Abstract
Quantum dots, or nanoscale semiconductors, are one of the most important materials for various research and development purposes. Due to their advantageous photoluminescence and electronic properties, namely, their unique photostability, high brightness, narrow emission spectra from visible to near-infrared wavelengths, convey them significant advantages over widely used fluorochromes, including organic dyes, fluorescent probes. Quantum dots are a unique instrument for a wide range of immunoassays with antibodies. The paper provides an overview of the developed and already applied methods of quantum dot surface modification, quantum dots conjugation to different antibodies (non-covalent, direct covalent linkage or with the use of special adapter molecules), as well as practical examples of recent quantum dot-antibody applications in the immunofluorescence microscopy for cell and cell structure imaging, fluorescent assays for biomolecules detection and in diagnostics of various diseases. The review presents advantages of quantum dot-antibody conjugation technology over the existing methods of immunofluorescence studies and a forward look into its potential prospects in biological and biomedical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Luminescent semiconductor nanocrystals, so-called quantum dots (QDs), are currently considered as promising materials for a variety of research and development [1, 2]. Such nanocrystals, most often spherical in morphology, have a semiconductor core formed by elements of groups II-VI or III-V of the periodic table, surrounded by a shell consisting of other semiconductor materials. Typically, quantum dots contain from 100 to 10,000 atoms, have a diameter in the range of 2–10 nm and are characterized by unique optical and optoelectronic properties [3]. In particular, semiconductor quantum dots absorb light in a wide range, from near ultraviolet to the red region of the spectrum. The spectrum of their radiation is quite narrow and depends on the size of QDs [4, 5]. In addition, they are extremely photostable, which gives them significant advantages over widely used fluorochromes, in particular, with organic dyes, fluorescent proteins, lanthanide chelates, etc. [6, 7]. Given such unique photophysical properties, QDs are considered as a new class of inorganic fluorophores that have begun to be rapidly tested and used in a variety of research and development. In particular, they are used in optoelectronics as LED elements, solar panels, and in biological and biomedical research to visualize individual cells, intracellular structures, toxin detection, immunofluorescent labeling of proteins, antitumor therapy, diagnostics (teranostics), etc. [4, 5, 8,9,10,11,12,13]. One of the major directions in the use of such luminescent nanostructures as fluorescent labels in biological research is related not only to the development of methods for the QDs’ synthesis (chemical or “green” synthesis using different biomatrices) [4, 5], but also to improvement of methods for modifying their surface for further effective binding to target compounds or certain biomolecules [14, 15]. The conjugation process can be achieved either through various direct chemical reactions between functional groups on the surface of QDs and organic compounds, or through the use of specific linkers that perform as bridges between QDs and certain substances, or by bio-recognitions (avidin-biotin interaction) [16,17,18]. Therefore, it is important to develop effective methods for obtaining QD-antibody(Ab) conjugates, as they can be used in bioimaging, diagnostics of various diseases and a targeted therapy. In particular, antibodies can effectively target the QD-Ab conjugates to their targets, significantly reducing both the dosage and possible side effects of QDs that may occur on non-target sites during transport in the body. Compared to free nanoparticles, the nanoparticles conjugated to antibodies are more site-specific, which results in their greater accumulation in the target areas and, consequently, allows to reduce their dosing. The existing methods of direct and indirect immunofluorescence microscopy using primary antibodies against different types of antigens (both of plant and animal origin) and secondary antibodies labeled with fluorescent labels, such as FITC, TRITC, Cy2, Cy3, Alexa Fluor, etc. [19,20,21,22,23] are characterized with low photostability, long time requirements to perform and high self-costs. Consequently, the search for new photostable, non-toxic, cheaper probes using quantum dots, and the development of effective methods based on bioimaging and fluorescence microscopy is a topical issue for biological and medical research.
The objective of this review is to outline the progress in obtaining QD-Ab conjugates, their direct application in immunofluorescence microscopy, methodological challenges in preparing such conjugates that have already been overcome, as well as potential prospects for the application of such composites in biology and medicine. It also discusses the potential for further development of this area, its applicability in other technologies and identifies barriers that limit further progress, including insufficiently studied surface chemistry of QDs, the potential for changes in the functions of biological molecules in combination with QDs, their conjugates, etc.
Antibody Conjugation to Nanoparticles
There are several strategies for conjugating QDs with antibodies, specifically, non-covalent (adsorption by electrostatic interaction at the isoelectric point of the antibody), direct covalent bonding or with the use of special adapter molecules (mainly streptavidin and biotin) [17, 18] (Fig. 1). In case of non-covalent conjugation (Fig. 1, A) biomolecules (mainly oligonucleotides and various serum albumins) can be adsorbed in a non-specific manner to the surface of the soluble QDs depending on pH, temperature, ionic strength and surface charge of the molecule. The QDs surface charge is determined by the free surface reactive groups and plays an important role in ensuring electrostatic interaction and the possibility of conjugation. This way of obtaining conjugates provides good photoluminescence but at the same time limits the use in biological environment since electrostatic interaction is unspecific and is weaker than covalent binding [24]. A number of biomolecules (including antibodies) have a thiol group that allows them to participate in a mercapto metabolism, which leads to conjugation with QDs, although the resulting bond is rather weak and dynamic [24].
An electrostatic non-covalent self-assembly approach can be used to conjugate QDs with antibodies via bridging proteins (positive charge, neutral or engineered) [16, 25]. The first step is to add a positively charged leucine zipper interaction domain to the C-terminus of recombinant proteins (bridging proteins) to enable its interactions with dihydrolipoic acid (DHLA)-capped QDs. For example, the immunoglobulin G-binding β2 domain of streptococcal protein G, modified by genetic fusion with a positively charged leucine zipper interaction domain, is used as a bridging protein for the QD-antibody conjugation. Another way of QD-Ab conjugation involves the adsorption on the QD surface of a genetically linked protein that binds avidin and maltose, and a positively charged domain of leucine zipper interaction with the QD surface [16]. Mixed surface approaches using antibody-bridging proteins that are further immobilized on each QD and further purification are quite simple and reproducible. At the same time this approach has some disadvantage due to the charge-induced agglomeration, therefore there is a limit to the capacity of QD surface assembled avidin molecules.
