Abstract
A novel fluorescence probe for the detection of Al3+ was developed based on methionine protected gold nanoclusters (Met-AuNCs). A fluorescent Schiff base (an aldimine) is formed between the aldehyde group of salicylaldehyde (SA) and the amino groups of Met on the AuNCs, and developed for selective detection of Al3+ in aqueous solution. Al3+ can strongly bind with the Schiff base ligands, accompanied by the blue-shift and an obvious fluorescence emission enhancement at 455 nm. The limits of detection (LODs) of the probe are 2 pmol L−1 for Al3+. Moreover, the probe can successfully be used in fluorescence imaging of Al3+ in living cells (SHSY5Y cells), suggesting that the simple fluorescent probe has great potential use in biological imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal ion contamination has induced great threats to the human health and environment, especially when they are present at high concentrations. Among the various metal, aluminum is extensively used in many fields, such as medicines, cookware, food additives and aerospace, automobile and construction industries [1, 2], which results in the increasing of Al3+ concentration in the environment. Nevertheless, excess aluminum can induce some diseases due to its toxicity, such as Parkinson’s disease, Alzheimer’s disease and breast cancer [3,4,5,6]. Therefore, selective trace detection of Al3+ is of great importance for ecological environment and human health.
Numerous methods have been used to detect Al3+, such as chromatography, atomic absorption spectrometry, fluorescent chemical sensor, colorimetry and electrochemistry [7,8,9,10,11]. Particularly, fluorescent chemical sensor has been paid considerable attention with advantages of superb selectivity, high sensibility, non-invasiveness and low cost [12,13,14,15,16]. Nevertheless, fluorescent chemical sensor has many disadvantages, such as complex synthetic routes, poor water solubility and being unsuitable for bioimaging, which make it difficult to be used easily and conveniently. It is known that Schiff base derivatives fluorescent probes are highly appealing for optical sensing of Al3+ due to their strong coordination with metal ions and good water solubility [16,17,18,19,20,21,22]. However, the synthesis of Schiff base derivatives generally suffers from sophisticated and tedious preparation process and strict experimental conditions.
Fluorescent gold nanoclusters (AuNCs) have been extensively used in chemo/biosensing and imaging due to their low toxicity, good biocompatibility, easy synthesis, excellent chemical stability and photostability. Despite many small biomolecules, such as glutathione, tyrosine, L-proline and methionine have been applied in the preparation of AuNCs [23,24,25,26,27,28,29], a facile and rapid synthesis of fluorescent AuNCs still remains a great challenge.
The aim of the present work is the construction of a fluorescent switching assay for the detection of Al3+ via a donor-π conjugation-acceptor (D-π-A) Schiff base as the linker. Firstly, fluorescent AuNCs modified with Met can be rapidly prepared using a domestic microwave oven within 50 s. Further, a Schiff base can be formed between the amino (-NH2) group of Met on the AuNCs surface and aldehyde (-CHO) group in salicylaldehyde. Al3+ is capable of coordination with Schiff base ligands, which leads to a blue shift of the fluorescence peak from 500 to 455 nm and significant fluorescence emission enhancement at 455 nm. Therefore, the new fluorescence assay for monitoring Al3+ has the advantages of rapidity, simplicity, high sensitivity, which has been further applied in living cell imaging.
Materials and Methods
Reagents
HAuCl4·4H2O and L-methionine were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Salicylaldehyde (SA) was bought from Aladdin Ltd. (Shanghai, China). rhodamine 6G was bought from Sangon Biotechnology Company, Ltd. (Shanghai, China). NaOH and other inorganic salts of cations were obtained from Laiyang Fine Chemical Plant (Shandong, China). All aqueous solutions were prepared with high purity deionized water (18.2 MΩ cm−1).
Microwave Synthesis of Met-AuNCs
All glasswares were cleaned with aqua regia (HCl/HNO3 3:1 v/v) and rinsed thoroughly with deionized water. 200 µL HAuCl4 solution (10 g L−1) was firstly mixed with 1 mL methionine solution (0.18 mol L−1). And then, the mixture was heated by microwaves for 50 s at 800 W in a domestic microwave oven and slowly cooled to ambient temperature. Afterwards, the solution was centrifugated at 10,000 rpm for 10 min to remove impurities.
