposed and data fitting demonstrated a good agreement between the experimental and theoretically calculated fluorescence data. The facile preparation and excellent sensing performance of BSA-Ag NCs in the real samples (plasma and juice) make sure that synthesized probe material is a promising candidate for advanced enzyme-free glucose sensing approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The urgent want for detection of glucose isn’t restrained to the field of medical diagnostics but also sensing glucose is critical in the process control of food, and pharmaceutical industries [1,2,3,4,5]. Glucose (C6H12O6, a six-membered ring main structure carries four hydroxyl groups and one hydroxymethyl) is an essential factor in the health condition of men’s lifetime and is a crucial active substance in biological processes, supplies the energy for living cells and join in synaptic neurotransmission [2,3,4]. Great efforts have been made for susceptible monitoring of glucose in subjects with diabetes mellitus management because diabetic diseases problem rapidly growing worldwide. The development of determination methods for simple and fast sensing of glucose in interstitial fluids, food, and so on has gained attention for a long time [1, 2, 4, 6,7,8,9,10]. In this context, various enzyme-based and non-enzymatic sensors have been extensively developed [6,7,8, 11,12,13]. The most sensors have been reported relying on an indirect glucose sensing involving enzyme glucose oxidase (GOx) based or intermediates detection (as peroxidase-like activity) [12, 14, 15]. However, despite the high selectivity and sensitivity of the enzyme-based sensors, they suffer from short-term stability due to the intrinsic nature of the enzymes. Moreover, there are some other drawbacks in their usage, for instance, high fabrication cost, temperature, pH level and humidity dependence, discontinuity, replenishment and immobilization problems [9, 10]. Non-enzymatic biosensors are better alternatives because of their superior properties, such as cost-effectiveness, necessitating minimum sample pre-treatment, stability, and reliability without mentioned shortcomings [6, 9, 10, 16]. Therefore, the non-enzymatic sensors for glucose hold a special leading position among glucose improved sensors. The evolution of nanotechnology has overcome limitations and tedious processes in the sensors area [17, 18]. As nanoparticle-based sensors are same in size to biological systems [1], desirable physiochemical properties are predictable. Analytical performance of such technologies also leads to the development of sensors with merits like non-invasiveness rapid measurement and improved precision, as well as sensitivity characteristics [4, 18, 19]. The main nanomaterial-based sensor topics for glucose detection in past few decades have been reviewed frequently [1, 6,7,8, 12, 13, 16]. Ultrafine size noble metal nanoclusters are one emerging fluorescent-emitting chemicals with fascinating features as strong photoluminescence quantum yield, size-dependent tunable emission, good photostability (especially compared to traditional organic dyes), excellent biocompatible properties, and lower toxicity often faced by quantum dots [18,19,20,21]. Metal clusters are missing link among nanoparticles (displaying plasmons) and metal atoms (by incredible optical properties), showing molecule-like behavior [18]. Clusters trigger a great variety of possible applications were extensively implemented in analysis subject for particular target readouts via different mechanisms [17,18,19,20]. Metal nanoparticles have been shown to have a high affinity to interact with glucose. The bonding between metal clusters and D-glucose is reported to occur by two major bonding factors through the anchoring of metal atoms (as example in Au, Cu, and Ag metal clusters) to the electron-rich oxygen atoms, and hydrogen bond between metal clusters as a proton acceptor with hydroxyl groups [10]. Notably, compared with other techniques, the fluorescent nanomaterial-based sensing probe is a proper method to fulfill the requirements with features such as high sensitivity, simplicity, easy synthesis and implementation, low detection limit, low cost, and less time-consuming [2, 18].
Over the past decade, an emphasis on “green” chemistry with the aim of minimization of generated waste has been the subject of several investigations. The three main steps in the green approach toward the synthesis of nanostructures include the choice of solvent medium, selection of an environmentally benign reducing agent, and a nontoxic material for the stabilization [22]. The absence of any toxic organic solvents and ingredients, rigorous reaction conditions (e.g., neither chemical reducing agents nor high temperature) and complicated procedure has attracted widespread attention in green chemistry. The essential harsh conditions (e.g., pH ≥ 12) or very strong reducers (e.g., BH4−) used in the synthesis protocols would retard biomolecular activities in some wise as well [19, 23]. Bovine serum albumin biomolecule-stabilized nanoclusters (BSA-NCs) have been widely drawn the interest of researchers [24] owing to their relatively cost-effective materials needed and the ease of green one-pot protein-directed synthesis [17, 20]. BSA proteins have functional and chelating groups (as carboxyl, amine, thiol and hydroxyl groups), that can specifically have a combination interaction with metal in clusters (include of Ag, Au, Cu, and Pt) [15]. Highly luminescent clusters concurrently synthesized and stabilized from aggregation and precipitation by bulkiness BSA molecules represent a special category of bionanomaterials with outstanding physical and chemical properties and desirable applications [18, 25]. Although NCs have been applied in fluorescent assays for the glucose sensing [1, 3, 4, 9, 25,26,27,28], no further study of direct interaction with Ag NCs probe for glucose detection in enzyme-free system has been introduced yet. Reports of BSA-protected Ag NCs are rare rather than gold nanocluster (Au NCs). Au atoms are relatively less reactive than Ag atoms, therefore the Ag metals have more tendency to oxidize [1, 17, 23], thus, stabilizers must be introduced truly using a robust synthesis method to prepare stable Ag nanostructures. However, Ag is cost-effective and described as more sensitive than Au [20, 27], which is a promising emerging trend. Synthesis of well-defined Ag NCs with a controlled size and specific number of silver atoms is a vital issue in obtaining suitable properties. Notably, the ratio of functional groups of stabilizer to Ag ions consists of electronegative elements (–SH, -COOH, –OH, -NH2 and, or = NH), which can strongly interact with Ag atoms, play a dominant role in their size, and their fluorescence characterization [18, 20, 23]. Fluorescence emission under UV light, is related to the nanoclusters size, solution pH, the interaction of surface ligands with the metal core and the metal atom quantum effect [27, 29].