One of the simplest, albeit expensive, methods for conjugating QDs with antibodies is affinity-based conjugation with streptavidin (avidin) and biotin [26,27,28,29]. The chemical structure of QDs surface allows to conjugate with bioligands (proteins, nucleic acids, peptides etc.). Streptavidin can react with biotinylated ligands which allows conjugation. Moreover, this process is quite simple and requires only for two materials to be mixed together. Conjugation of biotinylated immunoglobulin G antibodies with streptavidin-coated QDs was also performed [27]. In that case, antibodies had four to eight biotin molecules attached to them at random locations, resulting in conjugation of biotinylated antibodies with QDs in all possible spatial formations. Due to the high affinity of streptavidin and biotin, the reaction usually ends up with fully saturated products which makes it difficult to optimize the characteristics of the conjugates [28, 29]. Other functional carboxyl or amine groups are less commonly used, though available. Such a complex procedure of conjugation can cause changes in the conformation of antibodies and reduce the ability of the antigen to recognize. This method of conjugates obtaining can also be dependant on the pH that manifests in lowering the nanoparticles stability in acidic environment and presents poor biospecificity.
Proteins are often used for modifying the surface of QDs, and bovine serum albumin (BSA) is one of the most commonly used agents for this purpose [30, 31]. Successful application of BSA for modifying QDs and further conjugation with antibody was recently performed by Sahoo et al. [31]. BSA was used as a stabilizing agent for CdSe/ZnS QDs via a two step-route. The first step was the activation of the QDs carboxyl groups by carbonyldiimidazole (CDI) linker, and the second step was the BSA conjugation with the activated QDs. After that, the antibodies against human immunoglobulin were oxidized by sodium periodate to form reactive aldehyde groups to enable a spontaneous reaction with the amine groups of previously modified BSA-QDs. The final step was to mix the BSA-modified QDs with the oxidized anti-human IgG antibodies [31].
Covalent conjunction of QDs with antibodies using the commercially available conjugation kits is also often applied [27, 32]. For example, immunoglobulin G was reduced by dithiothreitol resulting in formation of three types of chains (25 kD light, 50 kD heavy and a 75 kD partially cleaved chain which is basically the light and the heavy chains grouped together by an unreduced disulfide bond) [27]. Subsequently, these fragments were covalently conjugated by succinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (SMCC) linkage bond producing three types of QD-Ab conjugates, respectively [27]. Reducing antibodies to smaller fragments prior to conjugation instead of the whole molecule is what distinguishes this method from the biotin-streptavidin-mediated approach. Tada et al. [32] performed QD conjugation with trastuzumab using Qdot 800 Antibody Conjugation Kit, wherein probes were functionalized with amine-derivatized polyethylene glycol (PEG). Activation of QDs was also performed by SMCC, the reduction and fragmentation of antibody was followed with the covalent coupling. H-mercaptoethanol was used for the reaction attenuation [32]. SMCC-mediated conjugation was also performed using the QD605 (aminefunctionalized CdSe/ZnS) and monoclonal antibody against a prostate stem cell antigen (PSCA) [33]. Despite considerable advantages of these approaches there is still an issue with relatively low number of functional antibodies gained compared to streptavidin-avidin conjugates.
Another approach to QD-Ab conjugation is the coupling via direct covalent interactions of biomolecule residues (Fig. 1, B). An example of this can be a conjugation using carboxyls and primary amines. This approach allows to optimize the characteristics of the obtained materials via the alteration of QDs’ surface density in the group of conjugated ligand. For example, this method modified with the addition of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) was successfully applied for obtaining of highly fluorescent QD-Ab conjugates with high biological activity [34]. This method of coupling carboxylamines to form QD-Ab conjugate is quite unpredictable, so despite its simplicity and cost-effectiveness, it is not widely used nowadays. Conjugation through targeted covalent binding can provide stable QD-Ab conjugates with control of available protein binding sites. The CdSe/CdS/ZnS nanoparticles in the core/shell were modified with the 3-mercaptopropionic acid (MPA) and mixed with the ligand solution to launch the substitution of the surface-bound MPA with ligand, resulting in the zwitterionic ligand-capped QD nanoparticles [35]. The isolated immobilization of the whole antibody to QD nanoparticles capped with the zwitterionic ligand was performed by covalently binding the QDs to the intermediate layer of protein A and subsequent immobilization of the antibody to this layer [35].
The selection of an appropriate binding agent for the synthesis of antibody conjugated QDs is an important step that can determine the size and properties of QD-Ab conjugates [36]. Investigation of the effect of the three different coupling methods for the synthesis of conjugated QDs-anti-HER2 antibodies (HER2Ab-QDs) on the formed QD-Ab conjugates, which stain the ability of human epidermal growth factor receptor 2 (HER2) to overexpress breast cancer cell lines, was conducted by Tiwari et al. [36]. Several coupling methods were used, namely, EDC/sulfo-3-sulfo-N-hydroxysuccinimide sodium (NHS) salt, iminothiolane/sulfo-SMCC, and sulfo-SMCC coupling. The EDC coupling method enables blocking of the antibody’s antigen-binding sites by non-selective formation of amide bonds in the Fab region of the antibody. Therefore, SMCC was used as a substitute for the selective conjugation of partially reduced antibodies to QDs. In contrast to these covalent QD-Ab conjugation methods, a noncovalent conjugation of streptavidin-coated QDs and biotinylated antibodies was also tested. As a result, three types of QD-Ab conjugates were obtained with different hydrodynamic size, dispersibility and molecular weights. At the same time, they possessed negative feauters like relative instability (no more then 2 months of complex stability), limited number of antibodies that can bind to QDs due to steric hindrance between antibody molecules [36]. Water dispersible and NIR luminescent Si-QDs, functionalized with amino and epoxy groups, were obtained using silane binders [37]. Three types of silane binders were applied, specifically, (3-aminopropyl) dimethylethoxysilane (APDMES), (3-aminopropyl) trimethoxysilane (APTMS), and (3-glycidyloxypropyl) trimethoxysilane (GOPTS). These amino-functionalized QDs were further successfully conjugated to IgG antibodies featuring high sensitivity and compatibility with bioimaging. However this method demonstrated a certain level of nonspecific absorption, which impacts the sensitivity of the antigen detection [37].
