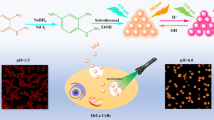
Abstract
Accurate monitoring of intracellular pH in living cells is critical for developing a better understanding of cellular activities. In the current study, label-free carbon dots (p-CDs), which were fabricated using a straightforward one-pot solvothermal treatment of p-phenylenediamine and urea, were employed to create a new ratiometric pH nanosensor. Under single-wavelength excitation (λex = 500 nm), the p-CDs gave dual emission bands at 525 and 623 nm. The fluorescent intensity ratio (I525/I623) was linearly related to pH over the range 4.0 to 8.8 in buffer solutions, indicating that the ratiometric fluorescence nanoprobe may be useful for pH sensing. In pH measurements, the p-CDs also demonstrated outstanding selectivity, reversibility, and photostability. Owing to the advantages outlined above, the nanoprobe was used to monitor the pH of HeLa cells effectively. The label-free CD-based ratiometric nanoprobe features comparatively easy manufacturing and longer excitation and emission wavelengths than the majority of previously reported CD-based ratiometric pH sensors, which is ultimately beneficial for applications in biological imaging.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracellular pH is a key target parameter in cell growth and death, muscle contraction, metabolism of organs, ion transport and homeostasis [1]. Abnormal intracellular pH values may cause various cardiopulmonary and neurological diseases, such as ischemic/reperfusion injury and Alzheimer’s and Parkinson’s disease [2]. Therefore, accurate intracellular pH monitoring is critical for developing a better understanding of cellular activities and providing crucial support in early disease identification. To date, the glass pH electrode is the most common tool used for pH measurement. However, the disadvantages of electrical interference or mechanical damage to cells cannot be avoided, making it unsuitable for intracellular pH studies [3]. In contrast, fluorescence-based sensors are more applicable to detection of intracellular pH changes because they are noninvasive and exhibit excellent spatial and temporal resolution, good sensitivity and ease of sample preparation [4, 5]. However, the majority of fluorescent pH sensors are dependent on changes of a single emission intensity, which can be affected by a range of parameters, such as fluorescence probe concentration and dye bleaching, thereby reducing the reliability of the measured data [6]. Keeping this in mind, considerable effort has been devoted to developing ratiometric pH sensors that can self-calibrate to overcome interference by environmental factors and successfully exceed the limits of single emission intensity measurements.
Carbon dots (CDs) have recently attracted much interest due to their small particle sizes, nonblinking properties, low toxicity, excellent solubility and biocompatibility [7,8,9,10,11]. In recent years, several groups have created many ratiometric fluorescent pH sensors based on CDs and used them to monitor intracellular pH [12,13,14,15,16,17,18]. Ma et al., for example, combined pH-insensitive rhodamine B isothiocyanate, pH-sensitive fluorescein isothiocyanate, and amino-coated CDs into a single device to create a programmable ratiometric pH sensor [19]. Using CD-fluorescein isothiocyanate composites, Wang et al. fabricated a fluorescent ratiometric pH probe [20]. However, these ratiometric pH-sensing devices involved cross-attachment or embedding of dyes in the CDs, and extensive separation and purification operations were still required [21]. To circumvent the disadvantages highlighted above, numerous ratiometric fluorescence nanosensors based on label-free CDs have been developed [22, 23]. For example, Yang et al. synthesized novel ratiometric fluorescent CDs for enantioselective sensing of L-lysine and pH in vivo and in vitro [24]. A novel ratiometric fluorescence nanosensor incorporating label-free CDs was constructed by Feng et al. for intracellular water pH monitoring and lysosome imaging [25]. Nevertheless, the development of CD-based fluorescent probes appropriate for measuring pHs in living cells is at an initial level [26, 27]. As a result, it is critical to construct CDs with pH-sensitive ratiometric fluorescence emission to develop ratiometric fluorescence nanoprobes for pH assessments in living cells.
A ratiometric fluorescent pH nanoprobe comprising label-free CDs was constructed in this work and utilized for intracellular pH sensing, as shown in Scheme 1. The preparation of CDs (p-CDs) exhibiting dual emission at 525 and 623 nm was achieved with a one-pot solvothermal treatment of p-phenylenediamine (p-PD) and urea. The p-CDs showed inherent ratiometric fluorescence emission (I525/I623) as a function of pH change without the necessity for dye labeling, and exhibited a strong linear relationship with pH over the response range 4.0−8.8 in buffer solutions. The CDs, owing to their unique emission features, were subsequently used as a ratiometric fluorescent pH nanoprobe to monitor the pHs of HeLa cells.