Herein, a simple green preparation method for highly stable, water-soluble and fluorescence emitting BSA capped Ag NCs synthesis was utilized. BSA-Ag NCs sensing performance was exhibited in neutral pH as a rapid sensitive enzyme-free glucose sensor at room temperature. The used approach reduces the number of sensing steps and avoids the using of harsh chemical reagents. To our best knowledge, this study is the first report for glucose determination without any intermediate by a label-free sensor response in the widest concentration ranges (see Table S1 for the comparison study and detailed information, including method advantages, enhancement/quenching mechanism, synthesis conditions and simplicities, response time, stability of NCs, limit of detection and linear range). The observations may guide promising strategies for simple glucose recognition with emissive stable BSA-Ag NCs.
Experimental
Instrumentation and Materials
All fluorescence measurements were performed on a Perkin-Elmer Model LS 55 B spectrofluorimeter (PerkinElmer, USA), equipped with a xenon lamp, using emission and excitation band passes of 10 and 10 nm and a scan rate of 300 nm min− 1. UV–vis absorption spectra were recorded using a Model Scinco UV S-2100. The pH values of the solutions were adjusted employing a Metrohm pH meter Model 632. HRTEM analysis was carried out using a 200 kV FEI Tecnai G2 F20 Super Twin transmission electron microscope. Brookhaven instruments at 25°C and ZetaPlus Particle Sizing Software Version 5.23, were used for dynamic light scattering (DLS) submicrometer particle size analysis.
All chemicals such as D-glucose, D-maltose, D-galactose, D-fructose, silver nitrate (AgNO3), sodium hydroxide (NaOH), hydrochloric acid (HCl), BSA (≥ 98.0%) and so on, were of analytical grade and were procured from Merck and Sigma-Aldrich companies. The appreciated solutions were produced by double distilled water (DDW). Different varieties of juices (orange and lemon verbena (Aloysia citrodora)) and human plasma samples were obtained from local store and a clinical laboratory respectively.
Green Synthesis of BSA-Ag NCs
Herein, the BSA-Ag NCs were synthesized using a good biocompatibility and environmental/cost advantages methodology comparable with the Au NCs synthesis [30], except some simplifications. The mild reduction and stabilization ability of BSA molecules was employed in the green preparation of subnanometer-sized Ag clusters in the BSA matrix [21]. Typically, 250 µL of 0.04 mmol L− 1 AgNO3 solution was added to the reaction vessel containing 4 mg BSA in 250 µL DDW under vigorous stirring at about physiological temperature 37℃ in a water bath. The mixture was left for 5 min stirring before the addition of 25 µL NaOH solution (1.0 mol L− 1). Just after NaOH was added to the former solution (the reduction ability of BSA molecules was activated by adjusting the reaction pH to 12 approximately [30]), the solution color turned to reddish-brown. Note that Ag ions are added into the stabilizer solution to form an intermediate of stabilizer-Ag+ n complex or stabilizer protected Ag+ n clusters instantaneously, transferred to the clusters by reduction (mixing the solutions, entrapped Ag+ ions underwent progressive reduction to form NCs in situ) [20, 23]. The solution followed stirring for about 12 h in a water bath [30] until the color of the suspension changed to yellowish-brown, indicating the formation of Ag nanoclusters. Notably with excess NaOH present, elevated temperatures and vigorous mixing in the protein-directed synthesis of BSA–Ag NCs, the initial formation of Ag(OH)3 is highly favorable due to the transformation of silver nitrate to silver hydroxide [25] in the presence of extra OH− ions. All BSA–Ag NCs solutions were stored in darkness to prevent light-induced flocculation and was kept at 4°C. The highly stable luminescence of NCs then remained almost unchanged for at least 60 days, without discernable changes in terms of peak position and spectral shape. The relative standard deviation for more than three measurements for the emission intensity demonstrates the good stability and repeatability of the synthesis method (RSD% = 2.4). Prepared BSA-Ag NCs also can be preserved in the refrigerator for more than 6 months with above 85% fluorescence intensity retention. There is a small amount of larger size particles produced owing to the aggregation of ultra-small NCs by nanoparticle properties (such as the plasmon effect) that can be removed by precipitation. A small amount (5 µL) of the colorless supernatant of synthesized BSA–Ag NCs was diluted with DDW to obtain the appropriate probe concentration.
