Abstract
Current study was aimed to determine the antibacterial, antioxidant and cytotoxic potential of Titanium dioxide nanoparticles (TiO2NPs) and Zinc oxide nanoparticles (ZnONPs). Nanoparticles were characterized by UV–Vis spectrophotometry, particle size analyzer (PSA), fourier transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). The Minimum inhibitory concentration (MIC) was determined by standard agar dilution method. Antibacterial potential of nanoparticles was analyzed by standard disc diffusion method against bacterial strains including Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumonia. Different concentrations of NPs (0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 mg/mL) were incorporated to evaluate the antimicrobial activity. Antioxidant activity and cytotoxicity of these NPs was analyzed by DPPH method and brine shrimp cytotoxicity assay, respectively. The MIC of TiO2NPs against E. coli, P. aeruginosa and K. pneumoniae was 0.04, 0.08 and 0.07 mg/mL respectively while the MIC of ZnONPs against the above strains was 0.01, 0.015 and 0.01 mg/mL. The maximum zone of inhibition was observed for K. pneumoniae i.e., 20mm and 25mm against TiO2 and ZnO NPs respectively, at 1.4 mg/mL concentration of NPs. The susceptibility of NPs against bacterial strains was evaluated in the following order: K. pneumoniae > P. aeruginosa > E. coli. The antioxidant activity of nanoparticles increased by increasing the concentration of NPs while cytotoxic analysis exhibited non-toxic effect of ZnO NPs while TiO2 had toxic effects on 1.2 and 1.4 mg/mL concentrations. Results revealed that ZnO NPs have more antibacterial and negligible cytotoxic potential in contrast to TiO2 NPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abrupt surge in prevalence of various dreadful diseases through pathogenic infections has compelled the global scientific community in continuous struggle to find some efficacious antibacterial alternatives [1, 2]. Genetic mutations and resistance in microbes have made the frontline synthetic drugs inefficacious along with multiple non-targeted damages to the healthy cells which have driven the researchers to develop some novel and cost-effective bactericidal agents with negligible damage and maximum efficacy [3,4,5]. Focus must shift to alternate approaches for treating infections as more antibiotics have become ineffective due to drug-resistant bacteria. Although there are a number of natural alternatives, it might be difficult to put them into practice in therapeutic settings. Synthetic chemistry, genetic engineering, and biotechnology advancements have created new opportunities for the quest for antibiotic-replacement medicines [6]. Previous research has revealed that widespread antibiotic use has led to the emergence of bacterial strains that are multidrug resistant. Additionally, recent antibiotic abuse has led to the emergence of superbugs that are virtually immune to all antibiotics [7]. In addition to these demerits, conventional therapeutic options are also the leading cause of environmental contaminations across the globe with huge amount of economic burden specifically for developing or under-developed counties [8]. Among the numerous novel approaches to tackle such biomedical dilemmas, nanotechnology has emerged and considered as a sole solution to multiple healths related problems owing to their peculiar physiochemical properties and tunable nature. In this regard, metals and metal oxide NPs have emerged as potential antibacterial agents to which microbes might not develop resistance [9].
Nanotechnology has fetched the ample attention globally due to multifacet roles in live sciences and bioengineering for the development of commercial products in different areas like food, agriculture, drug delivery, clinics and biomedicine [10, 11]. Metal oxide NPs have been the subject of intense research over the past ten years due to their wide range of applications in numerous technical fields [12]. In this regard, particular metal oxides nanoparticles (MeONPs) have become the point of focus for their distinguished physiochemical, mechanical, catalysis and optical properties [13]. MeONPs are also being preferred over their bulk counterparts due to their optimal shape, size, surface to volume ratio, selectivity and electromagnetic properties [14]. These MeONPs can be fabricated using different approaches like physical, chemical and biological/green methods. Physical and chemical method involves conventional or catalysts, reagents or reducing entities with synthetic origin while the biological method uses the biomaterials of natural origin like proteins, lipids, carbohydrates or even bacteria and fungi [15,16,17,18]. NPs could be a very helpful therapy for a various ailments like cardiovascular, cancer, microbial infections, and other inflammatory conditions [19].
The traditional methods used for synthesis of nanoparticles have certain disadvantages like high costs, less production rate, and high energy requirements [20]. On the other hand, chemical approaches include the use of toxicants which result in generation of perilous side-products and cause pollution from noxious substances [21]. As a consequence, there is an urgency to develop NPs with biocompatible routes, involving the plants/microbes or waste biomass as a reductant, that are nontoxic and ecologically friendly [22]. One of the key benefits of green synthesis includes the fact that they may be created in short time while micro-organism-assisted techniques are slower and require longer time due to microbial culture and slow growth rate [23].
Among the various kinds of MeONPs including zinc, silver, gold, silica, copper and zinc, have gained much attention due to flexible, modifiable, and long-range intrinsic properties like biocompatibility, biodegradability, photostability and varied electrochemical coupling for the synthesis of NPs using various reducing agents [24, 25]. ZnONPs have also been used in making ointments, lotions, and targeted drug delivery. Moreover, zinc oxides NPs have shown significant activity against various fungi and pathogenic bacteria including Bacillus subtilis and Klebsiella pneumoniae [10, 26]. Additionally, the ZnO NPs are employed as a catalyst or enhancer in combination with antibiotics and other pharmaceutical products, especially antibiotics, to combat bacteria that have evolved resistance to numerous kinds of medications [27].
Similarly, titanium oxide nanoparticles (TiO2NPs), a novel candidate with multiple biomedical applications could also be synthesized by physiochemical methods including doping, hydrolysis and precipitation [28, 29]. Being highly photocatalytic and photoreactive, TiO2NPs are considered as best industrial dyes degraders and waste removers [30]. Furthermore, in biomedical context, TiO2NPs nanoparticles have also been regarded as the promising antibacterial agents like against Streptococcus mutants [8, 31,32,33]. These nanoparticles are now also being incorporated into sunscreens to avoid the skin damage through UV radiations in developed countries. Moreover, cytotoxicity of the TiO2NPs have also been documented through doping of metals like copper, gold and silver with promising anti-cancerous activity using human cell lines [34,35,36].