Experimental Section
Materials
p-PD, urea, glutathione (GSH), cysteine (Cys), homocysteine (Hcy), alanine (Ala), arginine (Arg), serine (Ser), leucine (Leu), and metal ions were procured from Aladdin Ltd. (http://www.aladdin-e.com/, Shanghai, China). The rest of the chemicals were of analytical grade, and no additional purification was carried out before utilization in experiments. Throughout the research, distilled water was used.
Characterization
TEM micrographs were obtained using a GZF2.0 FEI Electron Optics transmission electron microscope (Thermo Scientific, America https://www.thermofisher.com/). An ESCALAB 250Xi multitechnique surface analyzer was used to perform X-ray photoelectron spectroscopy (XPS) studies (Thermo Scientific, America https://www.thermofisher.com/). KBr pellets were used to acquire Fourier transform infrared (FT-IR) spectra on a Tensor 27 FT-IR spectrometer (Bruker, Germany https://www.bruker.com/). A Shimadzu UV-2550 spectrophotometer was used to record absorption values (Shimadzu, Japan https://www.shimadzu.com). An RF5301 fluorescence spectrometer was used to measure all fluorescence spectra (Shimadzu, Japan https://www.shimadzu.com). The fluorescent lifetimes and absolute quantum yields were determined with an Edinburgh Analytical FLS1000 instrument (Techcomp, China) (Techcomp, China http://www.techcomp.cn/). A Sartorius PB-10 pH meter was used to measure pH (Sartorius, Germany https://www.sartorius.com.cn/). A Xiangyi H1650-W centrifuge was used to separate mixtures (Xiangyi, China http://www.xiangyihn.com/). A Carl Zeiss LSM 880 confocal laser scanning microscope was used for fluorescence imaging (Zeiss, Germany https://www.zeiss.com/corporate/int/home.html).
Preparation of CDs
The p-CDs were prepared through a one-pot solvothermal method according to literature reports [28, 29]. Carbonization of p-PD and urea with the help of concentrated HNO3 was used to generate p-CDs via a solvothermal technique. First, 0.1 g of p-PD and 0.1 g of urea were dissolved in C2H5OH (10 mL). Subsequently, the solution was poured into a 25 mL poly(tetrafluoroethylene)-lined stainless steel autoclave. Afterward, 40 µL of pure HNO3 was added dropwise with vigorous stirring. A dark-red suspension was formed after heating for 12 h at 180 °C, and the solution was left to cool to room temperature. To eliminate the nonfluorescent precipitates, the solution was subjected to centrifugation for 15 min at 12,000 rpm (with a 10,000×g centrifugation force). The products were purified on a silica column using methanol and methylene dichloride as the eluent, and the p-CDs were separated for use as nanosensors. The CD sample was eventually obtained following evaporation of the solvent and drying in a vacuum, and the absolute quantum yield of p-CDs emission was 8%.
Fluorescence Assays of pH Values
To make a stock solution, the p-CDs were dissolved in ethanol. pH values were determined using a 20 mM buffer containing disodium hydrogen phosphate-citric acid buffer at pHs 2.2−8.0 and a glycine-sodium hydroxide buffer at pHs 8.5−10.6. A 100 µL CD dispersion (1.0 mg/mL) was added to 4.90 mL of buffer at various pH levels in a series of test tubes. The resultant solutions were mixed well before fluorescence spectra were taken. By altering the pH values between 5.0 and 8.0 with HCl and NaOH solutions, the reversibility of CD suspension was studied after repetition for four continual cycles, and the fluorescence spectra were obtained. The tests were carried out in triplicate.
Cytotoxicity Assay
A conventional 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2 H-tetrazolium bromide (MTT) test was employed to examine the cytotoxicity of the p-CDs. Ninety-six-well plates were used to seed HeLa cells for 24 h prior to treatment, and they were subsequently cultured for 12 h with various doses of p-CDs (0−60 µg/mL). Next, 20 µL of MTT solution (5.0 mg/mL) was added to the individual wells and incubated for 4 h at 37 °C. Then, 100 µL of supernatant was withdrawn, and 150 µL of dimethyl sulfoxide (DMSO) was added to the individual wells for solubilization of formazan. The plate was agitated for 10 min before a microplate reader was employed for absorbance measurements of the solutions at 562 nm. All MTT tests were carried out four times in total.