Fluorescent Behavior of NCs in Glucose Detection
The BSA–Ag NCs show molecule-like characteristics due to its comparable small size to the Fermi wavelength of electrons (about 0.5 nm for Ag) [18, 23]. Therefore, the Ag NCs have discrete energy levels of electrons, resulting discrete electronic transitions and intense emission. The fluorescence of prepared Ag NCs was well suited for involving glucose sensing. D-glucose solutions with different concentrations were obtained via serial dilution of two stock solutions (100.0 and 1000.0 mmol L− 1). An appropriate volume of stock solution, were added to BSA–Ag NCs mixture (5 µL in 2 mL) at room temperature. The complex between glucose and the BSA–Ag NCs was allowed to form at ambient conditions for seconds. The fluorescent intensity of the reaction solutions was measured immediately using the spectrofluorimeter (λex = 240 nm, λem = 415 nm). To investigate the selectivity, the influence of similar sweets such as sucrose, lactose, galactose and fructose (at a 10-fold higher concentration than glucose) on the BSA–Ag NCs response was investigated. All experiments were performed in triplicate.
Results and Discussion
Characterization of the BSA–Ag NCs
As shown in Fig. 1, the UV-Vis absorption spectrum of BSA–Ag NCs exhibited an absorption band located at 280 nm. Such pure absorption spectrum without surface plasmon resonance peak in around 400–500 nm region due to the large Ag nanoparticles, indicating the successful preparation of the Ag NCs [27, 31, 32]. No significant absorbance signals were observed for as prepared BSA and glucose solutions (Fig. 1).
UV absorption spectra of BSA–Ag NCs (5 µL in 2 mL DDW), BSA (20 mg L− 1) and glucose (300 mmol L− 1) (a); excitation (1, gray) and emission (2, black) fluorescence spectra for the BSA-Ag NCs (20 µL in 2 mL DDW), and the fluorescent spectra of (brown) BSA 10 mg L− 1 (b); the excitation wavelength was λex = 240 nm at room temperature using spectrofluorimeter by the emission and excitation band passes of 10 nm and a scan rate of 300 nm min− 1
As well known, the fluorescent properties are highly dependent on the size of the metal nanocluster, ambient factors and ligands or stabilizing scaffolds on cluster surface. Here, the fluorescent properties including the excitation and emission spectra of the BSA-Ag NCs and BSA protein were investigated. Shown in Fig. 1 is the maximum fluorescence excitation and emission wavelengths of the prepared Ag NCs as 240 and 415 nm, respectively. It is observed that no change in emission maximum of NCs was occurred by altering the excitation wavelength indicated a real luminescence from the relaxed states instead of scattering effects. Glucose solution has no absorbance and no fluorescence in the visible range [1]. Furthermore, we explored the stability of the prepared NCs by monitoring the change of fluorescence intensity of the Ag NCs solution over time. Results shown that the BSA–Ag NCs solution could maintain ≥ 98% of its fluorescence intensity even after 2 months (after 6 months more than 85% emission retention).
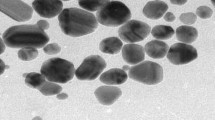
HR-TEM analysis (Fig. 2) was conducted to better identify the shape, size and morphology of the nanocclusters. Figure 2a and b illustrate that the average diameter of Ag NCs is below 5 nm. Also the HR-TEM image clearly revealed the presence of a lattice pattern with calculated inter fringe distance of 2.14 Å by DigitalMicrograph software (GMS) (Fig. 2b), and well-known (111) plane of Ag NCs corresponding to f.c.c crystal structure (according to selected area electron diffraction )SAED( pattern shown in Fig. 2c) [33, 34]. For particle size determination, DLS analysis of diluted NCs solution was investigated. In Fig. 2d number (%) size distribution DLS studies confirmed TEM analysis results, except that the mean sizes increased somewhat due to the DLS hydrodynamic diameter and solvent-swollen aggregates while TEM shows the size of dry particles.
Optical Response of BSA–Ag NCs to Glucose
To study the interaction between sensor and analyte, the influence of varying concentrations of glucose on the fluorescent emission intensity of the BSA–Ag NCs was studied. It was noteworthy that upon the addition of glucose solution, the fluorescent intensity of the BSA–Ag NCs increased in correspondence with increasing glucose concentration, but without any change in shape or position of cluster emission spectra (Fig. 3a). Obtained results showed that reasonably good linearity existed among the fluorescence intensity at 415 nm and the solutions concentration under the experimental conditions, suggested that the system should probably be used as a fluorescent probe for quickly glucose monitoring. All the experiments were carried out at room temperature, because of the system’s stability and simplicity of operation. For the glucose sensing platform, a solution of 200-fold diluted synthesis silver nanoclusters from the originally prepared mixture was used as the optical probe. The fluorescence emission of prepared NCs was used to fit the calibration curve by addition of a different amounts of freshly produced glucose to probe in aqueous solution and exhibited promising results for the sustainable improvement of non-enzymatic sensing applications. Fluorescence intensity increased significantly as a function of the added glucose concentration, and ΔF = F – F0 where F0 and F are fluorescence intensities of the probe before and after adding the analyte, respectively. The plot of ΔF verses glucose concentration was related to the two linear ranges from 0.1 to 8 and 8 to 92 mmol L− 1 with correlation coefficients > 0.99 (Fig. 3b). The detection limit obtained as low as 31 µmol L− 1 (by three times the standard deviation of the blank signal divided by slope of the regression equation of calibration curve). As a result of the above two linear ranges, we were able to quantitatively detect glucose at low and high concentration levels.
pH, time and Temperature Influencing on the D-glucose Detection
The rapidly response time of the glucose sensor based on fluorescence is much shorter than the electrochemical or spectrophotometric sensors reported findings (Table S1 shows the comparative nanocluster based spectroscopic reported sensors for glucose) [1, 9]. Results shown the fluorescence increasing reached completion within seconds. Rest time of 1 min was selected as equilibrium time for sure complication of interaction between BSA–Ag NCs and glucose for all further experiments. Considering the stability of the system and the simplicity of operation, the process was carried out at room temperature after removing from the refrigerator for convenience as no obvious changes was found in relative temperature fluctuation. The robustness to environmental changes is a key factor to explore in the biochemical applications of such fluorophore due to the strong interactions of thiol bonds of BSA molecules with nanoclusters surface [35].