Conjugation of QDs is performed not only with full size Abs but also with partially reduced antibodies or their single domain fragments (sdAbs) (Fig. 2). Preparation of QDs conjugates with full size, partially reducesd fragments and single domain Abs was suggested by Bilan et al. [18]. Full size Abs can be partially reduced by 2-mercaptoethanol-amine-HCl or dithiothreitol at low concentrations. To ensure the functional activity the Abs fragments were conjugated with QDs using the SMCC. It was also used the approach to integrate cysteine residue into the C-terminus of sdAbs for their covalent conjugation with QDs via the thiol group of the cysteine residue. Such sdAbs were conjugated to QDs that had amino groups on their surface. In the case when QDs had only hydroxyl groups on their surface the conjugation with sdAbs was performed using the p-maleimidophenyl isocyanate (PMPI) crosslinking agent. Studies have shown that the obtained QDs conjugates with sdAbs are very efficient for detection of the cell-surface cancer biomarkers CEA and HER2 both on live cells and on fixed tissue specimens [18].
Advanced strategies to targeted bioconjugation are being developed to avoid antigen-biding sites. These should provide an increased likelihood of interaction with antigens, and therefore more accurate targeting tools. One of the approaches is to form aldehyde groups by oxidizing the carbohydrate moieties on the Fc region of the antibody. These aldehyde groups may chemically bind to hydrazide-activated surfaces. This method allows two antibody Fab regions to interact with antigens and does not dissociate the framework of the antibody. The comparison of the non-oriented and oriented conjugation was suggested by Zhang et al. [38]. The synthesized hydrophobic CdSe/ZnSe/ZnS core/shell QDs were transferred into water phase by replacing hydrophobic ligands with glutathione (GSH) molecules. Аdditional stability of the GSH-coated QDs was achieved by adding so called spacer arms (bifunctional carboxyl PEG molecules). Both GSH-coated and PEGylated QDs with carboxy groups were activated by EDC·HCl and sulfo-NHS, and then coupled with antibodies. GSH-coated QDs with amino group(s) were then functionalized by succinimidyl valerate-PEG-maleimide (SMPEG) and coupled with free thiol antibody fragments. [38]. The successful QDs conjugation to antibodies was observed both with the oriented (SMPEG method) and non-oriented (EDC method) bioconjugation. However, higher detection sensitivity was achieved for the QD-Ab conjugates obtained with oriented bioconjugation. PEGylation was also useful for reducing non-specific binding and maintaining the biological activity of QD-Ab conjugates [38].
QD-Ab conjugates need to maintain their chemical and optical stability as well as biochemical functionality, high sensitivity and compatibility with bioimaging. As we can conclude, the most suitable way to obtain QD-Ab conjugates that meet those requirements is covalent conjugation namely by avidin-streptovidin binding and the use of BSA for the sensitivity increase. It is also promising in terms of specific binding to use single domain fragments of Abs for conjugation with QDs. Although the methodology for obtaining conjugates has been experimentally established, the procedure still remains a challenge and the technology for preparing stable targeted fluorescent conjugates still needs to be improved.
QD-Antibody Conjugates Applied in Immunofluorescence Microscopy
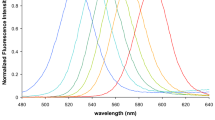
Immunofluorescence microscopy, as noted above, is a powerful method of detecting specific targets using fluorescent labeling, which is widely used in both research and clinical diagnostics. Organic dyes are most often used as fluorescent probes, but they quickly discolour and are not suitable for long-term observations. Therefore, semiconductor QDs are considered a promising tool for the replacement of organic fluorescent dyes due to their favorable physical and optical properties, in particular: wide excitation and narrow radiation spectra, which allow to excite different QDs simultaneously at the same wavelength; dependence of fluorescent radiation on the composition of QDs and the size of the nucleus; high photostability, which is very resistant to degradation and photobleaching; clear and symmetrical photoluminescence spectra, which provide better sensitivity; greater stokes shift of QDs, which significantly reduces their autofluorescence and increases sensitivity [7, 39,40,41].
QD-Ab сonjugates are currently one of the most promising fluorescent labels for intracellular imaging of individual structures compared to the existing similar tools. The results indicate that QD-based probes can be very effective in detecting cells because they provide a stable optical image both in vitro and in vivo [27, 42, 43]. For example, CdSe/ZnS core-shell QDs conjugated to anti- HER2 antibodies were used to establish a known marker for the identification of most breast cancers. Monoclonal anti- HER2 antibodies and biotinylated goat anti-human IgG secondary antibodies were used in this study to detect breast cancer cells SK-BR-3 [44]. Other authors have also used this approach to detect breast cancer cell lines (MCF-7, BT-474 and MDA-MB-231) using QDs conjugates with specific antibodies against five tumor markers, including HER2, estrogen receptor (ER), progesterone receptor (PR), epidermal growth factor receptor (EGFR) and mammalian target of rapamycin (m-TOR) [45]. Insulin-like growth factor receptor type I, involved in the proliferation and metastasis of breast cancer cells, has been identified using conjugates based on Cd/Te QDs and humanized anti-IGF1R monoclonal antibodies [46].