Cell Fluorescence Imaging
HeLa cells were seeded on 6-well culture plates with sterile coverslips and left to adhere for 24 h before investigation. The media was withdrawn, and the cells were subjected to treatment for 5 h at 37 °C with p-CDs (40 µg/mL) in RPMI medium. Phosphate buffered saline (PBS) (pH 7.4) was then utilized to wash the cells three times. It was subsequently incubated for 30 min with 5 µg/mL nigericin in a high concentration K+ buffer at various pH values (5.0, 7.0, and 8.0). A Carl Zeiss LSM 880 confocal laser scanning microscope was used to image the cells immediately. The excitation wavelength was 458 nm, while the emission collection wavelength range was 475–575 nm.
Results and Discussion
Characterization of p-CDs
TEM was first employed to assess the morphological features of the p-CDs. The p-CDs were spherical and exhibited good dispersion in aqueous solutions, as shown in Fig. 1. Furthermore, their size distribution was determined to be 2.6−5.4 nm, with a mean size of 3.8 nm (based on measurements of more than 100 particles in the TEM photograph) (inset of Fig. 1).
The surface functional groups and elemental compositions were then determined using XPS analysis and FT-IR spectroscopy. According to the survey spectrum, the main components of the targeted CDs were carbon, oxygen, and nitrogen: 78.48% carbon, 7.69% oxygen, and 13.83% nitrogen (Fig. 2 A). The high-resolution C 1s XPS spectrum (Fig. 2B) was deconvoluted into three peaks at 284.78, 287.13, and 290.43 eV that were ascribed to C-C/C = C, C-N/C = O, and O-C = O bonds, respectively [24, 30]. The high-resolution N 1s spectrum (Fig. 2 C) showed a pair of peaks at 399.48 and 402.08 eV, corresponding to pyridinic and amino N bonds, respectively [31, 32]. Oxygens on carbon atoms (C = O/C-OH) (Fig. 2D) were identified in the high resolution O 1s spectrum with a peak at 531.63 eV [33]. FT-IR characterization was also carried out for analysis of chemical bonding on the surfaces of the p-CDs. The FT-IR spectrum of the p-CDs is shown in Fig. 3 A, which demonstrates that the p-CDs contained –NH2 (3301 and 3203 cm− 1), –CH2 (3008 cm− 1), O = C − O− (1759 cm− 1), C = C (1630 cm− 1), –C = N (1517 cm− 1), C − N−C (1261 cm− 1), and C-O-C (1128 cm− 1) functional groups [34,35,36]. The results of the FT-IR and XPS analyses were similar, indicating that –NH2 and –COOH groups existed on the p-CD surface, rendering it highly water-soluble. The chemically reactive entities also allowed further functionalization and surface passivation.
Optical Features of the p-CDs
The optical features of the p-CDs were examined by collecting their absorption and fluorescence spectra. The p-CDs in aqueous solutions exhibited numerous absorption bands at 241, 296, and 400–550 nm, as shown in Fig. 3B. The aromatic C = C bonds gave rise to n-π* transitions, which were responsible for the absorption bands at 241 and 296 nm [37], and the wide absorption between 400 and 550 nm was ascribed to n-π* transitions occurring with the surface states of p-CDs [38, 39]. Next, the fluorescence characteristics of the p-CDs were thoroughly investigated. The fluorescence intensities of the p-CDs at 525 and 623 nm increased with increasing excitation wavelength, as shown in Fig. 3B. However, the emission spectrum at 525 nm was not fully displayed when the wavelength excitation was 510 nm. Therefore, an excitation wavelength of 500 nm was selected as the excitation maximum in this study, which was facilitated by ratiometric detection of pH. Furthermore, when the excitation wavelength was varied from 325 to 405 nm, the emission wavelengths of the p-CDs remained nearly unchanged. This excitation independence may be attributed to the remarkable chemical composition and content uniformity of the p-CDs [40], which may enable expanded biological applications by avoiding autofluorescence [41].
Investigation of pH-Response Properties
Using 20 mM buffer with various pH values in the range 4.0 to 8.8, standard fluorescence pH titrations of the p-CDs were carried out. As shown by the 3D emission-pH spectra (Fig. 4 A), the fluorescence emission intensity at 525 nm underwent a progressive increase with increasing pH values, whereas the emission intensity at 623 nm for the p-CDs remained more or less constant. Fluorescence emission spectra also confirmed this result (Fig. S1). The intensity ratios of the two emission channels (I525/I623) manifested a linear relationship with pH over the range 4.0 to 8.8 and were fit with the linear regression equation I525/I623 = 0.1279pH + 0.3057, with a linear coefficient of 0.9968 (Fig. 4B). The linear range was wide enough to suit the needs of intracellular pH monitoring and in vivo imaging. The aforementioned findings showed that p-CDs might be used as a novel nanoprobes for quantitatively determining pH levels.