NCs’ size and optical properties were strongly influenced by the reaction solution pH [18, 20]. But then, since the intrinsic NCs are enfolded within the BAS protein, so the fluorescence is fairly photostable and restricted to a wide range of environmental perturbations including pH changes [2]. It is also shown that the functional groups like carboxyl found on the Ag NCs surfaces are similar to ‘elastic cushions’, which can reduce pH changes’ impact on the nanoclusters and cause slower agglomeration among them [36]. With regard to presence of the carboxylic protecting groups, the prepared Ag nanoclusters have excellent stability in aqueous solutions. The NCs’ fluorescence is therefore not speculated to change significantly with pH changes in the solution, which makes them suitable for use as fluorescent probes [24]. In this work the effect of pH (ranges 6.0 − 9.0) on the BSA-Ag NCs’ fluorescence intensity was ignorable (data not shown). A drop in fluorescence intensity as pH increases (> 9), suggesting that the protein unfolds at higher pH levels [35]. While a too alkali medium led to hydrolysis and potentially altered the protein structure, in acidic media (pH below 5.0), Ag NCs were expected to aggregate and conformational change as well [15, 37, 38]. For convenience, the glucose detection was carried out under neutral pH conditions. It has already been reported, coordination by electron-rich groups is favored at neutral pH, too [18]. Sensing of glucose at a neutral pH is recommended and essential for improving sensitivity [16]. Specifically Tang et al. pointed out that, when performed at a pH outside the neutral range, results are less accurate, especially at diabetic glucose levels [39].
Mechanism of the Assay
There are a number of primary mechanisms used in fluorescent based nanosensing of glucose, which lead to changes in fluorescence. For example, direct glucose binding, competitive binding, fluorescent dye release, or other assays involving glucose catalysis oxidation products like gluconic acid and hydrogen peroxide are used [1, 11, 40]. Also, glucose can be indirectly detected by using several glucose binding moieties [9]. As usual, the principles of a non-enzymatic fluorimetric approach involves glucose sensing through aggregation/agglomeration processes [1, 7, 19, 21, 32] (According to the IUPAC definition, aggregation is the joining of colloidal particles by strong chemisorption, while agglomeration holds dispersed particles together by weak reversible interactions [36]), or by a method of measuring the transfer of fluorescence resonance energy (FRET) between fluorescent donors and fluorescent acceptors and so on [1, 2, 10, 40]. It is interesting to note, some of the former methods claimed that fluorescence sensors had a signal-off or quenching detection mode through the accumulation of non-fluorescent large aggregations [19, 21, 32]. Meanwhile, others conversely established increasing fluorescence upon on aggregation-induced enhancement [7]. Aslan et al. reported the prominent mechanism as cross-linked Ag NP–saccharide interactions, result in aggregation of the Ag nanoparticles. They revealed that since the distance between aggregating spheres is smaller than their radius, there is an increase in absorbance at arbitrary wavelengths that are longer than the surface plasmon peak [41]. However, Ren and coworkers surmised a non-fluorescence form due to the growth of Ag NCs’ aggregation by an interaction between Ag NCs and a-L-fucosidase [21]. Investigating all the methods, the exact mechanisms and influence of parameters such as scaffolds and environmental factors on fluorescence emissions of nanomaterials are not yet clear [9, 11, 31]. A study by Wu et al. showed that electron-rich ligands could stabilize NCs, and fluorescence was significantly enhanced by stabilization through complexation formation [42]. While, it is reported that the coordination structure of thiol groups can not only stabilize, but also destabilize Ag NCs leading to thiol-induced fluorescence quenching due to the thiol-adsorption-accelerated oxidation of silver nanoclusters [43, 44]. Also, it is suggested that anchoring with a higher refractive index organic ligand reduces the luminescence peak of noble metal nanomaterials [45]. No studies have been performed into glucose sensing by directly fluorescence enhancement of Ag NCs without any intermediate to our best knowledge. The proposed sensor simplifying the operation process, while overcome the challenge of obtaining false positive results in fluorescence quenching nanocluster-based assays [11]. Glucose chemically attaches to proteins spontaneously without the aid of enzymes through glycation, while the main covalent attachment forces are Van der Waals or hydrogen bonds [46]. Non-enzymatic glycation is a biomarker for diabetes and is implicated in some diseases and aging [47]. Based on the fact that NCs’ properties are highly dependent on their scaffold templates, so it was possible to manipulate their response to a specific analyte by simply selecting appropriate capped proteins [30, 48,49,50]. Glucose is the most prevalent glycating sugar at the highest concentration in the body. Additionally, serum albumin is one of the most crucial glucose transmitters and the primary target interacting with glucose in the blood [12, 51]. It is also reported that small amounts of glucose locked serum albumin structure, and albumin fluorescence intensity results indicated that it was “shocked” under normal glucose conditions. The mentioned structural shock was obtained by low levels of glucose, even though albumin and glucose were not incubated [12, 40, 52]. As a result, anchoring BSA-Ag NCs with glucose in terms of this structural rigidity reduce the possibility of a normal collisional quenching phenomenon and increase the quantum yield [53]. As glucose interacts with albumin in BSA-Ag NCs, glycation suffers breakage of BSA molecules and stays partially apart from Ag NCs. Glycation decreases the refractive index of the surrounding medium and the dielectric constant of the Ag NC, since clusters are sensitive to change in local refractive index [45]. Hence, enhancement