These metals oxide nanoparticles are also being preferred over their conventional counterparts due to their novel mechanistic pathways of action including DNA damage, protein synthesis blockage, oxidative stress, mitochondrial damage for various bacterial pathogens and even modifications of the apoptotic pathways for imparting their cytotoxic effects. Taking the sensitivity of the matter into consideration, current study was structured to evaluate the antibacterial, antioxidative and cytotoxic effects of the ZnONPs and TiO2NPs.
Materials and Methods
Titanium Dioxide and Zinc Oxide Nanoparticles Synthesis
Titanium dioxide and Zinc oxide nanoparticles were a kind gift from U.S Research Nanomaterials Inc. United States of America and were brought to department of Zoology, Lahore College for Women University Lahore (LCWU) and were dissolved in autoclaved distilled water for further use in present study. Both of these NPs were of green origin. All concentrations were vortexed vigorously before conducting the experiment.
Characterization of Titanium Dioxide and Zinc Oxide Nanoparticles
To confirm the synthesis of the titanium dioxide and zinc oxide nanoparticles, various characterization techniques including UV–Vis spectrophotometry, particle size analysis, scanning electron microscopy, fourier transform infrared spectroscopy and thermogravimetric analysis were used.
UV–Vis Spectrophotometric Study
Characteristic absorption peaks of TiO2NPs and ZnONPs were analyzed from 200 to 800 nm range of wavelength [37] using UV-SHIMADZU at central research laboratory Lahore College for Women University Lahore while the data was interpreted and graphs were generated at Applied Entomology and Medical Toxicology Laboratory, Department of Zoology, Government College University Lahore.
Evaluation of Particle Size
Size of TiO2NPs and ZnONPs was calculated by U.S Research Nanomaterials Inc. United States of America.
Morphological Study
Shape, size and distribution of TiO2NPs and ZnONPs were photographed by scanning electron microscopy (NANOSEM 450) at central research laboratory Lahore College for Women University Lahore.
Analysis of Bonding Pattern and Functional Groups
Fourier transform infrared spectroscopy (FTIR) (ATR-3000) of TiO2NPs and ZnONPs was performed to evaluate the bonding pattern and functional groups arrangement at central research lab. LCWU, Lahore while the data was interpreted and graphs were generated at Applied Entomology and Medical Toxicology Laboratory, Department of Zoology, GCU Lahore.
Thermal Behavior Study
Thermogravimetric analysis was performed to access the thermal behavior (stability) and percentage weight loss with respect to varied temperature exposure of TiO2NPs and ZnONPs.
Chemicals, Growth Media and Bacterial Strains
Different clinical isolates (20) of medical importance were obtained from different hospitals of Lahore, Pakistan. Out of these, 3 β-lactamases producing strains were selected as panel of test strains i.e., E. coli, P. aeruginosa and K. pneumonia. The strains were grown on nutrient agar and Luria-Bertina (LB) broth (Sigma-Aldrich, USA). Muller-Hinton Agar (Oxoid, USA) was used to check the inhibitory effect of NPs against the bacterial strains by disc-diffusion assay. For this, different concentrations of TiO2 and ZnONPs i.e., 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 mg/mL were used. The dilutions of the NPs were loaded on paper disc of 5 mm size.
Determination of Minimum Inhibitory Concentration (MIC)
The MIC was determined by using the standard agar dilution technique. The optical density of bacterial cells was retained at 0.8–1.0. The bacterial suspension containing 105 colony-forming units CFU/mL−1 was transferred to each Muller-Hinton agar plate having different concentration of nanoparticles and the bacterial growth was assessed after 24 h of incubation at 37 ºC.
Antimicrobial Susceptibility Test
To investigate the susceptibility of bacterial strains to TiO2 and ZnO NPs, the standard disc diffusion method was employed [38]. Prepared Muller-Hinton agar media was transferred into labeled sterilized petri dishes. Single colony of test organisms was grown overnight in LB broth on a rotatory shaker (200 rpm). This overnight culture swabbed on the solidified Muller-Hinton agar using a micro-pipette.
The sterile filter paper discs (5mm) were used to carry disc diffusion assay. These discs were soaked in 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 mg/mL of TiO2 nanoparticles solution. Same experiment was done by using ZnONPs solution. Once, the discs had diffused, all the plates were kept in incubator at 37 ºC for a period of 18–24 h. Experiments were performed in triplicates. The zone of inhibition (ZOI) was measured after 24 h incubation. The antibacterial activity of both nanoparticles i.e., TiO2 and ZnONPs were recorded and compared.
Determination of Antioxidant Activity and Cytotoxicity of Nanoparticles
Atioxidant activity of nanoparticles was assessed by using L-ascorbic acid as a standard antioxidant agent. DPPH assay was used to measure the scavenging potential according to method described by Ajmal et al. [39] with few modifications. Scavenging activity was evaluated for both NPs by mixing 0.1 mM DPPH in methanol with different concentrations (0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 mg/mL) of ZnO, TiO2 and (Vitamin C) ascorbic acid and incubated for 90 min in dark. The absorbance for each run was measured at 517nm. Tests were conceded out in triplicates [40].
Calculations
The cytotoxicity of nanoparticles was also evaluated using brine shrimp lethality assay by following the method of Simorangkir et al. [41]. Artemia salina eggs and sea salt were among the supplies and ingredients required for the activity. One liter of distilled water was mixed with 38 g of sea salt, and the pH was then raised to 7.40. Filtered brine solution was used to hatch brine shrimp eggs, and 50mg of the eggs were scattered over it in a rectangle hatching tray. The eggs were then incubated at 37 °C for 24 h to produce larvae. As a stock solution, 10 mg of the test extract was dissolved in 1mL of the appropriate solvents. From this stock solution, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 mg/mL concentrations were made. Tests were conceded out in triplicates [42].