We initially looked at the UV–vis spectra of p-CDs as the pH was increased from 4.0 to 8.8 to see if we could determine the cause of the response to pH. There was no discernible change, as indicated in Fig. 5 A. As a result, we concluded that the increased fluorescence of the p-CDs at high pH levels could not be due to aggregation [42]. Then, fluorescence lifetime measurements were measured (Fig. 5B). The fluorescence decays of the p-CD solutions remained biexponential as the pH was increased from 4.0 to 8.0, and the mean lifetimes rose from 1.70 to 4.31 ns, showing that the rate of nonradiative transitions was reduced. As a result, we hypothesized that the increased fluorescence intensity was due to the carboxylate-based negatively charged protective shell present on the surfaces of the p-CDs, which, in turn, restricted the rate of nonradiative decay from the photoexcited state of the p-CDs and resulted in a significant improvement in fluorescence [43].
Photostability and Reversibility of p-CDs
Since excellent photostability and reversibility are essential for long-term and real-time cellular imaging, we further investigated the photostability and reversibility of the p-CDs. Timed scans for measurements of fluorescence intensity in 20 mM PBS buffer with varying pH values (5.8, 7.0, and 8.0) were conducted for 12 h to examine the photostability. The ratiometric fluorescence intensities (I525/I623) of the p-CDs remained uniform during the scanning process (Fig. 6 A), which performed that they were stable to air and light at appropriate pH values. The fluorescence reversibility as a function pH was then tested by repeatedly altering the pH between 5.0 and 8.0. The ratiometric fluorescence intensity (I525/I623) of the carbon dots showed high consistency for the alkaline (pH 8.0) and acidic (pH 5.0) solutions, as shown in Fig. 6B. Thus, the pH-sensitive CDs demonstrated satisfactory fluorescence reversibility and photostability in solutions with a range of pH values, indicating that they might be utilized as intracellular pH nanoprobes.
Influence of Potentially Interfering Substances in Solutions with Varying pH Values
The possible interference resulting from high quantities of amino acids (Ala, Arg, Ser, and Leu), biothiols (GSH, Cys, and Hcy), and metallic ions (Ca2+, Mg2+, Al3+, Zn2+, Cu2+, Ni2+, Mn2+, Pb2+, Hg2+, Fe2+, Fe3+, Cd2+, and Ag+) was explored. As demonstrated in Fig. 7, there were no substantial variations in the fluorescence intensities of the p-CDs in the presence of these interfering chemicals, indicating remarkable selectivity for the nanoprobe.
Fluorescence intensity of p-CDs in the presence of interfering substances in 20 mM PBS buffer with different pH values. 1, Blank; 2, Ca2+; 3, Mg2+; 4, Al3+; 5, Zn2+; 6, Cu2+; 7, Ni2+; 8, Mn2+; 9, Pb2+; 10, Hg2+; 11, Fe2+; 12, Fe3+; 13, Cd2+; 14, Ag+; 15, Ala; 16, Arg; 17, Ser; 18, Leu; each concentration was 1 mM. 19, 5 mM GSH; 20, 0.5 mM Hcy; 21, 0.5 mM Cys. (A) pH at 5.0, (B) pH at 8.0
pH Imaging in Living Cells
The use of p-CDs for determining the pH values of HeLa cells was attempted after the abovementioned results were obtained. To assess the toxicity of p-CDs to live cells, a conventional MTT test was first used [44]. As demonstrated in Fig. S2, there was no substantial reduction in cell viability, demonstrating that p-CDs had limited cytotoxicity toward HeLa cells at concentrations below 60 µg/mL. HeLa cells were then treated with p-CDs (40 µg/mL) for 5 h at 37 °C to look for subtle alterations in the intracellular pH. The cells were then treated for 30 min with buffers establishing pH values of 5.0, 7.0, and 8.0, which allowed for H+/K+ exchange and maintained an equal pH in the buffer media and inside the cells [45, 46]. As depicted in Fig. 8, the emission intensity of the treated cells in channel 1 (green pseudocolor) increased progressively as the pH was raised from 5.0 to 8.0, but channel 2 (red pseudocolor) remained nearly unaltered. The merged graphs of channel 1 and channel 2 exhibited a noticeable hue shift with pH changes. In addition, Picture-Pro Plus software was used to create a fluorescence ratio image (Igreen/Ired) (Fig. S3). The intensity ratio (I525/I623) was linearly related to pH over range 5.0−8.0, as predicted, demonstrating that the CD-based nanoprobe is capable of detecting subtle changes in intracellular pH.