in emission occurs by lowering refractive indices around NCs, rather than increasing/decreasing output signal through aggregation suggestions. This fact is in good agreement with literature that shows weakens the interaction between the NCs and scaffolds by the addition of a ligand [18, 32, 53]. Also glucose and albumin both have functional groups that lengthened the distance between Ag NCs metal centers and caused the scaffold groups to lose contact with the metal centers [36]. On the other hand, glucose is known to have an affinity for getting adsorbed over Ag NCs [12]. Electron-rich oxygen atoms in glucose’s hydroxyl groups donate electron density to metal clusters’ unoccupied orbitals (Ag atoms as acceptors with low-lying vacant d orbitals) [10]. Glucose could penetrate the BSA protective layer and combine with the Ag NCs core due to the affinity of the oxygen group toward Ag [32]. An increase in the NC’s emission intensity by each of the binding types (Ag-O forming anchor bond or Ag…H-O hydrogen bond) that has a main contribution to the complexation of glucose and gold clusters which made complexed states is predicted [10]. In addition, glucose as an organic ligand and mild reducing agent that inhibiting the oxidation of Ag on the surface can provide Ag NCs stability [9, 32, 54]. Ag NCs were not only firmly stabilized, but also fluorescence increasing by the interaction of glucose with the prepared Ag NCs was expected. All in all, the mentioned reasons lead to an increase in the Ag NCs fluorescence intensity in proportion to the glucose concentration. The enhancing fluorescence intensity of BSA-Ag NCs as probe was modeled by interactions between glucose and probe with the following equations:
where, Probe, n, Glu, and Probe-Glun are a symbol of the BSA-Ag NCs, number of glucoses interacting, glucose and complex of probe and glucose, respectively. The Kbin is the corresponding equilibrium binding constant for the complexation reaction, Eq. (1). According to the reaction path given in Eq. (1), the binding constant could be defined as:
at the experimental conditions following equation can be supposed:
where [Probe]t, [Probe]f and [Probe-Glun] are total, free and complex of probe concentrations, respectively. Following model can be supposed for relating fluorescence signal to concentration of system ingredients in such solutions (Probe and Probe-Glun):
where F is fluorescence intensity of solution, C1 and C2 are fluorescence sensitivities of corresponding compounds. Substitution of Eqs. (2) (3) and (4) into (5), leads to:
where F0 is fluorescence intensity of probe in the absence of glucose and (C2 – C1). [Probe]t is constant in an experimental condition. Equation (6) can be used as a general response function for other fluorescence probe based on binding interaction of analyte to a probe. The experimental evidence work is carried on by recording the fluorescence spectra of probe in presence of different concentrations of glucose. Data fitting based on the non-linear least-squares Fit-o-mat software [55] demonstrated a good agreement between the experimental (points) and theoretically calculated (solid line) fluorescence data in the glucose and probe interaction (Fig. 4). The values of n and Kbin were found to 1.8 (≈ 2) and 6030 (L.mol− 1)2, respectively. Furthermore, UV–vis absorption spectra of probe in the absence and presence of different concentrations of glucose were investigated in the same experimental conditions and are shown in Fig. S1. The probe exhibit two absorption peaks located at 220 and 230 nm, the absorbance in 230 nm decrease without any remarkable shift with the increasing of glucose concentration (insert of Fig. S1). This end result shows that the glucose interacts in ground state of probe and agreement with the fluorescence results.
Interferences Studies
To address the selectivity of the assay, common interfering biological species like sucrose, lactose, galactose and fructose were tested (Fig. 5). The probe exhibited a good selectivity to glucose detection, as supported by the negligible changes of the fluorescence responses when interferences present. However, the total concentration of glucose in human blood is 100-fold higher than all other carbohydrates, thus, their effects are very small and could be ignored [9, 11, 56]. Unexpectedly, the method was strongly selective for glucose in the presence of typical sugars that participate in glycation as fructose, or their derivatives relative to glucose in the wavelength range of interest even if in 10-fold higher concentration (Fig. 5). This selectivity over glucose maybe justify due to their conformational structure or can be attributed to the higher tendency of glucose to albumin. BSA has dual hydrophobic and hydrophilic properties that selectively partitions glucose from interfering analytes. Notably, tryptophan like structures such as aspartame (a synthetic organic amino acid derivative used as an artificial sweetener) have lower attraction to albumin [46, 51]. As an experimental evidence, tendency of glucose and fructose to BSA study by fluoresce method. The fluorescence intensity (λem = 345 nm) of the BSA is quenched with increasing of glucose and fructose concentrations (Figure not shown). The Stern–Volmer plot is used to represent the fluorescence quenching emission data of the BSA which is illustrated in Fig. S2. The plot of F0/F versus [sugar] examined is nonlinear and shows a negative deflection at the higher sugar concentration (0.68–6.3 mM) with an intercept equal to unity (Fig. S2a). Quenching data due to this cause can be displayed by a modified Stern–Volmer equation or Lehrer Eqs. [57, 58] given by Eq. (7):
where f is the fraction of available fluorophores and is ≤ 1. By plot of the left hand of Eq. (7) versus 1/[Q] is linear and shown in Fig. S2b with intercept = 1/f and intercept/slope = KSV. The f values for glucose and fructose are the same value (0.24), but KSV for glucose (3845.5 L.mol− 1) is 3.6 times more than fructose (1059.5 L.mol− 1). Therefore, selectivity behavior of probe to glucose relative to other sugars can be describe by more tendency sequence with the BSA interaction.