The following formula was used to calculate the mortality rate (M):
where:
-
A = Total number of dead larvae after 24 h exposure
-
B = Av. number of dead larvae in blind samples after 24 h
-
N = No. of dead larvae before starting of the experiment
-
G = Total number of the larvae
Results
Characterization
UV–Vis Spectra Analysis
UV spectra analysis (UV-1700 SHIMADZU) produced diagnostic peaks at 350-365 nm and 370-400 nm for TiO2NPs and ZnONPs respectively (Fig. 2).
Particle Size Analysis
Average particle size of TiO2NPs and ZnONPs was found to be 18nm.
Surface Morphology of NPs
External structure by SEM images revealed that TiO2NPs and ZnONPs were of rectangular, triangular, round and spherical in shape. SEM also showed the uniform distribution of NPs with minor aggregation (Figs. 3 and 4). The polydispersity index of the NPs evaluated by the particle size analyzer was also less 0.5 which tells the normal distribution of the NPs with less chance of the complex formation within the NPs of various natures that involves the biomolecules as reducing or capping agents [43, 44].
Elemental and Functional Group Analysis
Unidentified biomolecules involved in the reduction of metals were spectroscopically identified by using Fourier Transform Infrared Spectroscopic analysis [43, 44]. FTIR also shows the various banding patterns on the basis of different functional groups present in the biomolecules [43,44,45]. FTIR peaks of TiO2NPs and ZnONPs were produced at a wavenumber of 500-4000 cm−1. Common FTIR peaks for TiO2NPs and ZnONPs were present at 3400-3500 cm−1, 2600-2700 cm−1 and 1500 to 1550 cm−1. Diagnostic and sharp peaks for TiO2NPs and ZnONPs were generated at 1350–1450 cm−1 (Fig. 5). Usually during the NPs synthesis, FTIR spectral peaks shifts are also observable.
Thermogravimetric Analysis (TGA)
To evaluate the temperature stability of any substance alongside the percent mass loss can be analyzed using the TGA. The thermogravimetric analysis produced diagnostic peaks at various temperatures ranging from 100–600 °C which produced diagnostic peaks at weight % loss peak. Both nanoparticles weight loss occur in 3 stages i.e., 1st weight loss (3–4%) for Titanium oxide NPs till 210 °C, 2nd weight loss (2–3%) till 510 °C and 3rd weight loss (1–2%) till 660 °C. An almost similar pattern of weight loss percentage at various temperatures was followed by zinc oxide NPs (Fig. 6). The total weight percent loss was almost 4%.
Minimum Inhibitory Concentration (MIC) of the NPs
The Minimum Inhibitory Concentration (MIC) for TiO2 NPs against E. coli, P. aeruginosa and K. pneumoniae was found to be 0.04 mg/mL, 0.07 mg/mL and 0.08 mg/mL respectively. On the other hand, the MIC for ZnO NPs against the above mentioned strains was calculated as 0.01 mg/mL, 0.015 mg/mL and 0.01 mg/mL, respectively.
Antibacterial Activity
The effect of TiO2NPs and ZnONPs at different concentrations i.e., 0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL, 1.2 mg/mL and 1.4 mg/mL was examined against Gram-negative bacterial strains. The highest inhibition zone obtained for all bacterial strains was observed at 1.4 mg/mL of the nanoparticles used. Antibacterial effects of nanoparticles are exhibited in Table 1.
Effect of TiO2 Nanoparticles Against Bacterial Strains
Results showed that TiO2NPs act as antibacterial agent against the tested bacteria. Among all bacterial strains, the maximum zone of inhibition was observed against K. pneumoniae i.e., 20 mm, while the minimum zone of inhibition was observed against E. coli i.e., 11 mm at 1.4 mg/mL concentration of NPs. Antibacterial effects of TiO2 are presented in Fig. 7.
Effect of ZnO Nanoparticles Against Bacterial Strains
Results showed that these nanoparticles act as antibacterial agents against the bacterial strains. Amongst all bacterial strains, K. pneumoniae exhibited maximum zone of inhibition 24 mm and E. coli showed minimum zone of inhibition i.e., 12 mm at 1.4 mg/mL concentration of NPs. Antibacterial effects of ZnO are exhibited in the following Fig. 8.
Comparison of Zones of Inhibition Obtained with TiO2 and ZnO Nanoparticles
The consequence of different concentrations of TiO2 was checked against the Gram-negative bacteria used in the study. The effect of same concentrations of ZnO nanoparticles was also evaluated against given bacterial strains. The highest zones of inhibition were formed when 1.4 mg/mL concentration of NPs was used. The results demonstrated that K. pneumoniae is more vulnerable to both nanoparticles with zone of inhibition of 20 and 24 mm with TiO2 and ZnO nanoparticles, respectively. Among all the three bacterial strains, E. coli showed lowest zone of inhibition i.e., 11 and 12 mm with TiO2 and ZnO nanoparticles, respectively. The susceptibility of bacterial strains was identified to be in the following order: K. pneumoniae > P. aeruginosa > E.coli. This is illustrated in the Table 1.
The comparison of results showed that ZnONPs are more effective antibacterial agents against the bacterial strains as compared to TiO2 NPs.
Antioxidant Activity of Nanoparticles
ZnO and TiO2 NPs were evaluated for their free radical scavenging activity using DPPH assay. Results depict that TiO2NPs has more ability to scavenge free radicals as compared to ZnONPs. The %age scavenging activity of TiO2 and ZnO nanoparticles was calculated as 77% and 73% respectively. The antioxidant activities of ZnO and TiO2NPs were increased as we increased the concentration of nanoparticles i.e., from 0.2 to 1.4 mg /mL as depicted in Figs. 9 and 10.