Finally, comparisons between this ratiometric nanosensor and other published CD-based ratiometric sensors used for pH detection in materials, including their diameters, excitation and emission wavelengths, and measurement ranges, are summarized in Table 1 [16, 23, 25, 47,48,49,50,51,52,53]. This nanosensor presented longer excitation and emission wavelengths (λex = 500 nm; λem = 526/623 nm), a wider linear range (4.0−8.8) and simpler synthetic procedures (one-pot solvothermal method) than the other CD-based pH sensors, making this nanosensor comparable to previous CD-based ratiometric sensors. The relatively small Stokes shift is the major limitation of the p-CDs, and this can be modified by constructing CDs with larger Stokes shifts to extend the scope of applications involving sensitive measurements of intracellular pH.
Conclusion
A simple one-pot solvothermal carbonization process was used to generate novel nanoprobe p-CDs. Detailed spectroscopic characterization revealed that this novel fluorescent nanoprobe may be utilized to monitor pH in ratiometrically by using emission intensities at the two wavelengths 525 and 623 nm. The fluorescence intensity ratios (I525/I623) for p-CDs increased with increasing pH from 4.0 to 8.8 and showed a linear relationship. This sensor could be used effectively to ratiometrically monitor pH changes in living cells due to its excellent selectivity, strong photostability, and fluorescence reversibility, suggesting practical applications for detection of pH-related diseases and disorders.
Code Availability
Not applicable.
Data Availability
All data generated or analyzed of this study are available within the article.
References
Ohkuma S, Poole B (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci 75(7):3327–3331. https://doi.org/10.1073/pnas.75.7.3327
Kennedy RT, Huang L, Aspinwall CA (1996) Extracellular pH is required for rapid release of insulin from Zn-insulin precipitates in β-cell secretory vesicles during exocytosis. J Am Chem Soc 118(7):1795–1796. https://doi.org/10.1021/ja953271w
Wan SL, Zheng Y, Shen J, Yang WT, Yin MZ (2014) “On-off-on” switchable sensor: a fluorescent spiropyran responds to extreme pH conditions and its bioimaging applications. ACS Appl Mater Inter 6(22):19515–19519. https://doi.org/10.1021/am506641t
Chen Y (2021) Recent advances in fluorescent probes for extracellular pH detection and imaging. Anal Biochem 612:113900. https://doi.org/10.1016/j.ab.2020.113900
Dangi V, Baral M, Kanungo BK (2020) Study on the development of a cyclohexane based tripodal molecular device as “off-on-off” pH sensor and fluorescent iron sensor. Curr Anal Chem 16(5):620–630. https://doi.org/10.2174/1573411015666-190314154126
Liu LY, Zhao YP, Zhang N, Wang KN, Tian MG, Pan QL, Lin WY (2021) Ratiometric fluorescence imaging for the distribution of nucleic acid content in living cells and human tissue sections. Anal Chem 93(3):1612–1619. https://doi.org/10.1021/acs.analchem.0c04064
Li S, Li L, Tu HY, Zhang H, Silvester DS, Banks CE, Zou GQ, Hou HS, Ji XB (2021) The development of carbon dots: from the perspective of materials chemistry. Mater Today 51:188–207. https://doi.org/10.1016/j.mattod.2021.07.028
Wareing TC, Gentile P, Phan AN (2021) Biomass-based carbon dots: current development and future perspectives. ACS Nano 15(10):15471–15501. https://doi.org/10.1021/acsnano.1c03886
Sk MP, Sailapu SK, Chattopadhyay A (2015) Luminescent carbon dots for logic operations in two phases. ChemPhysChem 16(4):723–727. https://doi.org/10.1002/cphc.201402747
Arshad F, Pal A, Alam T, Khan JA, Sk MP (2020) Luminescent carbogenic dots for the detection and determination of hemoglobin in real samples. New J Chem 44(16):6213–6221. https://doi.org/10.1039/D0NJ00401D
Arshad F, Sk MP (2019) Aggregation-induced red shift in N, S-doped chiral carbon dot emissions for moisture sensing. New J Chem 43(33):13240–13248. https://doi.org/10.1039/C9NJ03009C