Sensor Performance Evaluation
To test the practicability of sensor, glucose levels in real samples (plasma and juices) were determined by the proposed assay. The fluorescence property of BSA-Ag NCs at λem = 415 nm was chosen to monitor the glucose concentration. Human blood sample was collected from a laboratory. The plasma was extracted after collection. The preparation of plasma sample solution involves precipitation with acetone in order to total protein extraction. It is notable that every other material such as TCA or acetonitrile caused NC’s BSA albumin precipitation as well, and destruction of the BSA-Ag NCs was occurred to some extent. In this case the fluorescence intensity increased dramatically overlapping the BSA proteins emission. An advantage of the sensor is that small sample of plasma is used to check the blood glucose levels in the simple same procedure (taking into account the system’s low detection limit and human plasma glucose levels, a certain volume of plasma samples could be diluted more than 35 fold). For investigations the 2 fold diluted samples were centrifuged for 10 min at 4000 rpm before quantification (Table 1). The detected glucose content in plasma samples was derived from the standard curve and the regression equation. Concentration of glucose in plasma was cross checked using local clinical laboratory analyses. Moreover, for recovery studies, the glucose in the real samples was detected by spike and recovery experiment for known concentrations of glucose solutions. As shown in Fig. 3, plotting the value of (F − F0) versus the concentration of glucose gave two calibration curves, therefore we had chosen the spiked concentrations in the both parts. Table 1, summarized our sensor recoveries ranged from 94.9 to 103.4% for the samples, and the relative standard deviations (RSD) were no more than 5%, which proved the feasibility of the proposed sensor for glucose detection within normal glucose range of 3.9 to 6.9 mmol L− 1 (70 to 125 mg dL− 1) [upon World Health Organization] and so on for diabetes diagnosis. In addition, measurement of glucose was investigated in diluted lemon verbena and orange juices samples using standard addition method and the results verified in good agreement with those commercial glucose contents on their packages (Table 1).
Conclusion
Great interest has been sparked in the further development of enzyme-free precise determination of glucose level. A well applicable non-enzymatic fast response glucose sensor with a wide linear determination range and high stability and durability is proposed using familiar BSA biomolecule-stabilized Ag NCs. Based on measuring the fluorescence enhancement of BSA-Ag NCs without significant interference effects, the proposed nanostructures are strong candidates for fluorescent glucose sensing. Utilizing green facile method, NCs can be easily prepared and scaled up. Over two months, BSA-Ag NCs exhibited stable performance, in terms of fluorescence peak position and intensity, making them promising for long-term glucose monitoring. Ultra-small emissive Ag NCs showed increasing relation between intensity and concentration of glucose at ambient conditions in the widest concentration range of 0.1 to 92 (0.1 to 8 and 8 to 92) mmol L− 1 of glucose concentration, which covers the normal and diabetics glucose concentration in blood, and overcame all the challenges compared to the previous sensors such as requiring complicated conditions and reactions. This study reported the impact of glycation on albumin structure (BSA-Ag NCs) for sensitive glucose determination. The mechanism is easier to use, provides accurate data, and is sensitive even at very low concentrations of glucose. Comparing results of binding constants of Glu-BSA and Glu-BSA-Ag NCs shown that presence of Ag NCs on albumin structure not only caused the enhancing fluorescence signal but also increasing binding affinity of glucose (3845 to 6030). The sensor accuracy in determining glucose in juices and human plasma as real samples were demonstrated, which makes the robust species ideal for practical use.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen L, Hwang E, Zhang J (2018) Fluorescent nanobiosensors for sensing glucose. Sensors 18(5):1440
Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ (2005) Fluorescence-based glucose sensors. Biosens Bioelectron 20(12):2555–2565
Jin L, Shang L, Guo S, Fang Y, Wen D, Wang L, Yin J, Dong S (2011) Biomolecule-stabilized au nanoclusters as a fluorescence probe for sensitive detection of glucose. Biosens Bioelectron 26(5):1965–1969
Sabu C, Henna T, Raphey V, Nivitha K, Pramod K (2019) Advanced biosensors for glucose and insulin. Biosens Bioelectron 141:111201
Malhotra S, Tang Y, Varshney PK (2020) Fabrication of highly sensitive non-enzymatic sensor based on Pt/PVF modified Pt electrode for detection of glucose. Iran Chem Soc 17(3):521–531