Cytotoxicity of Nanoparticles
The brine shrimp lethality assay (BSLA) was used to evaluate the cytotoxicity of both nanoparticles. The percentage mortality of brine shrimp larvae is presented in Figs. 11 and 12. The results demonstrated that TiO2NPs showed toxic effect at concentration of 1.2 mg/mL and 1.4 mg/mL with average mortality rate of 5.55% and 12.05%, respectively while other concentrations had no toxic effect on the shrimps. The cytotoxicity of ZnONPs revealed that the ZnONPs had no significant toxic effects on the shrimps i.e., 0% mortality. The results revealed that both nanoparticles had toxic effects on the shrimps as the concentration of nanoparticles increased. Thus, it showed that the effects of nanoparticles on brine shrimp larvae are concentration dependent.
Discussion
Titanium oxide nanoparticles and zinc oxide nanoparticles produced diagnostic UV–Vis peaks at 350-365nm and 37–400nm respectively which confirmed the nanoparticles synthesis without any oxidative damage which could decrease their efficacy. Durairaj et al. [46] and Panda et al. [47] also documented the TiO2NPs peaks at range of 350-370nm which are in accordance with the present study results. Similarity of the current study peaks with the previous researches is an indication of successful fabrication of TiO2NPs. Furthermore, many researchers globally have also reported the ZnONPs UV–Vis peaks between 400-500 nm [48,49,50]. Similarly, present study ZnONPs also produced same UV–Vis peaks 370-400 nm. Size of the nanoparticles was found to be 18 nm which is a good sign for promising antibacterial, antioxidant with minimum cytotoxic potential as many researchers describes that nanoparticles with size below 50 have the maximum bactericidal potential. This is due to the fact that small size NPs could easily pass the outer membrane of the bacteria and produce the best antibacterial results [51, 52].
SEM analysis of the Titanium oxide nanoparticles and zinc oxide nanoparticles produced nanoparticles with multiple shapes which are without/negligible aggregates which is confirmed from the SEM images. Many authors also reported that nanoparticles with aggregates or large size particles have lower/effected bactericidal potential [53, 54]. Visual results from SEM images authenticated the normal distribution of the nanoparticles with PDI < 0.5 [55, 56].
FTIR analysis produced diagnostic peaks for TiO2NPs and ZnONPs at 3200-3300 cm−1(OH-Stretching), 2340-2500 cm−1(CH-Stretching) and 1500 to 1550 cm−1(CO-Stretching). Diagnostic and sharp peaks for TiO2NPs and ZnONPs were generated at 1350–1450 cm−1 and 550–650 cm−1 which confirm the successful reduction and formation of the TiO2NPs and ZnONPs as also reported by many researchers [57,58,59]. Same results of the previous studies are a certification of the viable synthesis of the NPs of the present study.
TGA analysis showed the percent weight loss for both the nanoparticles at various temperatures which is attributed to the water content evaporation from the samples if any [60]. Initial weight loss till 100–200 °C could be due to water [61] but weight loss later at 300–500 °C could be due to the decomposition of the materials which is very less (1%) in present study results. After 500 °C, the decomposition of the titanium or zinc become constant and further no loss suggests the presence of pure salts [62]. Similar TGA results in the study were reported (Fig. 5).
The Minimum Inhibitory Concentration (MIC) for TiO2NPs against E. coli, P. aeruginosa and K. pneumoniae was found to be 0.04 mg/mL, 0.07 mg/mL and 0.08 mg/mL respectively. On the other hand, the MIC for ZnO NPs against the above mentioned strains was calculated as 0.01 mg/mL, 0.015 mg/mL and 0.01 mg/mL respectively. The results are in accordance with the study of Masoumi et al. [63] where ZnONPs induced broad-spectrum bactericidal effect on K. pneumoniae, and P. aeruginosa and restricted their growth at mean MIC concentration 4 ± 0.2 mg/L; in contrast. No growth inhibition was noticed for TiO2 NPs (MIC ≥ 1280 ± 0.2 mg/L). Similar findings of such studies authenticate the results of present study.
Moreover, results showed TiO2NPs as antibacterial agent also against the tested strains. Significant zones of inhibition were produced against all three pathogens. Similar results were also documented by Rosi and Kalyanasundaram [64] where they synthesized TiO2NPs and evaluated their antimicrobial activity against Gram negative bacteria i.e., E. coli and P. aeruginosa. Lowest zone of inhibition was observed against E. coli. ZnONPs also produced promising bactericidal results i.e., highest inhibition zone against K. pneumoniae. A similar study conducted by Hoseinzadeh et al. [65], evaluated the effect of ZnONPs against E. coli and P. aeruginosa. Antibacterial studies revealed greater effectiveness for P. aeruginosa as compared to E. coli. In another study, ZnO nanoparticles have displayed inhibition zone of 13mm against E. coli [66]. The slight difference of inhibition zone in the present study with previous study could be due to the small size (18 nm) of the present study nanoparticles due to the highest penetration ability of the nanoparticles with smaller size. These results are an indicative of the potent efficacy of the present study nanoparticles.
One of our previous articles showed synergistic effect of ZnONPs with the conventional antibiotics in which results indicated an increased antibacterial effect of antibiotics when used in combination with ZnONPs as compared to antibiotics alone [67]. A similar study conducted by Faisal et al. [68], showed that the activity of antibiotics was significantly increased against tested strains when coated with ZnONPs.
Probable mechanisms of action of nanoparticles include adherence to cell membrane, protein component of the cell membrane and DNA structure, resulting in functional modification, release of ions that impacts the membrane, nucleic acids (DNA) and protein content of the cell. Moreover, oxidative stress rise as a result of reactive oxygen species release which also subsequently effects cell membrane, nuclei acids (DNA) and protein content of the membrane, which ultimately becomes the reason for bacterial cell death as reported by Crisan et al. [69]. Furthermore, Chen et al. [70] recommended the mechanisms which may involve in the interaction of nanoparticles with the infectious microorganisms. It is thought that microorganisms carry a negative charge whereas nanomaterials carry a positive charge. The fact that NPs tend to collect predominantly on the outer surface of bacterial membranes may be the cause of their antibacterial effect, which was supported by the study of Faisal et al. [71].