To detect thyroid carcinoma cells (SW1736), CdSe carboxyl QDs were associated with the specific antibody JT95 IgM. QD-JT95 conjugates directly detected the target molecules on the plasma membrane, and quantitative analysis showed the presence of antigen in the range of 1.56–100 g / mL [47].
A multifunctional nanoprobe has been developed using a specific antibody (anti-HER2) conjugated to RNase A-associated CdTe quantum dot cluster (HER2-RQDs). As a result of in vivo studies using the obtained nanoprobes, gastric cancer cells MGC803 were detected. It has also been shown that inhibition of cancer tissue growth by RNase A, which is released from HER2-QD nanoprobes and prevents protein synthesis and induces cell apoptosis [48]. QDs antibody conjugates (proteins p53 and Hsp70) have recently been found to have better photostability to track apoptosis in cancer cells over a longer period of time than organic dyes [49]. Also, a rapid multiplex approach has been proposed for the diagnosis of colorectal cancer using a combination of enzyme-sensitive fluorescent dye and AgInS2/ZnS QD conjugates with a specific antibody (matrix metalloproteinase-14) [50].
Most studies using QD antibody conjugates have been developed based on natively synthesized QDs, but commercial ones have also been used to immunolabeling cellular structures. Conjugates of QDs (CdSe/CdZnS, CdSe/CdS and InAs/CdZnS) with antibodies have been used for in vivo visualization of bone marrow cells. The resulting conjugates have been shown to diffuse into the bone marrow and effectively target individual cells belonging to rare populations of hematopoietic stem and progenitor cells (Sca1 + c-Kit + cells). The results obtained may be useful for studying the interactions between cells, as well as between the cell and its environment in intact and diseased tissues [28].
A new two-step approach to the functionalization and bioconjugation of QDs (InPZnS/ZnSe/ZnS, CdSe/ZnS, CdSeTe/ZnS) with the formation of ultracompact, stable and high-luminescent QDs conjugates with antibody fragments (usingfor immuno FRET) has been proposed [51]. To achieve better affinity, the surface of CdSe/ZnS QDs was modified with BSA and then with an antibody against human IgG expressed in recombinant CHO cells. Cell viability has shown that the developed conjugate is non-toxic and can be used to label cells and quantify antibodies in immunoassays [31]. Succinimidyl valerate-PEG-maleimide cross-linker (SMPEG) was used for targeted bioconjugation of antibodies on the surface of CdSe/ZnSe/ZnS QDs, which can significantly improve the sensitivity of detection of the resulting QD-antibody conjugates compared to those prepared by traditional methods [38].
Fluorescent immunoprobes have been developed using CdSe/ZnS QD and anti-morphine antibody conjugates that can be used as a convenient tool for rapid morphine detection [52]. A new immunofluorescent method for the detection of neutrophils and somatic cells using anti-bovine neutrophil elastase antibody and QD conjugates has been developed. Studies have shown that QD-BNE conjugate has better fluorescence intensity and stability than FITC conjugate and is better suited for detecting somatic cells in milk samples [53]. QD-based multiplex imaging technology using specific antibodies (anti-TLR4, anti-AR) was the first to develop diabetes mellitus in the kidney tissues of diabetic rats [54]. In vitro and in vivo assays for fluorescence imaging are presented in Table 1.
Immunofluorescence Staining of the Cytoskeleton
Several studies have reported the use of QD conjugates with specific antibodies to stain cytoskeletal structures, in particular microtubules – tubulin and microtubule associated proteins. Microtubules are polymeric structures with a diameter of 24 nm, consisting of the heterodimeric protein tubulin. They play an important role in understanding fundamental cellular processes such as maintenance of cell division, cell motility, cell architecture, intracellular transport, and cell differentiation. As for the study of microtubules, Jeong and Hollingsworth [55] obtained bioconjugates of nanocrystalline QDs with intact tubulin (NQD-tubulin bioconjugates), which were capable of polymerizing microtubules. Although the kinetics of their polymerization was found to be significantly lower compared to unmodified tubulin and tubulin modified with small-molecule dyes or biotin. In parallel with the development of this method, an approach was developed for the indirect labeling of microtubules in PtK2 cells using secondary Ab conjugated with QDs (QDot 605 goat anti-rabbit IgG conjugate) and Oregon Green conjugated secondary Abs [56]. Since it was clearly demonstrated that the staining details of QDs and Oregon Green microtubules are virtually indistinguishable, the authors recommended this approach for routine biological measurements [56]. Also for indirect visualization of microtubule network in HeLa cells the primary anti-β-tubulin monoclonal antibody and secondary anti-mouse IgG antibody conjugated to QD655 quantum dots were used also. Unfortunately, the authors concluded that this staining resulted in poor imaging of the microtubule network, background presence, and visualization of QD aggregates that were not selectively associated with β-tubulin [57]. Although previously the similar approach using primary antibodies against β-tubulin and secondary antibodies - lipid-coated Qdot 605 (CdSe QDs) that couples to the secondary goat F(ab0)2 anti-mouse antibody was successfully used for microtubule visualization in the human ovarian cancer cell line SKOV3 [58]; as well as in U373 cells (human, glioblastoma-astrocytoma) [59].
For the first time, a direct immunofluorescence method based on the use of CdSe-based semiconductor nanocrystals conjugated to anti-tubulin antibodies has been developed to visualize plant microtubules [60]. To do this, bioconjugation was performed in two ways: by covalent coupling of anti-tubulin antibodies with (1) coated BSA nanocrystals and (2) with functionalized nanocrystals coated with silica shells. This method, although in need of refinement, is extremely promising for visualizing and studying the functional role of posttranslational modifications of tubulin or, for example, the action of certain antimitotic compounds on microtubules compared to indirect immunofluorescence microscopy [19,20,21, 61].