Detection of Al3+ Based on SA-Met-AuNCs Complex
Firstly, 94 nmol L−1 SA was added into 100 µL Met-AuNCs solution. Then, 0.2 mol L−1 NaOH was added into the above mixture solution, which was mixed and incubated for 30 min at room temperature. Then, Al3+ solutions with various concentrations were added into the mixture and reacted for 1 min. The fluorescent spectra with an excitation wavelength at 370 nm and an emission wavelength at 455 nm were recorded.
Fluorescence Cellular Imaging
SHSY5Y cells were inoculated a 12-well plate and adhered 24 h at 37 °C and 5% CO2. Then these cells were incubated with SA-Met-AuNCs complex for 2 h at 37 °C and fixed with methanol, and then washed three times with PBS buffer (0.1 mol L−1, pH 7.4) prior to imaging. And then, Al3+ was added into the precultured cells with SA-Met-AuNCs complex, cultured for 30 min at 37 °C, fixed with methanol and washed with PBS buffer for three times, then imaging.
Results and Discussion
Preparation and Characterization of Met-AuNCs
A household microwave furnace is used to prepare Met-AuNCs in order to achieve fast and efficient synthesis [30,31,32]. Different conditions for microwave-facilitated prepared of Met-AuNCs were optimized (Fig. S1). We found that microwave-assisted synthesis of Met-AuNCs at 800 W took only 50 s. As shown in Fig. 1A, Met-AuNCs prepared with microwave irradiation exhibit much sharper UV/vis absorption band and a sharp emission peak at 565 nm upon 370 nm excitation (Fig. 1B). A luminescence QY of 16.8% is attained (relative to rhodamine 6G in ethanol, Fig. S2). Figure 1C shows the TEM and HRTEM images of microwave-synthesized Met-AuNCs, which reveals that the AuNCs are well-dispersed. The average diameter is about 3.5 nm and the clear lattice fringe is about 2.34 Å, corresponding to the d-spacing of the (111) lattice plane of face-centered cubic Au. XRD is performed to characterize the crystal structure of Met-AuNCs (Fig. 1D). Four peaks at 2θ = 38.2, 44.37, 64.62 and 77.68º are observed, corresponding to the diffractions from the (111), (200), (220), and (311) planes of the face-centered cubic Au, which is in accordance with TEM results. XPS of Met-AuNCs is carried out to determine the valence states of Au and the data are shown in Fig. 1E. The binding energy (BE) of the Au 4f5/2 and Au 4f7/2 at 87.8 and 84.1 eV is observed in Au 4f XPS spectrum, respectively, demonstrating that both Au+ and Au0 exist in Met-AuNCs. Moreover, Met-AuNCs demonstrate a good photostability between pH 4 and 9 and possess high salt stability even in 1 M NaCl (Fig. S3). Therefore, the results demonstrate that Met-AuNCs have been successfully prepared with microwave irradiation.
Feasibility of Detection of Al3+ Based on SA-Met-AuNCs
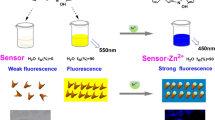
A fluorescence switch for the determination of Al3+ using SA-Met-AuNCs was designed as illustrated in Scheme 1. Upon the addition of SA into Met-AuNCs, SA-Met-AuNCs complex is formed under aqueous solution by a Schiff base reaction (-C = N-) between the amino (-NH2) group of Met on the Met-AuNCs surface and the aldehyde (-CHO) group of SA [33, 34]. And that, the Schiff base (-C = N-) of the SA-Met-AuNCs has a fluorescent emission peak at around 500 nm. When Al3+ is further added into SA-Met-AuNCs complex, there is a coordination interaction among Al3+, the imine groups (C = N) of Schiff base, carboxyl group (-COOH) of Met and the hydroxyl groups (-OH) of SA. The coordination interaction reduces charge transfer efficiency and restrains the isomerization of C = N, which can cause blue-shift of fluorescent peak wavelength of Schiff base from 500 to 455 nm, accompanied by a remarkable fluorescence enhancement around 455 nm. Thereby, an ultrasensitive fluorescent assay is proposed for Al3+ detection based on the change in fluorescent intensity at 455 nm.