Macairan JR, Zhang I, Clermont-Paquette A, Naccache R, Maysinger D (2020) Ratiometric pH sensing in living cells using carbon dots. Part Part Syst Char 37(1):2070002. https://doi.org/10.1002/ppsc.202070002
Du FK, Ming YH, Zeng F, Yu CM, Wu SZ (2013) A low cytotoxic and ratiometric fluorescent nanosensor based on carbon-dots for intracellular pH sensing and mapping. Nanotechnology 24(36):365101. https://doi.org/10.1088/0957-4484/24/36/365101
Hu SL, Meng X, Tian F, Yang WL, Li N, Xue CR, Yang JL, Chang Q (2017) Dual photoluminescence centers from inorganic-salt-functionalized carbon dots for ratiometric pH sensing. J Mater Chem C 5(38):9849–9853. https://doi.org/10.1039/C7TC03266H
Lesani P, Singh G, Lu Z, Mirkhalaf M, New EJ, Zreiqat H (2022) Two-photon ratiometric carbon dot-based probe for real-time intracellular pH monitoring in 3D environment. Chem Eng J 433(3):133668. https://doi.org/10.1016/j.cej.2021. 133668
Long RQ, Tang C, Li T, Tong X, Tong CY, Guo Y, Gao QP, Wu LH, Shi SY (2020) Dual-emissive carbon dots for dual-channel ratiometric fluorometric determination of pH and mercury ion and intracellular imaging. Microchim Acta 187(5):307. https://doi.org/10.1007/s00604-020-04287-7
Pal A, Ahmad K, Dutta D, Chattopadhyay A (2019) Boron doped carbon dots with unusually high photoluminescence quantum yield for ratiometric intracellular pH sensing. ChemPhysChem 20(8):1018–1027. https://doi.org/10.1002/cphc.201900140
Wang X, Zhao L, Hu JS, Wei H, Liu XY, Li E, Yang SH (2022) Rational design of novel carbon-oxygen quantum dots for ratiometrically mapping pH and reactive oxygen species scavenging. Carbon 190:115–124. https://doi.org/10.1016/j.carbon.2022.01.006
Shi W, Li XH, Ma HM (2012) A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells. Angew Chem Int Edit 51(26):6432–6435. https://doi.org/10.1002/anie.201202533
Zhu XX, Jin H, Gao CL, Gui RJ, Wang ZH (2017) Ratiometric, visual, dual-signal fluorescent sensing and imaging of pH/copper ions in real samples based on carbon dots-fluorescein isothiocyanate composites. Talanta 162(1):65–71. https://doi.org/10.1016/j.talanta.2016.10.015
Xu SM, He X, Huang YB, Liu X, Zhao LH, Wang XH, Sun Y, Ma PY, Song DQ (2020) Lysosome-targeted ratiometric fluorescent sensor for monitoring pH in living cells based on one-pot-synthesized carbon dots. Microchim Acta 187:478. https://doi.org/10.1007/s00604-020-04462-w
Xia C, Cao MM, Xia JF, Zhou GH, Jiang DY, Zhang DF, Wang J, Li HL (2019) An ultrafast responsive and sensitive ratiometric fluorescent pH nanoprobe based on label-free dual-emission carbon dots. J Mater Chem C 7:2563–2569. https://doi.org/10.1039/C8TC05693E
Hamd-Ghadareh S, Salimi A, Fathi F, Soleimani F (2019) Dual-emission carbon dots as biocompatible nanocarrier for in vitro/in vivo cell microenvironment ratiometric pH sensing in broad range. J Iran Chem Soc 16(10):2081–2092. https://doi.org/10.1007/s13738-019-01678-3
Chang D, Zhao ZH, Shi H, Feng JY, Yang YX, Shi LH (2022) Ratiometric fluorescent carbon dots for enantioselective sensing of L-lysine and pH discrimination in vivo and in vitro. Sens Actuat B-Chem 362:131792. https://doi.org/10.1016/j.snb.2022.131792
Li SR, Song XR, Wang YH, Hu ZR, Yan F, Feng GD (2021) Developed a ratiometric fluorescence pH nanosensor based on label-free carbon dots for intracellular lysosome imaging and water pH monitoring with a smartphone. Dyes Pigm 193:109490. https://doi.org/10.1016/j.dyepig.2021.109490
He C, Xu P, Zhang XH, Long WJ (2022) The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: current state and future perspective. Carbon 186:91–127. https://doi.org/10.1016/j.carbon.2021.10.002
Das P, Maruthapandi M, Saravanan A, Natan M, Jacobi G, Banin E, Gedanken A (2020) Carbon dots for heavy-metal sensing, pH-sensitive cargo delivery, and antibacterial applications. ACS Appl Nano Mater 3(12):11777–11790. https://doi.org/10.1021/acsanm.0c02305