To label the microtubules and actin filament in the living cancer cells, the commercial amino functionalized PEG coated quantum dots QD 655 (Qdot® nanocrystal) were linked to anti-bovine α-tubulin monoclonal antibodies and phalloidin (binds to actin and prevents its depolymerization) molecules. Whereas, to study kinesin (microtubule associated motor protein) movement, kinesin-1 with biotin-tag at C-terminal was conjugated to streptavidin functionalized PEG coated QDs 655 via streptavidin-biotin reaction [62]. To deliver OD-conjugates into cancer cells without damaging their cellular functions a lipid transfection based delivery method was used. As the authors conclude, the developed ODs-based method can be considered as an excellent method for the investigation of microtubules and the behavior of purified protein, in particular intracellular-specific behavior of kinesin, in biology and medicine [62].
Also, by direct immunofluorescence microscopy, Francis et al. [63] stained tubulin (for microtubule imaging) and extracellular fibronectin using produced conjugates between specific antibodies and commercially available QDs. The most recent study [64] describes the development of a method for the synthesis of different QD conjugates with anti-tubulin antibodies for microtubules labeling in HeLa cells. For this, Ma et al. [64] used near infrared (NIR) quantum dots with 720 nm emission (Table 1) to enable sensitive detection of tubulin in autofluorescent cells and focus on visualization of microtubules forming a dense network within cells. As a result, it was found that QD-sa-PA-sa-Ab conjugates prepared are the best material for intracellular microtubule labeling, at least in cancer HeLa cells [64].
Therefore, the above given examples of creating and using QD-Ab conjugates to detect different cell types, their internal structures, as well as to diagnose various diseases, in particular cancer, predict their possible development, through, for example, detecting markers of metastasis of individual tumor cells are promising direction in cell biology and medicine. Thus, the development of new and improved strategies for the conjugation of QDs with antibodies opens up opportunities for the development of advanced techniques for both fluorescence microscopy and biological imaging, including new opportunities for the diagnosis of a number of diseases.
The study of cellular mechanisms using QD-antibody conjugates can significantly improve our understanding of the functioning of different cell types and subcellular structures, and provide faster and more accurate diagnostics for the development and development of new test systems and drugs for different disease types. However, there are still issues that need further study and refinement, such as efficiency, accuracy, biodelivery and stability of the developed QD-Ab complexes, as well as expanding the possibilities of their application.
Conclusions
The unique photoluminescent and electronic properties of quantum dots (QDs), nanosized semiconductors, namely, their extraordinary photostability, high brightness, narrow spectra of radiation from visible to near infrared wavelengths, give them significant advantages over other widely used fluorescents. Accordingly, QDs is a unique material for a variety of studies using antibodies (Abs) for different immunoassays. The analyzed material of developed and already used methods of QDs surface modification, QD conjugation with different Abs (non-covalent, direct covalent bonding or using special adapter molecules) provides a good basis for concluding that they are one of the most important materials for various studies and practical developments in cell biology and biovisualization. An available example of recent applications of QDs-Abs in immunofluorescence microscopy to visualize cells and cell structures, fluorescence assays to detect biomolecules, and in the diagnosis of various diseases demonstrates the enormous potential of this approach. The advantages of QD-Ab conjugation technology over existing immunofluorescence methods can be a useful tool for further prospects for its use in experimental biology and biomedicine.
Data Availability
All data analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Cotta MA (2020) Quantum dots and their applications: what lies ahead? ACS Appl Nano Mater 3(6):4920–4924. https://doi.org/10.1021/acsanm.0c013864
García de Arquer FP, Talapin DV, Klimov VI, Arakawa Y, Bayer M, Sargent EH (2021) Semiconductor quantum dots: Technological progress and future challenges. Science 373:6555. https://doi.org/10.1126/science.aaz8541
Alami AH, Faraj M (2022) Quantum dots: types and characteristics. In: Olabi AG (Ed.), Encyclopedia of smart materials, Elsevier, pp 183–191. https://doi.org/10.1016/B978-0-12-815732-9.00018-8
Borovaya MN, Burlaka OM, Yemets AI, Blume YaB (2015) Biosynthesis of quantum dots and their potential applications in biology and biomedicine. In: Fesenko O, Yatsenko L (Eds.), Nanoplasmonics, Nano-Optics, Nanocomposites, and Surface Studies, Springer-Verlag: Springer Proceedings in Physics, 167, pp 339–362. https://doi.org/10.1007/978-3-319-18543-9_24