UV–vis and fluorescence spectroscopy are carried out to further investigate the feasibility of the fluorescent method. As displayed in Fig. 2A, a new absorption peak at 392 nm in a range of 300–500 nm is observed after the addition of SA into Met-AuNCs, which can be attributed to the C = N absorption band of SA-Met-AuNCs complex [35]. Upon successive addition of Al3+ ions, the band at 392 nm was blue-shifted to 342 nm, demonstrating the formation of a new compound between Al3+ and SA-Met-AuNCs complex. Fluorescence investigation results (Fig. 2B) demonstrate a new characteristic peak of Schiff base centered at 500 nm after adding SA into Met-AuNCs [36], accompanied by an obvious fluorescence color change from pink into green under UV lamp (Fig. 2A). After the addition of Al3+ to the SA-Met-AuNCs complex solution, a blue-shift in the emission peak of Schiff base from 500 to 455 nm is also observed (Fig. 2B). Concomitantly, the fluorescence intensity around 455 nm enhances dramatically, along with a noticeable fluorescence color change from green into blue (Fig. 2A), which is attributed to the formation of a Al3+ and SA-Met-AuNCs complex. Hence, all above results affirm that SA-Met-AuNCs complex fluorescent sensor can detect Al3+ with high sensitivity and high selectivity.
A UV–vis absorption spectra of SA (a), Al3+ (b), Met-AuNCs (c), Met-AuNCs + SA (d) and Met-AuNCs + SA + Al3+ (e). Inset: corresponding photographs image under UV light (λex = 365 nm); B Fluorescence emission spectra of Met-AuNCs, Met-AuNCs + Al3+, Met-AuNCs + SA, Met-AuNCs + SA + Al3+, SA, SA + Al3+
Optimization of the Conditions
The main experimental parameters including pH, incubation time between SA and Met-AuNCs and SA concentration are optimized for Al3+ detection. pH is very important for the formation of a Schiff base between the Met-AuNCs and SA. As shown in Fig. S4A, the maximum fluorescence intensity is observed when 0.2 mol L−1 NaOH is added, which is therefore used in all subsequent experiments. The fluorescence intensity at 500 nm increases with the increase of SA concentration (Fig. S4B). Simultaneously, the solution color under UV lamp change from red to orange, and then to green (Fig S4C). When 94 nmol L−1 SA is added, the obvious fluorescence solution color changes can be observed, implying that SA is excessive. Therefore, 94 nmol L−1 SA is used for selective and sensitive detection of Al3+. The results in Fig. S4D demonstrate that the fluorescence intensity at 500 nm decreases gradually as an increase of the incubation time between SA and Met-AuNCs, and then tends to level off after 30 min. Accordingly, 30 min incubation time is chosen for the next experiments.
Sensitive Detection of Al3+
At the optimal conditions, the sensing performance of SA-Met-AuNCs complex probe toward Al3+ was investigated. As shown in Fig. 3A, the fluorescence intensity of the probe at 455 nm enhances obviously with increase of Al3+ concentration. As demonstrated in Fig. 3B, a good linear relationship is obtained between the fluorescence intensity at 455 nm and Al3+ concentration over the 0.002 to 1.6 µmol L−1 range. The limit of detection (LOD) was estimated to be 2 pmol L−1 (3α/S, where α and S represent the standard deviation of blank and slope of the linear plot), which is far below the permitted level in drinking water permitted by the World Health Organization (WHO) (7.41 µmol L−1). Moreover, Table S1 summarizes some other reported sensors for the analysis of Al3+, which demonstrates that the assay is comparable or superior to the previous approaches. Hence, all the results suggest that the developed fluorescent probe is sensitive enough to monitor Al3+ in the drinking water.