Tian K, Prestgard M, Tiwari A (2014) A review of recent advances in nonenzymatic glucose sensors. Mater Sci Eng C 41:100–118
Aslan K, Zhang J, Lakowicz JR, Geddes CD (2004) Saccharide sensing using gold and silver nanoparticles-a review. J Fluoresc 14(4):391–400
Scognamiglio V (2013) Nanotechnology in glucose monitoring: advances and challenges in the last 10 years. Biosens Bioelectron 47:12–25
Hussain A, Sarangi S, Kesarwani J, Sahu S (2011) Au-nanocluster emission based glucose sensing. Biosens Bioelectron 29(1):60–65
Jamshidi Z, Farhangian H, Tehrani ZA (2013) Glucose interaction with au, Ag, and Cu clusters: theoretical investigation. Int J Quantum Chem 113(8):1062–1070
Molaabasi F, Hosseinkhani S, Moosavi-Movahedi AA, Shamsipur M (2015) Hydrogen peroxide sensitive hemoglobin-capped gold nanoclusters as a fluorescence enhancing sensor for the label-free detection of glucose. RSC Adv 5(42):33123–33135
Singh K, Agarwal T, Kumar U, Pal S, Runthala A, Pan T-M, Wu CC (2022) Optical biosensors for diabetes management: advancing into stimuli-responsive sensing mechanisms. Smart Mater Med
Peng Z, Xie X, Tan Q, Kang H, Cui J, Zhang X, Li W, Feng G (2022) Blood glucose sensors and recent advances: a review. J Innov Opt Health Sci 15(02):2230003
Romeo A, Moya T, Leung G, Gabriel R Villa and, Sánchez S (2018) Appl Mater Today 10:133
Wang L-L, Qiao J, Qi L, Xu X-Z, Li D (2015) Construction of OVA-stabilized fluorescent gold nanoclusters for sensing glucose. Sci China Chem 58(9):1508–1514
Abunahla H, Mohammad B, Alazzam A, Jaoud M, Al-Qutayri M, Hadi S, Al-Sarawi S (2019) MoMsense: metal-oxide-metal elementary glucose sensor. Sci Rep 9:5524
Yu Y, Geng J, Ong EYX, Chellappan V, Tan YN (2016) Bovine serum albulmin protein-templated silver nanocluster (BSA‐Ag13): an effective Singlet Oxygen Generator for Photodynamic Cancer Therapy. Adv Health Mater 5(19):2528–2535
Li J, Zhu J-J, Xu K (2014) Fluorescent metal nanoclusters: from synthesis to applications. TrAC Trends Analyt Chem 58:90–98
Xia X, Long Y, Wang J (2013) Glucose oxidase-functionalized fluorescent gold nanoclusters as probes for glucose. Anal Chim acta 772:81–86
Mathew A, Sajanlal P, Pradeep T (2011) A fifteen atom silver cluster confined in bovine serum albumin. J Mater Chem 21(30):11205–11212
Ren X, Chen Z, Meng X, Chen D, Tang F (2012) Synthesis of fluorescent ag nanoclusters and their application in α-L-fucosidase detection. Chem Comm 48(76):9504–9506
Raveendran P, Fu J, Wallen SL (2003) Completely green synthesis and stabilization of metal nanoparticles. J Am Chem Soc 125(46):13940–13941
Hua D, Hongtao Y (2015) A mini review on controlling the size of Ag nanoclusters by changing the stabilizer to Ag ratio and by changing DNA sequence. Adv Nat Sci 8(2):1–9
Russell B, Jachimska B, Kralka I, Mulheran P, Chen Y (2016) Human serum albumin encapsulated gold nanoclusters: effects of cluster synthesis on natural protein characteristics. J Mater Chem B 4(42):6876–6882
Chevrier DM, Thanthirige VD, Luo Z, Driscoll S, Cho P, MacDonald MA, Yao Q, Guda R, Xie J, Johnson E (2018) Structure and formation of highly luminescent protein-stabilized gold clusters. Chem Sci 9(10):2782–2790
Meng F, Yin H, Li Y, Zheng S, Gan F, Ye G (2018) One-step synthesis of enzyme-stabilized gold nanoclusters for fluorescent ratiometric detection of hydrogen peroxide, glucose and uric acid. Microchem J 141:431–437
Wen T, Qu F, Li NB, Luo HQ (2012) Polyethyleneimine-capped silver nanoclusters as a fluorescence probe for sensitive detection of hydrogen peroxide and glucose. Anal Chim Acta 749:56–62
Zhou Z, Shu T, Sun Y, Si H, Peng P, Su L, Zhang X (2021) Luminescent wearable biosensors based on gold nanocluster networks for turn-on detection of uric acid, glucose and alcohol in sweat. Biosens Bioelectron 192:113530
Zheng J, Zhang C, Dickson RM (2004) Highly fluorescent, water-soluble, size-tunable gold quantum dots. Physic Rev Lett 93(7):077402
Xie J, Zheng Y, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131(3):888–889
Li D, Kumari B, Makabenta JM, Gupta A, Rotello V (2019) Effective detection of bacteria using metal nanoclusters. Nanoscale 11(46):22172–22181
Chen Z, Lu D, Cai Z, Dong C, Shuang S (2014) Bovine serum albumin-confined silver nanoclusters as fluorometric probe for detection of biothiols. Luminescence 29(7):722–727
Mondal MK, Banerjee PP, Saha SK, Chowdhury P, Sengupta A, Bandyopadhyay A, Bhattacharya S, Chattopadhyay A (2016) Selective reduction technique (SRT): a robust method to synthesize bioactive Ag/Au doped graphene oxide. Mater Des 102:186–195
Adhikari B, Banerjee A (2010) Facile synthesis of water-soluble fluorescent silver nanoclusters and HgII sensing. Chem Mater 22(15):4364–4371
Chib R, Butler S, Raut S, Shah S, Borejdo J, Gryczynski Z, Gryczynski I (2015) Effect of quencher, denaturants, temperature and pH on the fluorescent properties of BSA protected gold nanoclusters. J lumin 168:62–68