Borovaya M, Horiunova I, Plokhovska S, Pushkarova N, Blume Y, Yemets A (2021) Synthesis, properties and bioimaging applications of silver-based quantum dots. Int J Mol Sci 22:12202. https://doi.org/10.3390/ijms222212202
Jamieson T, Bakhshi R, Petrova D, Pocock R, Imani M, Seifalian AM (2007) Biological applications of quantum dots. Biomaterials 28(31):4717–4732. https://doi.org/10.1016/j.biomaterials.2007.07.014
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5:763–775. https://doi.org/10.1038/nmeth.1248
Valizadeh A, Mikaeili H, Samiei M, Farkhani S, Zarghami N, Kouhi M, Akbarzadeh A, Davaran S (2012) Quantum dots: synthesis, bioapplications and toxicity. Nanoscale Res Lett 7:480. https://doi.org/10.1186/1556-276X-7-480
Badilli U, Mollarasouli F, Bakirhan NK, Ozkan Y, Ozkan S (2020) Role of quantum dots in pharmaceutical and biomedical analysis, and its application in drug delivery. TrAC Trends Analyt Chem 131:116013. https://doi.org/10.1016/j.trac.2020.116013
Singh S, Dhawan A, Karhana S, Bhat M, Dinda AK (2020) Quantum dots: an emerging tool for point-of-care testing. Micromachines 11(12):1058. https://doi.org/10.3390/mi11121058
Fakayode OJ, Tsolekile N, Songca SP, Oluwafemi OS (2018) Applications of functionalized nanomaterials in photodynamic therapy. Biophys Rev 10:49–67. https://doi.org/10.1007/s12551-017-0383-2
Zare-Zardini H, Ferdowsian F, Soltaninejad H, Azam AG, Soleymani S, Zare-Shehneh M, Mofidi M, Rafati R, Ebrahimi L (2015) Application of nanotechnology in biomedicine: a major focus on cancer therapy. J Nano Res 35:55–66. https://doi.org/10.4028/www.scientific.net/jnanor.35.55
Walling MA, Novak JA, Shepard JR (2009) Quantum dots for live cell and in vivo imaging. Int J Mol Sci 10(2):441–491. https://doi.org/10.3390/ijms10020441
Chen Y, Liang H (2014) Applications of quantum dots with upconverting luminescence in bioimaging. J Photochem Photobiol B 135:23–32. https://doi.org/10.1016/j.jphotobiol.2014.04.003
Wang W, Liu Z, Lan X (2020) Quantum dot-based simultaneous multicolor imaging. Mol Imaging Biol 22:820–831. https://doi.org/10.1007/s11307-019-01432-40
Goldman ER, Balighian ED, Mattoussi H, Kuno MK, Mauro JM, Tran PT, Anderson GP (2002) Avidin: a natural bridge for quantum dot-antibody conjugates. J Am Chem Soc 124:6378–6382. https://doi.org/10.1021/ja0125570
Ferrari BC, Bergquist PL (2007) Quantum dots as alternatives to organic fluorophores for cryptosporidium detection using conventional flow cytometry and specific monoclonal antibodies: lessons learned. Cytom Part A 71A:265–271. https://doi.org/10.1002/cyto.a.20381
Bilan R, Brazhnik K, Chames P, Baty D, Nabiev I, Sukhanova A (2015) Oriented conjugates of single-domain antibodies and fluorescent quantum dots for highly sensitive detection of tumor-associated biomarkers in cells and tissues. Physics Procedia 73:228–234. https://doi.org/10.1016/j.phpro.2015.09.162
Yemets A, Stelmakh O, Blume YB (2008) Effects of the herbicide isopropyl-N-phenyl carbamate on microtubules and MTOCs in lines of Nicotiana sylvestris resistant and sensitive to its action. Cell Biol Int 32(6):623–629. https://doi.org/10.1016/j.cellbi.2008.01.012
Blume YB, Krasylenko YA, Demchuk OM, Yemets AI (2013) Tubulin tyrosine nitration regulates microtubule organization in plant cells. Front Plant Sci 4:530. https://doi.org/10.3389/fpls.2013.00530
Blume Y, Yemets A, Sheremet Y, Nyporko A, Sulimenko V, Sulimenko T, Draber P (2010) Exposure of beta-tubulin regions defined by antibodies on a Arabidopsis thaliana microtubule protofilament model and in the cells. BMC Plant Biol 10:29. https://doi.org/10.1186/1471-2229-10-29.
Combs C, Shroff H (2017) Fluorescence microscopy: a concise guide to current imaging methods. Curr Protoc Neurosci 10(79):2.1.1–2.1.25. https://doi.org/10.1002/cpns.29
Joshi S, Yu D (2017) Immunofluorescence. In: Basic science methods for clinical researchers, pp 135–150. https://doi.org/10.1016/B978-0-12-803077-6.00008-4
Rizvi SB, Ghaderi S, Keshtgar M, Seifalian M (2010) Semiconductor quantum dots as fluorescent probes for in vitro and in vivo bio-molecular and cellular imaging. Nano Rev 1:5161. https://doi.org/10.3402/nano.v1i0.5161
Goldman ER, Mattoussi HM, Anderson GP, Medintz IL, Mauro JM (2005) Fluoroimmunoassays using antibody-conjugated quantum dots. In: Rossenthal SJ, Wright DW (eds) Nanobiotechnology protocols. Humana Press, New Jersey, pp 19–35
Lyu Y, Martínez A, D’Incà F, Mancin F, Scrimin P (2021) The biotin–avidin interaction in biotinylated gold nanoparticles and the modulation of their aggregation. Nanomater 11:1559. https://doi.org/10.3390/nano11061559
Pathak S, Davidson MC, Silva GA (2007) Characterization of the functional binding properties of antibody conjugated quantum dots. Nano Lett 7:1839–1845. https://doi.org/10.1021/nl062706i
Han HS, Niemeyer E, Huang Y, Kamoun WS, Martin JD, Bhaumik J, Chen Y, Roberge S, Cui J, Martin MR, Fukumura D, Jain RK, Bawendi MG, Duda DG (2015) Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proc Natl Acad Sci USA 112:1350–1355. https://doi.org/10.1073/pnas.1421632111