Selectivity of Al3+ Detection
Selectivity is another important characteristic of a sensor for metal ions. To evaluate the high selectivity of the fluorescent probe for Al3+, the fluorescence responses of the assay were carried out in the presence of various metal cations under the same experimental condition. As shown in Fig. 4, for the detection of Al3+, no fluorescence response at 455 nm is observed upon addition of other metal ions even with high concentration (50 µmol L−1). Moreover, the mixture of Met-AuNCs, SA and Al3+ demonstrated almost no fluorescence variation against other metal cations. These results demonstrated that the simple D‑π‑A Schiff base probe exhibited a strong selectivity and could be as a fluorescent probe for sensing Al3+ over all other metal ions.
Real Sample Analysis
To evaluate the accuracy of the developed method, we applied it to the detection of Al3+ in lake water. The lake water was filtered through a 0.45 μm filter prior to measurement. Al3+ was spiked into the lake water at different concentrations. The results have been shown in Table 1. Satisfactory recoveries of the assay are 98–106% for Al3+, which further indicate the suitability of the assay for the determination of Al3+ in real samples.
Cell Imaging
To investigate the biological applications of the strategy, cellular uptake and fluorescence imaging of SA-Met-AuNCs to detect Al3+ were conducted using SHSY5Y cells as a model. As displayed in Fig. 5A, the intracellular imaging of SHSY5Y cells display no fluorescence signals. However, the cells display a pronounced blue fluorescence image inside the cells (Fig. 5B), when SHSY5Y cells were pretreated with SA-Met-AuNCs and Al3+ (1 µmol L−1) for 30 min under the same conditions. Hence, the results verify the internalization of the probe and its binding to Al3+. Moreover, no morphological changes can be seen in cells after pretreatment with probe, which demonstrates that the probe has a good membrane permeability and can be applied to detection of Al3+ in living cells.
Conclusions
In summary, a microwave-assisted method was applied in the synthesis of Met-AuNCs, which dramatically improved the QY of AuNCs and shortened the reaction time. Remarkably, a new Schiff base was formed between the amino groups of Met on the as-prepared AuNCs surface and aldehyde groups of SA, which exhibited a highly selective fluorescence response to Al3+ in aqueous media. Moreover, it had a good cell permeability and low cytotoxicity, which made it possible to monitor intracellular Al3+ in the living cells with high selectivity.
Data Availability
All data generated or analyzed of this study are available within the article.
Code Availability
Not applicable.
References
Zhao Y, Lin Z, Liao H, Duan C, Meng Q (2006) A highly selective fluorescent chemosensor for Al3+ derivated from 8-Hydroxyquinoline. Inorg Chem Commun 9:966–968
Li Y, Niu Q, Wei T, Li T (2019) Novel thiophene-based colorimetric and fluorescent turn-on sensor for highly sensitive and selective simultaneous detection of Al3+ and Zn2+ in water and food samples and its application in bioimaging. Anal Chim Acta 1049:196–212
Fasman GD (1996) Aluminum and Alzheimer’s Disease: Model studies. Coord Chem Rev 149:125–165
Perl DP, Gajdusek DC, Garruto RM, Yanagihara RT, Gibbs CJ (1982) Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and parkinsonism-dementia of guam. Science 217:1053–1055
Wen X, Fan Z (2016) Linear schiff-base fluorescence probe with aggregation-induced emission characteristics for Al3+ detection and its application in live cell imaging. Anal Chim Acta 945:75–84