ZnONPs may have an antibacterial effect since they tend to aggregate mostly on the bacterial membrane's outer surface. Reactive oxygen species (ROS), which are created by ZnO-NPs and bind to the bacterial membrane to cause permeability and cell death, are produced. Bacterial DNA and mitochondria are harmed by the production of Zn4 +, which is also capable of inhibiting vital bacterial enzymes and causing cell death [72].
The comparison of results showed that ZnONPs are more effective antibacterial agents against these bacterial strains as compared to TiO2NPs. There is no as such comparative study on the antibacterial activity of TiO2 and ZnO NPs with best of my knowledge against these specific strains. However, comparison on the doping of these nanoparticles has been studied previously. A study performed to assess the antibacterial activity of Ag-doped TiO2 and Ag-doped ZnO nanoparticles showed that Ag-ZnO NPs are highly efficient antibacterial agents as compared to TiO2NPs [73]. This comparative study could pave the pathways for further comparative analysis of the bactericidal studies to possibly make any synergistic studies for better control of pathogens in various biomedical fields.
Environmental stress, which causes reactive oxygen species (ROS) to degrade membrane, lipids, DNA, and proteins, is to be blamed for the alteration in metabolic pathways in plants. Many substances with metabolic importance, including terpenoids, flavonoids, and substances that respond to oxidative stress, hold a great potential in capping and stabilizing the nanoparticles [68]. The antioxidant activities of ZnO and TiO2NPs were evaluated by DPPH assay and results showed an increase in radical scavenging of these NPs as we increased their concentration i.e., from 0.2–1.4 mg /mL. A similar study was conducted by Boroumand Moghaddam et al. [74] which investigated the antioxidant activity of ZnO and found that radical scavenging activity increases by increasing the concentration of ZnONPs. A similar study on the DPPH activity of TiO2 NPs was evaluated and results indicated that the DPPH activity of the nanoparticles also increase in a dose-dependent manner [75]. The results indicated radical scavenging activity as 73% and 77% of ZnO and TiO2 respectively, at highest conc. of NPs i.e., 1.4 mg/mL. At lowest concentration the percentage radical scavenging activity was 39% and 42% for ZnO and TiO2 respectively. These studies endorsed the authentication of the current study findings.
To evaluate the possible safety of NPs for use in biological systems for multiple purposes like drug delivery, cytotoxicity bioassay was performed. Cytotoxicity of nanoparticles is assessed by using the Artemia salina test to determine the cell viability because this method is more rapid and convenient and less expensive when compared to other methods [76]. The results revealed that TiO2NPs showed toxic effect at concentration of 1.2 mg/mL and 1.4 mg/mL with average mortality rate of 5.55% and 12.05%, respectively while other concentrations had no toxic effect on the shrimps. This is illustrated in Fig. 5. The death of the larvae could be due to the accumulation of the NPs inside the gut. The results demonstrated that with the increase in concentration of nanoparticles, the %age mortality of Artemia salina was also increased. The result was supported by the study of Kumar et al. [77], in which the cytotoxicity of AgNPs was checked by the brine shrimp lethality assay with the conclusion that the lethality was directly proportional to the concentration of extract. As limited work is done to evaluate cytotoxicity of TiO2 using brine shrimp lethality assay. This study provides novel approach to study the cytotoxic potential of TiO2 in future. Moreover, ZnONPs had no significant toxic effects on the shrimps i.e., 0% mortality (Fig. 4). Our result was supported by the study of Supraja et al. [78], which indicated that the tested ZnO nanoparticles have poor brine shrimp larvicidal activity. Minimum mortality percentage (30%) was noted at a concentration of 50 ppm while no mortality was observed at 100 and 170 ppm. In light of the data, it can be concluded that the brine shrimp lethality of the different concentrations of ZnO was found to be concentration-dependent.
Conclusion
In the current study, ZnONPs exhibit stronger antibacterial activity when compared to TiO2NPs, which may be because of the fact that the nano-composition of ZnONPs contains more antibacterial active sites than that of TiO2 NPs. The ZnONPs can be investigated further in preclinical studies as potential antibacterial drug candidates.
Availability of Data and Material
All the appropriate data and material regarding the current study is present within the manuscript.
References
Chau TP, Brindhadevi K, Krishnan R, Alyousef MA, Almoallim HS, Whangchai N, Pikulkaew S (2022) A novel synthesis, analysis and evaluation of Musa coccinea based zero valent iron nanoparticles for antimicrobial and antioxidant. Environ Res 209:112770
Gangwar R, Ghosh A, Kumar S, Maurya VK (2023) Antibacterial, antioxidant and nutraceutical potential of new culinary-medicinal mushroom Russula lakhanpalii (Agaricomycetes) from India. Int J Med Mushrooms 25.