Lesani P, Lu Z, Singh G, Mursi M, Mirkhalaf M, New EJ, Zreiqat H (2021) Influence of carbon dot synthetic parameters on photophysical and biological properties. Nanoscale 13(25):11138–11149. https://doi.org/10.1039/D1NR01389K
Wang Q, Tang ZH, Li L, Guo JX, Jin LX, Lu JF, Huang P, Zhang SR, Jiao (2022) Highly efficient red-emitting carbon dots as a “turn-on” temperature probe in living cells. Spectrochim Acta A 280:121538. https://doi.org/10.1016/j.saa.2022.121538
Ji YY, Zou X, Wang WJ, Wang TF, Zhang SL, Gong ZJ (2021) Co-doped S, N-Carbon dots and its fluorescent film sensors for rapid detection of cr (VI) and ascorbic aci. Microchem J 167:106284. https://doi.org/10.1016/j.microc.2021.106284
Wang Q, Zhang SR, Zhong YG, Yang XF, Li Z, Li H (2017) Preparation of yellow-green-emissive carbon dots and their application in constructing a fluorescent turn-on nanoprobe for imaging of selenol in living cells. Anal Chem 89(3):1734–1741. https://doi.org/10.1021/acs.analchem.6b03983
Jia J, Lu WJ, Li L, Gao YF, Jiao Y, Han H, Dong C, Shuang SM (2020) Orange-emitting N-doped carbon dots as fluorescent and colorimetric dual-mode probes for nitrite detection and cellular imaging. J Mater Chem B 8(10):2123–2127. https://doi.org/10.1039/C9TB02934F
Lu S, Sui L, Liu J, Zhu SJ, Chen A, Jin MX, Yang B (2017) Near-infrared photoluminescent polymer-carbon nanodots with two-photon fluorescence. Adv Mater 29(15):1603443. https://doi.org/10.1002/adma.201603443
Espina-Casado J, Fontanil T, Fernández-González A, Cal S, Obaya ÁJ, Díaz-García ME, Badía-Laíño R (2021) Carbon dots as multifunctional platform for intracellular pH sensing and bioimaging. In vitro and in vivo studies. Sens Actuators B 346:130555. https://doi.org/10.1016/j.snb.2021.130555
Yang Y, Wang CF, Shu Q, Xu N, Qi SQ, Zhuo SJ, Zhu CQ, Du JY (2022) Facile one-step fabrication of Cu-doped carbon dots as a dual-selective biosensor for detection of pyrophosphate ions and measurement of pH. Spectrochim Acta A 268:120681. https://doi.org/10.1016/j.saa.2021.120681
Huo XM, Shen HL, Xu YJ, Shao J, Liu R, Zhang ZY (2022) Fluorescence properties of carbon dots synthesized by different solvents for pH detector. Opt Mater 123:111889. https://doi.org/10.1016/j.optmat.2021.111889
Bhati A, Anand SR, Gunture, Garg AK, Khare P, Sonkar SK (2018) Sunlight-induced photocatalytic degradation of pollutant dye by highly fluorescent red-emitting Mg-N-embedded carbon dots. ACS Sustain Chem Eng 6(7):9246–9256. https://doi.org/10.1021/acssuschemeng.8b01559
Liu JJ, Li DW, Zhang K, Yang MX, Sun HC, Yang B (2018) One-step hydrothermal synthesis of nitrogen-doped conjugated carbonized polymer dots with 31% efficient red emission for in vivo imaging. Small 14(15):1703919. https://doi.org/10.1002/smll.201703919
Zou GY, Chen S, Liu NZ, Yu YL (2021) A ratiometric fluorescent probe based on carbon dots assembly for intracellular lysosomal polarity imaging with wide range response. Chin Chem Lett 33(2):778–782. https://doi.org/10.1016/j.cclet.202108.076
Ge JC, Jia QY, Liu WM, Guo L, Liu QY, Lan MH, Zhang HY, Meng XM, Wang PF (2015) Red-emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv Mater 27(28):4169–4177. https://doi.org/10.1002/adma.201500323
Xu HB, Zhou SH, Xiao L, Wang HH, Li SZ, Yuan QH (2015) Fabrication of a nitrogen-doped graphene quantum dot from MOF-derived porous carbon and its application for highly selective fluorescence detection of Fe3+. J Mater Chem 3(2):291–297. https://doi.org/10.1039/C4TC01991A
Shi BF, Su YB, Zhang LL, Liu RJ, Huang MJ, Zhao SL (2016) Nitrogen-rich functional groups carbon nanoparticles based fluorescent pH sensor with broad-range responding for environmental and live cells applications. Biosens Bioelectron 82:233–239. https://doi.org/10.1016/j.bios.2016.04.003