Faller P, Hureau C (2012) A bioinorganic view of alzheimer’s disease: When misplaced metal ions (Re) direct the electrons to the wrong target. Chem A Eur J 18:15910–15920
Liu B, Wang P, Chai J, Hu X, Gao T, Chao J, Chen T, Yang B (2016) Naphthol-based fluorescent sensors for aluminium ion and application to bioimaging. Acta A Mol Biomol Spectrosc 168:98–103
Xing B, Zhu W, Zheng X, Zhu Y, Wei Q, Wu D (2018) Electrochemiluminescence immunosensor based on quenching effect of SiO2@PDA on SnO2/rGO/Au Nps-luminol for insulin detection. Sens Actuators B Chem 265:403–411
Yang L, Li Y, Zhang Y, Fan D, Pang X, Wei Q, Du B (2017) 3D nanostructured palladium-functionalized graphene-aerogel-supported Fe3O4 for enhanced Ru(bpy)32+-based electrochemiluminescent immunosensing of prostate specific antigen. ACS Appl Mater Interfaces 9:35260–35267
Ren X, Zhang T, Wu D, Yan T, Pang X, Du B, Lou W, Wei Q (2017) Increased electrocatalyzed performance through high content potassium doped graphene matrix and aptamer tri infinite amplification labels strategy: Highly sensitive for matrix metalloproteinases-2 detection. Biosens Bioelectron 94:694–700
Maity D, Govindaraju T (2012) A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem Commun Camb 48:1039–1041
Chandra R, Manna AK, Rout K, Mondal J, Patra GK (2018) A dipodal molecular probe for naked eye detection of trivalent cations (Al3+, Fe3+ and Cr3+) in aqueous medium and its applications in real sample analysis and molecular logic gates. RSC Adv 8:35946–35958
Fan L, Qin J, Li T, Wang B, Yang Z (2014) A novel rhodamine chromone-based “off-on” chemosensor for the differential detection of Al(III) and Zn(II) in aqueous solutions. Sens Actuators B Chem 203:550–556
Cao W, Zheng X, Sun J, Wong W, Fang D, Zhang J, Jin L (2014) A highly selective chemosensor for Al(III) and Zn(II) and its coordination with metal ions. Inorg Chem 53:3012–3021
Jiang J, Jiang H, Tang X, Yang L, Dou W, Liu W, Fang R, Liu W (2011) An efficient sensor for Zn2+ and Cu2+ based on different binding modes. Dalton Trans 40:6367–6370
Shellaiah M, Wu Y, Lin H (2013) Simple pyridyl-salicylimine-based fluorescence “turn-on” sensors for distinct detections of Zn2+, Al3+ and OH- ions in mixed aqueous media. Analyst 138:2931–2942
Wang L, Qin W, Liu W (2010) A sensitive schiff-base fluorescent indicator for the detection of Zn2+. Inorg Chem Commun 13:1122–1125
Udhayakumari D, Saravanamoorthy S, Ashok M, Velmathi S (2011) Simple imine linked colorimetric and fluorescent receptor for sensing Zn2+ ions in aqueous medium based on inhibition of ESIPT mechanism. Tetrahedron Lett 52:4631–4635
Gupta VK, Singh AK, Ganjali MR, Norouzi P, Faridbod F, Mergu N (2013) Comparative study of colorimetric sensors based on newly synthesized schiff bases. Sens Actuators B Chem 182:642–651
Al Zoubi W, Al Mohanna N (2014) Membrane sensors based on schiff bases as chelating ionophores - A review, Spectrochim. Acta A Mol Biomol Spectrosc 132:854–870
Kumar A, Dubey M, Pandey R, Gupta RK, Kumar A, Kalita AC, Pandey DS (2014) A schiff base and its copper(II) complex as a highly selective chemodosimeter for mercury(II) involving preferential hydrolysis of aldimine over an ester group. Inorg Chem 53:4944–4955
Manna AK, Chowdhury S, Patra GK (2020) Combined experimental and theoretical studies on a phenyl thiadiazole-based novel turn-on fluorescent colorimetric Schiff base chemosensor for the selective and sensitive detection of Al3+. New J Chem 44:10819–10832
Li QR, Zhou R, Sun Y, Xiao D, Liu M, Zhao D, Peng S, Chen Y, Lin Y (2021) Synthesis and antitumor application of antiangiogenetic gold nanoclusters. ACS Appl Mater Interfaces 13:11708–11720