Martelli G, Giacomini D (2018) Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur J Med Chem 158:91–105
Rajeswari VD, Eed EM, Elfasakhany A, Badruddin IA, Kamangar S, Brindhadevi K (2021) Green synthesis of titanium dioxide nanoparticles using Laurus nobilis (bay leaf): Antioxidant and antimicrobial activities. Appl Nanosci 1–8
Yuan S, Xue Z, Zhang S, Wu C, Feng Y, Kou X (2023) The characterization of antimicrobial nanocomposites based on chitosan, Cinnamon essential oil, and TiO2 for fruits preservation. Food Chem 135446
Ghosh C, Sarkar P, Issa R, Haldar J (2019) Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol 27(4):323–338
Habib S, Rashid F, Tahir H, Liaqat I, Latif AA, Naseem S, ... Jefri OA (2023) Antibacterial and cytotoxic effects of biosynthesized zinc oxide and titanium dioxide nanoparticles. Microorganisms 11(6):1363
Anupong W, On-Uma R, Jutamas K, Salmen SH, Alharbi SA, Joshi D, Jhanani GK (2023) Antibacterial, antifungal, antidiabetic, and antioxidant activities potential of Coleus aromaticus synthesized titanium dioxide nanoparticles. Environ Res 216:114714
Joshi KM, Shelar A, Kasabe U, Nikam LK, Pawar RA, Sangshetti J, ... Chaskar MG (2022) Biofilm inhibition in Candida albicans with biogenic hierarchical zinc-oxide nanoparticles. Biomater Adv 134:112592
Archana P, Janarthanan B, Bhuvana S, Rajiv P, Sharmila S (2022) Concert of zinc oxide nanoparticles synthesized using Cucumis melo by green synthesis and the antibacterial activity on pathogenic bacteria. Inorg Chem Commun 137:109255
Iqbal J, Abbasi BA, Ahmad R, Mahmood T, Ali B, Khalil AT, ... Munir A (2018) Nanomedicines for developing cancer nanotherapeutics: From benchtop to bedside and beyond. Appl Microbiol Biotechnol 102:9449–9470
Jan H, Shah M, Andleeb A, Faisal S, Khattak A, Rizwan M, ... Abbasi BH (2021) Plant-based synthesis of zinc oxide nanoparticles (ZnO-NPs) using aqueous leaf extract of aquilegia pubiflora: Their antiproliferative activity against HepG2 cells inducing reactive oxygen species and other in vitro properties. Oxid Med Cell Longev
Naiel B, Fawzy M, Halmy MWA, Mahmoud AED (2022) Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci Rep 12(1):20370
Han Y, Zhang S, Shen N, Li D, Li X (2017) MOF-derived porous NiO nanoparticle architecture for high performance supercapacitors. Mater Lett 188:1–4
Gudkov SV, Burmistrov DE, Serov DA, Rebezov MB, Semenova AA, Lisitsyn AB (2021) A mini review of antibacterial properties of ZnO nanoparticles. Front Phys 9:641481
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13(10):2638–2650
Subhani AA, Irshad M, Ali S, Jawad M, Akhtar MF, Summer M (2023) UV-spectrophotometric optimization of temperature, pH, concentration and time for eucalyptus globulus capped silver nanoparticles synthesis, their characterization and evaluation of biological applications. J Fluoresc 1–12
Summer M, Ali S, Tahir HM, Abaidullah R, Tahir H, Mumtaz S, ... Tariq M (2023) Silk sericin protein: Turning discarded biopolymer into ecofriendly and valuable reducing, capping and stabilizing agent for nanoparticles synthesis using sonication. Macromol Chem Phys 2300124
Faisal S, Ullah R, Alotaibi A, Zafar S, Rizwan M, Tariq MH (2023) Biofabrication of silver nanoparticles employing biomolecules of Paraclostridium benzoelyticum strain: Its characterization and their in-vitro antibacterial, anti-aging, anti-cancer and other biomedical applications. Microsc Res Tech
Shafiee A, Rabiee N, Ahmadi S, Baneshi M, Khatami M, Iravani S, Varma RS (2022) Core-shell nanophotocatalysts: Review of materials and applications. ACS Appl Nano Mater 5:55–86
Sarfraz N, Khan I (2021) Plasmonic gold nanoparticles (AuNPs): Properties, synthesis and their advanced energy, environmental and biomedical applications. Chem Asian J 16:720–742
Rai P, Pandey A (2022) Role of emerging green technology in remediation of toxic pollutants. Innov Environ Biotechnol 183–201
Zafar S, Faisal S, Jan H, Ullah R, Rizwan M, Abdullah, … & Khattak, A. (2022) Development of Iron nanoparticles (FeNPs) using biomass of enterobacter: Its Characterization, antimicrobial, anti-Alzheimer’s, and enzyme inhibition potential. Micromachines 13(8):1259
Thema FT, Manikandan E, Dhlamini MS, Maaza MJML (2015) Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater Lett 161:124–127
Saqib S, Nazeer A, Ali M, Zaman W, Younas M, Shahzad A, ... Nisar M (2022) Catalytic potential of endophytes facilitates synthesis of biometallic zinc oxide nanoparticles for agricultural application. BioMetals 35(5): 967–985
Alavi M, Nokhodchi A (2021) Synthesis and modification of bio-derived antibacterial Ag and ZnO nanoparticles by plants, fungi, and bacteria. Drug Discov Today 26(8):1953–1962
Al-Radadi NS, Faisal S, Alotaibi A, Ullah R, Hussain T, Rizwan M, ... Ali Z (2022) Zingiber officinale driven bioproduction of ZnO nanoparticles and their anti-inflammatory, anti-diabetic, anti-Alzheimer, anti-oxidant, and anti-microbial applications. Inorg Chem Commun 140:109274
Eadi SB, Kim S, Jeong SW, Jeon HW (2017) Novel preparation of Fe doped TiO2 nanoparticles and their application for gas sensor and photocatalytic degradation. Adv Mater Sci Eng 2017:1–6
Manzoor M, Rafiq A, Ikram M, Nafees M, Ali S (2018) Structural, optical, and magnetic study of Ni-doped TiO2 nanoparticles synthesized by sol–gel method. Int Nano Lett 8:1–8
Khairy M, Zakaria W (2014) Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt J Pet 23:419–426
Nithya N, Bhoopathi G, Magesh G, Kumar CDN (2018) Neodymium doped TiO2 nanoparticles by sol-gel method for antibacterial and photocatalytic activity. Mater Sci Semicond Process 83:70–82
Salehi P, Babanouri N, Roein-Peikar M, Zare F (2018) Long-term antimicrobial assessment of orthodontic brackets coated with nitrogen-doped titanium dioxide against Streptococcus mutans. Prog Orthod 19:35
Ahmad MA, Yuesuo Y, Ao Q, Adeel M, Hui ZY, Javed R (2019) Appraisal of comparative therapeutic potential of undoped and nitrogen-doped titanium dioxide nanoparticles. Molecules 24(21):3916
Ahamed M, Khan MAM, Akhtar MJ, Alhadlaq HA, Alshamsan A (2017) Ag-doping regulates the cytotoxicity of TiO2 nanoparticles via oxidative stress in human cancer cells. Sci Rep 7:17662