Dong JX, Gao ZF, Zhang Y, Li BL, Zhang W, Lei JL, Li NB, Luo HQ (2016) The pH-switchable agglomeration and dispersion behavior of fluorescent ag nanoclusters and its applications in urea and glucose biosensing. NPG Asia Mater 8(12):e335–e335
Soudahome AG, Catan A, Giraud P, Kouao SA, Guerin-Dubourg A, Debussche X, Le Moullec N, Bourdon E, Bravo SB, Paradela-Dobarro B (2018) Glycation of human serum albumin impairs binding to the glucagon-like peptide-1 analogue liraglutide. J Biol Chem 293(13):4778–4791
Cao X-L, Li H-W, Yue Y, Wu Y (2013) pH-Induced conformational changes of BSA in fluorescent AuNCs@ BSA and its effects on NCs emission. Vib Spectrosc 65:186–192
Tang Z, Du X, Louie RF, Kost GJ (2000) Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med 124(4):577–582
Amini-Geram P, Goshadrou F, Ebrahim-Habibi A, Yaghmaei P, Hesami Tackallou S (2017) Human serum albumin structure in presence of different concentrations of cortisol and glucose: an in vitro modeling under normal and hyperglycemic conditions. Biology Med Iran Red Crescent Med J 19:e40306. https://doi.org/10.5812/IRCMJ
Aslan K, Pérez-Luna VH (2002) Surface modification of colloidal gold by chemisorption of alkanethiols in the presence of a nonionic surfactant. Langmuir 18(16):6059–6065
Wu Z, Jin R (2010) On the ligand’s role in the fluorescence of gold nanoclusters. Nano lett 10(7):2568–2573
Shang L, Dong S (2009) Sensitive detection of cysteine based on fluorescent silver clusters. Biosens Bioelectron 24(6):1569–1573
Choi S, Dickson RM, Yu J (2012) Developing luminescent silver nanodots for biological applications. Chem Soc Rev 41(5):1867–1891
Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP (2008) Biosensing with plasmonic nanosensors. Nat Mater 7(6):442–453
Shahabadi N, Khodaei MM, Kashanian S, Kheirdoosh F, Filli SM (2014) Study on the interaction of a copper (II) complex containing the artificial sweetener aspartame with human serum albumin. Mol Biol Rep 41(5):3271–3278
Glenn JV, Stitt AW (2009) The role of advanced glycation end products in retinal ageing and disease. Biochim Biophys Acta - Gen Subj 1790(10):1109–1116
Le Guével X, Hötzer B, Jung G, Hollemeyer K, Trouillet V, Schneider M (2011) Formation of fluorescent metal (au, ag) nanoclusters capped in bovine serum albumin followed by fluorescence and spectroscopy. J PHYS CHEM C 115(22):10955–10963
Xavier PL, Chaudhari K, Verma PK, Pal SK, Pradeep T (2010) Luminescent quantum clusters of gold in transferrin family protein, lactoferrin exhibiting FRET. Nanoscale 2(12):2769–2776
Lin Y-H, Tseng W-L (2010) Ultrasensitive sensing of Hg2+ and CH3Hg + based on the fluorescence quenching of lysozyme type VI-stabilized gold nanoclusters. Anal Chem 82(22):9194–9200
Du S, Xie Y, Chen X (2013) Influence of glucose on the human serum albumin–flavone interaction and their antioxidant activity. Mol BioSyst 9(1):55–60
Mohamadi-Nejad A, Moosavi-Movahedi A, Hakimelahi G, Sheibani N (2002) Thermodynamic analysis of human serum albumin interactions with glucose: insights into the diabetic range of glucose concentration. Int J Biochem Cell B 34(9):1115–1124
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer
Moosavi R, Afkhami A, Madrakian T (2015) A simple cyanide sensing probe based on Ag/Fe3O4 nanoparticles. RSC Adv 5(21):15886–15891
Möglich A (2018) An open-source, cross-platform resource for nonlinear leastsquares curve fitting. J Chem Educ 95:2273–2278
Shiang Y-C, Huang C-C, Chang H-T (2009) Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose. Chem Comm 23:3437–3439
Lehrer S (1971) Solute perturbation of protein fluorescence. Quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry 10:3254–3263
Ghorbanian N, Kajinehbaf T, Alizadeh N (2023) Picomolar detection of as(III) ions by using hydrothermal synthesis of functionalized polymer dots as a highly selective fluorescence sensor. Talanta 261:124667
Acknowledgements
The authors acknowledge to Tarbiat Modares University Research Council for the financial support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
“Razieh Moosavi: Conceptualization; Methodology; Formal analysis; Investigation; Writing - original draft- review& editing and Naader Alizadeh: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing - review & editing.“
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing Interests
The authors have no relevant fnancial or nonfnancial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary information (ESI) available: Figures UV-vis spectra of probe, modified Stern–Volmer plot, Table of comparison reports.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moosavi, R., Alizadeh, N. Silver Nanoclusters as Label Free Non-enzymatic Fast Glucose Assay with the Fluorescent Enhancement Signal. J Fluoresc 34, 1865–1876 (2024). https://doi.org/10.1007/s10895-023-03407-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-023-03407-9