Yang P, Zhang A, Sun H, Liu F, Jiang O, Cheng X (2010) Highly luminescent quantum dots functionalized and their conjugation with IgG. J Colloid Interface Sci 345:222–227. https://doi.org/10.1016/j.jcis.2010.01.072
Selim KMK, Kang IK (2009) Albumin-conjugated cadmium sulfide nanoparticles and their interaction with KB cells. Macromol Res 17(6):403–410. https://doi.org/10.1007/BF03218881
Sahoo SL, Chi-Hsien Liu C-H, Kumari M, Wu W-C, Wang C-C (2019) Biocompatible quantum dot-antibody conjugate for cell imaging, targeting and fluorometric immunoassay: crosslinking, characterization and applications. RSC Adv 9:32791–32803. https://doi.org/10.1039/c9ra07352c
Tada H, Higuchi H, Wanatabe TM, Ohuchi N (2007) In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res 67(3):1138–1144. https://doi.org/10.1158/0008-5472.CAN-06-1185
Yuan R, Rao T, Cheng F, Yu WM, Ruan Y, Zhang XB, Larre S (2018) Quantum dot-based fluorescent probes for targeted imaging of the EJ human bladder urothelial cancer cell line. Exp Th Med 16:4779–4783. https://doi.org/10.3892/etm.2018.6805
Pellegrino T, Manna L, Kudera S, Liedl T, Koktysh D, Rogach AL, Keller S, Radler J, Natile G, Parak WJ (2004) Hydrophobic nanocrystals coated with an amphiphilic polymer shell: a general route to water soluble nanocrystals. Nano Lett 4(4):703–707. https://doi.org/10.1021/nl035172j
Tasso M, Singh MK, Giovanelli E, Fragola A, Loriette V, Regairaz M, Dautry F, Treussart F, Lenkei Z, Lequeux N, Pons T (2015) Oriented bioconjugation of unmodified antibodies to quantum dots capped with copolymeric ligands as versatile cellular imaging tools. ACS Appl Mater Interfaces 7(48):26904–26913. https://doi.org/10.1021/acsami.5b09777
Tiwari DK, Tanaka SI, Inouye Y, Yoshizawa K, Watanabe TM, Jin T (2009) Synthesis and characterization of anti-HER2 antibody conjugated CdSe/CdZnS quantum dots for fluorescence imaging of breast cancer cells. Sensors 9:9332–9354. https://doi.org/10.3390/s91109332
Yanagawa H, Inoue A, Sugimoto H, Shioi H, Fujii M (2019) Antibody-conjugated near-infrared luminescent silicon quantum dots for biosensing. MRS Commun 9(3):1079–1086. https://doi.org/10.1557/mrc.2019.98
Zhang B, Yu J, Liu C, Wang J, Han H, Zhang P, Shi D (2016) Improving detection sensitivity by oriented bioconjugation of antibodies to quantum dots with a flexible spacer arm for immunoassay. RSC Adv 6:50119–50127. https://doi.org/10.1039/c6ra09279a
Gao X, Yang Y, Petros JA, Marshall FF, Simons JW, Nie S (2005) In vivo molecular and cellular imaging with quantum dots. Curr Opin Biotechnol 16:63–72. https://doi.org/10.1016/j.copbio.2004.11.003
Wang Y, Cai E, Rosenkranz T, Ge P, Teng KW, Lim SJ, Smith AM, Chung HJ, Sachs F, Green WN, Gottlieb P, Selvin PR (2014) Small quantum dots conjugated to nanobodies as immunofluorescence probes for nanometric microscopy. Bioconjug Chem 25(12):2205–2211. https://doi.org/10.1021/bc5004179
Kargozar S, Hoseini SJ, Milan PB, Hooshmand S, Kim HW, Mozafari M (2020) Quantum dots: a review from concept to clinic. Biotechnol J 15(12):2000117. https://doi.org/10.1002/biot.202000117
Mashinchian O, Johari-Ahar M, Ghaemi B, Rashidi M, Barar J, Omidi Y (2014) Impacts of quantum dots in molecular detection and bioimaging of cancer. BioImpacts 4(3):149–166. https://doi.org/10.15171/bi.2014.008
Wagner AM, Knipe JM, Orive G, Peppas NA (2019) Quantum dots in biomedical applications. Acta Biomater 94:44–63. https://doi.org/10.1016/j.actbio.2019.05.022
Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP, Ge N, Peale F, Bruchez MP (2003) Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 21:41–46. https://doi.org/10.1038/nbt764
Yezhelyev MV, Al-Hajj A, Morris C, Marcus AI, Liu T, Lewis M, Cohen C, Zrazhevskiy P, Simons JW, Rogatko A, Nie S, Gao X, O’Regan RM (2007) In situ molecular profiling of breast cancer biomarkers with multicolor quantum dots. Adv Mater 19:3146–3151. https://doi.org/10.1002/adma.200701983
Zhang H, Zeng X, Li Q, Gaillard-Kelly M, Wagner CR, Yee D (2009) Fluorescent tumour imaging of type I IGF receptor in vivo: comparison of antibody-conjugated quantum dots and small-molecule fluorophore. Br J Cancer 101:71–79. https://doi.org/10.1038/sj.bjc.6605103
Watanabe M, Fujioka K, Akiyama N, Takeyama H, Manabe N, Yamamoto K (2011) Conjugation of quantum dots and JT95 IgM monoclonal antibody for thyroid carcinoma without abolishing the specificity and activity of the antibody. IEEE Trans Nanobiosci 10:30–35. https://doi.org/10.1109/TNB.2011.2125800. Manome Y
Ruan J, Song H, Qian Q, Li C, Wang K, Bao C, Cui D (2012) HER2 monoclonal antibody conjugated RNase-A-associated CdTe quantum dots for targeted imaging and therapy of gastric cancer. Biomaterials 33:7093–7102. https://doi.org/10.1016/j. biomaterials.2012.06.053
Matyushkin LB, Aleksandrova OA, Drobintseva AO, Kvetnoy IM, Krylova YS, Marchenko YY, Mazing DS, Moshnikov VA, Musikhin SF, Nikolaev BP, Polyakova VO, Shevtsov MA, Yakovleva LY (2020) Detection of apoptosis in cancer cells using heat shock protein 70 and p53 antibody conjugated quantum dot nanoparticles. In: Grigoryeva N (ed) Fluorescence methods for investigation of living cells and microorganisms. IntechOpen, London, pp 235–250. https://doi.org/10.5772/intechopen.92994