Ahmad J, Siddiqui M, Akhtar M, Alhadlaq H, Alshamsan A, Khan S, Wahab R, Al-Khedhairy A, Al-Salim A, Musarrat J et al (2018) Copper doping enhanced the oxidative stress–mediated cytotoxicity of TiO2 nanoparticles in A549 cells. Hum Exp Toxicol 37:496–507
Caratto V, Locardi F, Alberti S, Villa S, Sanguineti E, Martinelli A, Balbi T, Canesi L, Ferretti M (2016) Different sol–gel preparations of iron-doped TiO2 nanoparticles: Characterization, photocatalytic activity and cytotoxicity. J Sol-Gel Sci Technol 80:152–159
Liu Z, Jian Z, Fang J, Xu X, Zhu X, Wu S (2012) Low-temperature reverse microemulsion synthesis, characterization, and photocatalytic performance of nanocrystalline titanium dioxide. Int J Photoenergy
Ahmad W, Kalra D (2020) Green synthesis, characterization and antimicrobial activities of ZnO nanoparticles using Euphorbia hirta leaf extract. J King Saud Univ-Sci 32(4):2358–2364
Ajmal N, Saraswat K, Bakht MA, Riadi Y, Ahsan MJ, Noushad M (2019) Cost-effective and eco-friendly synthesis of titanium dioxide (TiO2) nanoparticles using fruit’s peel agro-waste extracts: characterization, in vitro antibacterial, antioxidant activities. Green Chem Lett Rev 12(3):244–254
Ullah R, Shah S, Muhammad Z, Shah SA, Faisal S, Khattak U, ... Taj Akbar M (2021) In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres. Green Process Synth 10(1):101–111
Simorangkir M, Nainggolan B, Juwitaningsih T, Silaban S (2021, March) The toxicity of n-hexane, ethyl acetate and ethanol extracts of sarangbanua (Clerodendrumfragrans Vent Willd) leaves by brine shrimp lethality test (BSLT) method. J Phys Conf Ser 1811(1):012053. IOP Publishing
Imran M, Jan H, Faisal S, Shah SA, Shah S, Khan MN, ... Syed S (2021) In vitro examination of anti-parasitic, anti-Alzheimer, insecticidal and cytotoxic potential of Ajuga bracteosa Wallich leaves extracts. Saudi J Biol Sci 28(5):3031–3036
Summer M, Tahir HM, Ali S (2023) Sonication and heat-mediated synthesis, characterization and larvicidal activity of sericin-based silver nanoparticles against dengue vector (Aedes aegypti). Microsc Res Tech. https://doi.org/10.1002/jemt.24333
Summer M, Tahir HM, Ali S, Abaidullah R, Mumtaz S, Nawaz S (2023) Bactericidal potential of different size sericin-capped silver nanoparticles synthesized by heat, light, and sonication. J Basic Microbiol. https://doi.org/10.1002/jobm.202200632
Mumtaz S, Ali S, Tahir HM, Mumtaz S, Mughal TA, Kazmi SAR, ... Zulfiqar A (2023) Biological applications of biogenic silk fibroin–chitosan blend zinc oxide nanoparticles. Polym Bull 1–24
Durairaj B, Xavier T, Muthu S (2014) Fungal generated titanium dioxide nanopartilces for UV Protective and bacterial resistant fabrication. Int J Eng Sci Technol 6(9):621
Panda J, Singh UP, Sahu R (2018, September) Synthesis, characterization of TiO2 nano particles for enhancement of electron transport application in DSSC with Cu-BPCA Dye. IOP Conf Ser Mater Sci Eng 410(1):012008. IOP Publishing
Fadillah R, Rati Y, Dewi R, Farma R, Rini AS (2021, February) Optical and structural studies on bio-synthesized ZnO using Citrullus lanatus peel extract. J Phys Conf Ser 1816(1):012019. IOP Publishing
Muhammad W, Ullah N, Haroon M, Abbasi BH (2019) Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. RSC Adv 9(51):29541–29548
Zak AK, Razali R, Majid WA, Darroudi M (2011) Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int J Nanomed 1399–1403
Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI (2005) Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol 5(2):244–249
Tang S, Zheng J (2018) Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater 7(13):170–178
Le Ouay B, Stellacci F (2015) Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today 10(3):339–354
Russel WB (1989) Formulation and processing of colloidal dispersions. MRS Proc Cambridge University Press 177:281
Muhammad Tahir H, Saleem F, Ali S, Ain QU, Fazal A, Summer M, ... Murtaza G (2020) Synthesis of sericin-conjugated silver nanoparticles and their potential antimicrobial activity. J Basic Microbiol 60(5):458–467
Mumtaz S, Ali S, Kazmi SAR, Mughal TA, Mumtaz S, Tahir HM, ... Rashid MI (2022) Analysis of the antimicrobial potential of sericin-coated silver nanoparticles against human pathogens. Microsc Resd Tech
Alamdari S, Sasani Ghamsari M, Lee C, Han W, Park HH, Tafreshi MJ, ... Ara MHM (2020) Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl Sci 10(10):3620
Handore K, Bhavsar S, Horne A, Chhattise P, Mohite K, Ambekar J, ... Chabukswar V (2014) Novel green route of synthesis of ZnO nanoparticles by using natural biodegradable polymer and its application as a catalyst for oxidation of aldehydes. J Macromol Sci Part A 51(12):941–947
León A, Reuquen P, Garín C, Segura R, Vargas P, Zapata P, Orihuela PA (2017) FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl Sci 7(1):49
Patil, P. R., & Joshi, S. S. (2007). Polymerized organic–inorganic synthesis of nanocrystalline zinc oxide. Mater Chem Phys 105(2–3):354–361
Look DC (2001) Recent advances in ZnO materials and devices. Mater Sci Eng B 80(1–3):383–387
Sangkhaprom N, Supaphol P, Pavarajarn V (2010) Fibrous zinc oxide prepared by combined electrospinning and solvothermal techniques. Ceram Int 36(1):357–363
Masoumi S, Shakibaie MR, Gholamrezazadeh M, Monirzadeh F (2018) Evaluation synergistic effect of TiO2, ZnO nanoparticles and amphiphilic peptides (Mastoparan-B, indolicidin) against drug-resistant Pseudomonas aeruginosa, Klebsiellapneumoniae and acinetobacterbaumannii. Arch Pediatr Infect Dis 6(3)
Rosi H, Kalyanasundaram S (2018) Synthesis, characterization, structural and optical properties of titanium-dioxide nanoparticles using Glycosmiscochinchinensis Leaf extract and its photocatalytic evaluation and antimicrobial properties. World News Nat Sci 17:1–15
Hoseinzadeh E, Alikhani MY, Samarghandi MR, Shirzad-Siboni M (2014) Antimicrobial potential of synthesized zinc oxide nanoparticles against gram positive and gram negative bacteria. Desalin Water Treat 52(25–27):4969–4976
Yusof NAA, Zain NM, Pauzi N (2019) Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int J Biol Macromol 124:1132–1136
Rashid Y, Ahmad I, Ahmad N, Aslam M, Alotaibi A (2022) Affective antidepressant, cytotoxic activities, and characterization of phyto-assisted zinc oxide nanoparticles synthesized using Sanvitalia procumbens aqueous extract. BioMed Res Int 2022.