Liu XX, Liu JR, Zheng BZ, Yan L, Dai JY, Zhuang ZJ, Du J, Guo Y, Xiao D (2017) N-doped carbon dots: green and efficient synthesis on a large-scale and their application in fluorescent pH sensing. New J Chem 41(19):10607–10612. https://doi.org/10.1039/C7NJ01889D
Wang K, Wang XY, Liu XY, Li ES, Zhao RS, Yang SH (2022) Facile synthesis of dual emission carbon dots for the ratiometric fluorescent detection of 2, 4, 6-trinitrophenol and cell imaging. J Mol Struct 1263(2022)133167. https://doi.org/10.1016/j.molstruc.2022.133167
Luo X, Yang HT, Wang HL, Ye ZW, Zhou ZN, Gu LY, Chen JQ, Xiao Y, Liang XW, Qian XH, Yang YJ (2018) Highly sensitive hill-type small-molecule pH probe that recognizes the reversed pH gradient of cancer cells. Anal Chem 90(9):5803–5809. https://doi.org/10.1021/acs.analchem.8b00218
Chen YC, Zhu CC, Cen JJ, Bai Y, He WJ, Guo ZJ (2015) Ratiometric detection of pH fluctuation in mitochondria with a new fluorescein/cyanine hybrid sensor. Chem Sci 6:3187–3194. https://doi.org/10.1039/C4SC04021J
Li B, Ma H, Zhang B, Qian J, Cao T, Feng HT, Li W, Dong YP, Qin WW (2019) Dually emitting carbon dots as fluorescent probes for ratiometric fluorescent sensing of pH values, mercury (II), chloride and cr (VI) via different mechanisms. Microchim Acta 186(6):1–10. https://doi.org/10.1007/s00604-019-3437-2
Lei XX, Fu YY, Wu Y, Chen L, Liang JG (2020) A ratiometric fluorescent probe for pH detection based on Ag2S quantum dots-carbon dots nanohybrids. Roy Soc Open Sci 7(7):200482. https://doi.org/10.1098/rsos.200482
Wang XY, Wang YS, Pan W, Wang JP, Sun XB (2021) Carbon-dot-based probe designed to detect intracellular pH in fungal cells for building its relationship with intracellular polysaccharide. ACS Sustain Chem Eng 9(10):3718–3726. https://doi.org/10.1021/acssuschemeng.0c08160
Shangguan JF, He DG, He XX, Wang K, Xu FZ, Liu JQ, Tang JL, Yang X, Huang J (2016) Label-free carbon-dots-based ratiometric fluorescence pH nanoprobes for intracellular pH sensing. Anal Chem 88(15):7837–7843. https://doi.org/10.1021/acs.analchem.6b01932
Chen YY, Zhao CX, Wang YY, Rao HB, Lu ZW, Lu CF, Shan Z, Ren B, Wu W, Wang XX (2020) Green and high-yield synthesis of carbon dots for ratiometric fluorescent determination of pH and enzyme reactions. Mat Sci Eng C 117(1–2):111264. https://doi.org/10.1016/j.msec.2020.111264
Song JQ, Zhao N, Qu Y, Zhao LS (2021) Natural deep eutectic solvent-assisted preparation of nitrogen-doped carbon dots for ratiometric determination of pirimicarb and pH. Dyes Pigm 193:109564. https://doi.org/10.1016/j.dyepig2021.109564
Li SR, Song XR, Hu ZR, Feng GD (2021) A carbon dots-based ratiometric fluorescence probe for monitoring intracellular pH and bioimaging. J Photoch Photobio A 409:113129. https://doi.org/10.1016/j.jphotochem.2021.113129
Funding
The authors acknowledge financial support from the National Natural Science Foundation of China (Nos. 22177066), Innovation Capability Support Program of Shaanxi (No. 2021KJXX-51), Coconstruction Project of Hanzhong city and Shaanxi University of Technology (SXJ-2103, SXJ-2105) and Open Foundation of Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education (KLSNFM2020007).
Author information
Authors and Affiliations
Contributions
Yanli Sun contributed to the methodology, investigations, probe preparation, data collection and curation, and writing-original draft preparation. Jin Liu, Zuoping Zhao, Lihua Li, and Zhifeng Liu contributed to methodology development, data collection, and sample pretreatment. Jiufu Lu and Lingxia Jin contributed to the formal analyses and validation. Qin Wang and Shengrui Zhang performed conceptualization, supervision, writing-reviewing and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no competing.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Wang, Q., Liu, J. et al. Ratiometric Sensing of Intracellular pH Based on Dual Emissive Carbon Dots. J Fluoresc 33, 653–661 (2023). https://doi.org/10.1007/s10895-022-03107-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03107-w