Park Y, Ryu Y-M, Wang T, Jung Y, Kim S, Hwang S, Park J, Bae D-J, Kim J, Moon H, Lim H-S, Kim S-Y, Chung E, Kim KH, Kim S, Myung S-J (2018) Colorectal cancer diagnosis using enzyme-sensitive ratiometric fluorescence dye and antibody–quantum dot conjugates for multiplexed detection. Adv Funct Mater 28(4):1703450. https://doi.org/10.1002/adfm.201703450
Mattera L, Bhuckory S, Wegner KD, Qiu X, Agnese F, Lincheneau C, Senden T, Djurado D, Charbonnière LJ, Hildebrandt N, Reiss P (2016) Compact quantum dot-antibody conjugates for FRET immunoassays with subnanomolar detection limits. Nanoscale 8:11275–11283. https://doi.org/10.1039/c6nr03261c
Zhang С, Han Y, Lin L, Deng N, Chen B, Liu Y (2017) Development of quantum dots-labeled antibody fluorescence immunoassays for the detection of morphine. J Agric Food Chem 65:1290–1295. https://doi.org/10.1021/acs.jafc.6b05305
Becheva Z, Godjevargova T (2017) Preparation of anti-elastase antibody conjugated with quantum dots 710 nm and fluorescein isothiocyanate for immunoassay of milk somatic cells. J Nanomater Mol Nanotechnol 6:3. https://doi.org/10.4172/2324-8777.1000217
Liu X, Hu R, Lian H, Liu Y, Liu J, Liu J, Lin G, Liu L, Duan X, Yong K, Ye L (2015) Dual-color immunofluorescent labeling with quantum dots of the diabetes-associated proteins aldose reductase and Toll-like receptor 4 in the kidneys of diabetic rats. Int J Nanomedicine 10:3651–3662. https://doi.org/10.2147/IJN.S81395
Jeong S, Hollingsworth JA (2006) Polymerization of nanocrystal quantum dot–tubulin bioconjugates. IEEE Transact Nanobiosci 5(4):239–245. https://doi.org/10.1109/TNB.2006.886561
Medda R, Jakobs S, Hell SW, Bewersdorf J (2006) 4Pi microscopy of quantum dot-labeled cellular structures. J Struct Biol 156(3):517–523. https://doi.org/10.1016/j.jsb.2006.08.013
Montón H, Roldán M, Merkoçi A, Rossinyol E, Castell O, Nogués C (2012) The use of quantum dots for immunochemistry applications. Methods Mol Biol 906:185–192. https://doi.org/10.1007/978-1-61779-953-2_13
Corezzi S, Urbanelli L, Cloetens P, Emiliani C, Helfen L, Bohic S, Elisei F, Fioretto D (2009) Synchrotron-based X-ray fluorescence imaging of human cells labeled with CdSe quantum dots. Anal Biochem 388:33–39. https://doi.org/10.1016/j.ab.2009.01.044
Heidbreder M, Endesfelder U, van de Linde S, Hennig S, Widera D, Kaltschmidt B, Kaltschmidt C, Heilemann M (2010) Subdiffraction fluorescence imaging of biomolecular structure and distributions with quantum dots. Biochim Biophys Acta 1803(10):1224–1229. https://doi.org/10.1016/j.bbamcr.2010.06.004
Eggenberger K, Merkulov A, Darbandi M, Nann T, Nick P (2007) Direct immunofluorescence of plant microtubules based on semiconductor nanocrystals. Bioconjug Chem 18(6):1879–1886. https://doi.org/10.1021/bc700188d
Yemets AI, Blume YB, Kundelchuk OP, Smertenko AP, Solodushko VA, Rudas VA, Gleba YY (2000) Transfer of amiprophosmethyl-resistance from Nicotiana plumbaginifolia mutant by somatic hybridization. Theor Appl Genet 100(6):847–857. https://doi.org/10.1007/s001220051361
Yoo J, Kambara T, Gonda K, Higuchi H (2008) Intracellular imaging of targeted proteins labeled with quantum dots. Exp Cell Res 314(19):3563–3569. https://doi.org/10.1016/j.yexcr.2008.09.014
Francis JE, Mason D, Lévy R (2017) Evaluation of quantum dot conjugated antibodies for immunofluorescent labelling of cellular targets. Beilstein J Nanotechnol 8:1238–1249. https://doi.org/10.3762/bjnano.8.125
Ma L, Geng J, Kolossov VL, Han Z, Pei Y, Lim SJ, Kilian KA, Smith AM (2021) Antibody self-assembly maximizes cytoplasmic immunostaining accuracy of compact quantum dots. Chem Mater 33(13):4877–4889. https://doi.org/10.1021/acs.chemmater.1c00164
Acknowledgements
The authors are grateful to Dr. Victor Kyrylenko (Institute of Food Biotechnology and Genomics, National Academy of Sciences of Ukraine) for final improving of English version of the manuscript.
Funding
This work was supported by the grant of the National Academy of Sciences of Ukraine for young research groups “Creating of eco-friendly Ag2S quantum dots as new tools for intracellular imaging” (2020–21) (State Registration N 0120U100930) for Svitlana Plokhovska and Nadia Pushkarova.
Author information
Authors and Affiliations
Contributions
Alla Yemets: Conceptualization, data analysis, writing; Svitlana Plokhovska and Nadia Pushkarova: Data analysis, writing, figure drawing; Yaroslav Blume: Review, critical remarks. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Conflict of Interest
No conflicts of interest to declare.
Competing Interest
The authors declare they have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yemets, A., Plokhovska, S., Pushkarova, N. et al. Quantum Dot-Antibody Conjugates for Immunofluorescence Studies of Biomolecules and Subcellular Structures. J Fluoresc 32, 1713–1723 (2022). https://doi.org/10.1007/s10895-022-02968-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02968-5