Faisal S, Jan H, Shah SA, Shah S, Khan A, Akbar MT, ... Syed S (2021) Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 6(14):9709–9722
Crisan CM, Mocan T, Manolea M, Lasca LI, Tăbăran FA, Mocan L (2021) Review on silver nanoparticles as a novel class of antibacterial solutions. Appl Sci 11(3):1120
Chen F, Shi Z, Neoh KG, Kang ET (2009) Antioxidant and antibacterial activities of eugenol and carvacrol‐grafted chitosan nanoparticles. Biotechnol Bioeng 104(1):30–39
Faisal S, Rizwan M, Ullah R, Alotaibi A, Khattak A, Bibi N, Idrees M (2022) Paraclostridium benzoelyticum bacterium-mediated zinc oxide nanoparticles and their in vivo multiple biological applications. Oxid Med Cellr Longev
Khan MI, Shah S, Faisal S, Gul S, Khan S, Abdullah, … & Shah, W. A. (2022) Monotheca buxifolia driven synthesis of zinc oxide nano material its characterization and biomedical applications. Micromachines 13(5):668
Nigussie GY, Tesfamariam GM, Tegegne BM, Weldemichel YA, Gebreab TW, Gebrehiwot DG, Gebremichel GE (2018) Antibacterial activity of Ag-doped TiO2 and Ag-doped ZnO nanoparticles. Int J Photoenergy
Boroumand Moghaddam A, Moniri M, Azizi S, Abdul Rahim R, Bin Ariff A, ZuhainisSaad W, ... Mohamad R (2017) Biosynthesis of ZnO nanoparticles by a new Pichiakudriavzevii yeast strain and evaluation of their antimicrobial and antioxidant activities. Molecules 22(6):872
Santhoshkumar T, Rahuman AA, Jayaseelan C, Rajakumar G, Marimuthu S, Kirthi AV, ... Kim SK (2014) Green synthesis of titanium dioxide nanoparticles using Psidiumguajava extract and its antibacterial and antioxidant properties. Asian Pac J Trop Med 7(12):968–976
Rajabi S, Ramazani A, Hamidi M, Naji T (2015) Artemiasalina as a model organism in toxicity assessment of nanoparticles. DARU J Pharm Sci 23:1–6
Kumar P, Selvi SS, Praba AL, Selvaraj M, Rani LM, Suganthi P, ... Govindaraju M (2012) Antibacterial activity and in-vitro cytotoxicity assay against brine shrimp using silver nanoparticles synthesized from Sargassumilicifolium. Dig J Nanomater Biostruct 7(4):1447–1455
Supraja N, Prasad TNVKV, Gandhi AD, Anbumani D, Kavitha P, Babujanarthanam R (2018) Synthesis, characterization and evaluation of antimicrobial efficacy and brine shrimp lethality assay of Alstoniascholaris stem bark extract mediated ZnONPs. Biochem Biophys Rep 14:69–77
Author information
Authors and Affiliations
Contributions
Hunaiza Tahir performed the experiment and wrote the manuscript. Farzana Rashid supervised the experiment and edited the manuscript. Shaukat Ali edited the manuscript and evaluated the manuscript. Muhammad Summer edited the manuscript and did the characterization analysis. Rimsha Abaidulah assisted in writeup and reference management.
Corresponding author
Ethics declarations
Ethical Approval
As no animal is being used/sacrificed in present study, ethical approval was not applicable
Conflict of Interest
There is no conflict of interest of author regarding the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Research Highlights
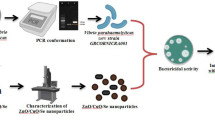
• 1st Comparative study of TiO2NPs and ZnONPs regarding their bactericidal, antioxidative and cytotoxic potential as shown in Fig. 1.
• Complete characterization of smaller (18 nm) TiO2NPs and ZnONPs using, SEM, FTIR, UV–Vis and PSA.
• Study of thermal behavior of both TiO2NPs and ZnONPs for their working efficiency at variable temperatures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tahir, H., Rashid, F., Ali, S. et al. Spectrophotometrically, Spectroscopically, Microscopically and Thermogravimetrically Optimized TiO2 and ZnO Nanoparticles and their Bactericidal, Antioxidant and Cytotoxic Potential: A Novel Comparative Approach. J Fluoresc (2023). https://doi.org/10.1007/s10895-023-03367-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10895-023-03367-0