Li D, Liu Q, Qi Q, Shi H, Hsu EC, Chen W, Yuan W, Wu Y, Lin S, Zeng Y, Xiao Z, Xu L, Zhang Y, Stoyanova T, Jia W, Cheng Z (2020) Gold nanoclusters for NIR-II fluorescence imaging of bones. Small 16:e2003851
Yang X, Luo Y, Zhuo Y, Feng Y, Zhu S (2014) Novel synthesis of gold nanoclusters templated with L-tyrosine for selective analyzing tyrosinase. Anal Chim Acta 840:87–92
Khan IM, Niazi S, Yu Y, Mohsin A, Mushtaq BS, Iqbal MW, Rehman A, Akhtar W, Wang Z (2019) Aptamer induced multicolored AuNCs-WS2 “turn on” FRET nano platform for dual-color simultaneous detection of aflatoxinB1 and zearalenone. Anal Chem 91:14085–14092
Sang F, Li M, Yin S, Shi H, Zhao Y, Zhang Z (2021) Highly sensitive and selective detection and intracellular imaging of glutathione using MnO2 nanosheets assisted enhanced fluorescence of gold nanoclusters. Spectrochim Acta A Mol Biomol Spectrosc 256:119743
Sang F, Zhang X, Shen F (2019) Fluorescent methionine-capped gold nanoclusters for ultra-sensitive determination of copper (II) and cobalt (II), and their use in a test strip. Microchim Acta 186:1–9
Xiu L, Huang K, Zhu C, Zhang Q, Peng H, Xia X, Chen W, Deng H (2021) Rare-Earth Eu3+/gold nanocluster ensemble-based fluorescent photoinduced electron transfer sensor for biomarker dipicolinic acid detection. Langmuir 37:949–956
Bilecka I, Niederberger M (2010) Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2:1358–1374
Larhed M, Moberg C, Hallberg A (2002) Microwave-accelerated homogeneous catalysis in organic chemistry. Acc Chem Res 35:717–727
Shang L, Yang L, Stockmar F, Popescu R, Trouillet V, Bruns M, Gerthsen D, Nienhaus GU (2012) Microwave-assisted rapid synthesis of luminescent gold nanoclusters for sensing Hg2+ in living cells using fluorescence imaging. Nanoscale 4:4155–4160
Gharagozlou M, Boghaei DM (2008) Interaction of water-soluble amino acid schiff base complexes with bovine serum albumin: Fluorescence and circular dichroism studies. Spectrochim Acta A Mol Biomol Spectrosc 71:1617–1622
Faiz Ur R, Ali A, Guo R, Tian J, Wang H, Li Z, Zhang D (2015) Methionine-derived schiff base as selective fluorescent “turn-on” chemosensor for Zn2+ in aqueous medium and its application in living cells imaging. Sens Actuators B Chem 211:544–50
Kumar A, Asthana SK, Upadhyay KK (2016) A dichloro-substituted salicylimine as a bright yellow emissive probe for Al3+. J Photochem Photobiol A 329:69e76
Liu X, Fu C, Ren X, Liu H, Li L, Meng X (2015) Fluorescence switching method for cascade detection of salicylaldehyde and zinc(II) ion using protein protected gold nanoclusters. Biosens Bioelectron 74:322–328
Funding
This work was funded by the Natural Science Foundation of China (NSFC) (No. 21407035), Shandong Provincial Natural Science Foundation (ZR2014BM021).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Writing-original draft preparation, data collection and analysis were performed by [Fuming Sang]. Writing-original draft preparation, data collection and editing: [Tiedan Xiong]. Material preparation, data collection and analysis: [Weijie Wang]. Data collection and analysis: [Jianxin Pan]. Material preparation: [Huahua Shi]. Writing-review and editing: [Yan Zhao]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sang, F., Xiong, T., Wang, W. et al. A Simple Schiff Base as Fluorescent Probe for Detection of Al3+ in Aqueous Media and its Application in Cells Imaging. J Fluoresc 33, 177–184 (2023). https://doi.org/10.1007/s10895-022-03047-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-03047-5