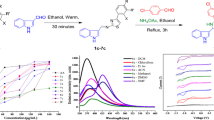
Abstract
Quinoxaline derivatives are well-known N-heterocycles with pharmacological and fluorescence activities. Almost all quinoxaline derivatives with extensive π-conjugation have been introduced as fluorophores which emit blue and green light. For the first time, we designed and synthesized 6-chloro-2,3 di(Pyridine-2yl) quinoxaline (2-CPQ) as a pink fluorophore in acetonitrile medium by simple route at room temperature whitin 30 min. The synthesized quinoxaline was identified using 1H, 13C NMR, MS, and FT-IR spectroscopy. Our results showed that the iodine-catalyzed method for both oxidation and cyclization during the synthesis of quinoxaline from pyridine 2-carbaldehyde was straightforward, efficient, and clean. All of the mentioned characterization devices confirmed the synthesis of 2-CPQ.
Moreover, we studied the photophysical properties of the synthesized fluorophore in which The UV–Vis absorption spectrum of 2-CPQ in DMF were three peaks at 451, 518 and 556 nm. Based on photophysical properties investigation, 2-CPQ shows good fluorescence with maximum peaks 607 and 653 nm in DMF as solvent (фF = 0.21). Hence, the fluorophore was applied in the peroxyoxalate chemiluminescence system. The reaction of imidazole, H2O2, and bis (2,4,6-trichlorophenyl) oxalate (TCPO) can transfer energy to a 6-chloro-2,3 di(pyridine-2yl) quinoxaline. In this process, dioxetane was synthesized, which chemically initiated the electron exchange luminescence (CIEEL) mechanism and led to pink light emission. We anticipate our synthesized fluorophores 2-CPQ will have great potential applications in imaging and medical markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quinoxaline derivatives are an essential class of aromatic heterocyclic compounds. Nitrogen as a heteroatom in their pyrazine ring extended π-conjugation [1]. The pyrazine ring with low π*orbitals acts as acceptors of metal d-orbital density. The quinoxalines represent rigid subunits in macrocyclic receptors. An extensive π-conjugation exists in quinoxaline-based chromophores that display a strong emission with almost low Stokes shifts. The synthesis of quinoxaline is practically simple to prepare. One of the widely used methods for synthesizing quinoxalines is the condensation of an o-phenylenediamine with a 1,2-dicarbonyl compound [2, 3]. The reaction was found individually by Korner and Hinsberg in 1884. A range of applications has been reported for quinoxaline derivatives. They have various biological properties, optoelectronic devices, and self-extinguishing flame-resistant polymers. They have other important functions: fluorophores, photosensitizers, and electron-transporter material. Their metal complexes also displayed multiple photochemical, photophysical, catalytic, and biological activities[4]. Numerous approaches have been described for the processes of quinoxaline derivatives synthesis involving 1,2-diamines with α-diketones condensation [5], 1,4-addition of diamines to the diazenylbutenes[6], cyclization-oxidation of phenacyl and epoxides coupling with ene-1,2-diamines. Many green synthetic methods have been developed using eco-friendly catalysts, one-pot synthesis, microwave-assisted technology, and reactions via an aqueous medium [7,8,9,10]. Almost all quinoxaline fluorophores introduced to date emit light in the range of blue and green. Almost all quinoxaline fluorophores introduced to date emit light in the range of blue and green due to the different strengths of the donor and acceptor [11]. Although, the synthesis of red or longer region fluorophores are quietly spares and seems attractive.
Nowadays, inspiring applications of sensors push scientists to incorporate them with nanotechnology, given that the intrinsic CL illumination of luminol and its low quantum yield can be improved by various compounds such as nanoparticles, metal oxides, metal ions, and enzymes. Therefore, one of the stimulating research interests of scientists is to alter nanomaterials to advance their particular physical–chemical properties. It has been demonstrated that the surface defects, chemical composition, crystal structure, size, and surface properties are critical factors of engineered nanomaterials for sensing applications. Rare earth metal doped nanomaterials have revealed exceptional chemical, physical, and mechanical properties, imperative for analytical approaches[12,13,14,15,16,17].
The benefits of luminescent technologies Chemiluminescence include ultra-sensitivity, rapid assay procedures, and diversity of analytical applications. These technologies are now used normally by clinical laboratories for immunoassay and DNA probe assays. Concurrently, the scope of applications for luminescent technologies in medical investigation has continued to develop, and the most active advance has been in reporter gene assays, nitric oxide studies, imaging of luminescent reactions, and cellular luminescence [18].
Chemiluminescence (CL) property happens when chemically formed molecules in excited states release energy with light emission. The decomposition of organic peroxides usually accompanies this phenomenon. Oxalic acid derivatives, the most studied branches of Chemiluminescence, are responsible for the emission. Peroxyoxalate Chemiluminescence (PO-CL) is a valuable reaction system that displays powerful analytical potential for many fluorescent compounds. A typical PO-CL consists of the reaction of the active oxalates, such as bis (2,4,6-trichlorophenyl) oxalate (TCPO), hydrogen peroxide as oxidant and a catalyst, in which light emits from the excited states of various externally added fluorescent activators. The detection method based on the PO-CL system proposes an improvement in chemiluminescence imaging. This system can also integrate determination, ascribed to the virtue of its constant Chemiluminescence and comparative high quantum yield [19,20,21,22]. In the peroxyoxalate system, 1,2-dioxetanones, including 1,2-dioxetanedione and cyclic peroxides, have been implied as to the high-energy vital intermediates. They can generate an excited species by their thermal decay occurring via an initiated electron exchange luminescence (CIEEL) process [23, 24]. These metastable intermediates form complexes with the fluorescent activator (fluorophore) to donate one electron to the intermediate. This electron is then transferred back to the fluorophore raising it to an excited state and liberating light. To find a new fluorophore with the capability of light emission, we worked on synthesizing quinoxaline derivatives with pyridine 2-carbaldehyd as a trigger. CL is a powerful tool for drug analysis since its detection limits are meager and its instrumentation very simple and low cost. Combined with derivatization techniques to increase sensitivity, it has a wide range of applications. Ultimately, we detected 6-chloro-2,3 di(pyridine-2yl) quinoxaline (2-CPQ), an analog of quinoxaline as a fluorophore with novel light. The synthesis and characterization of 2-CPQ and its chemiluminescence characteristics are discussed in detail.
Experimental
Pyridine 2-carbaldehyde and 4-Chloro-o-phenylenediamine were prepared from Sigma Aldrich Chemical Co. (Milwaukee, WI, USA) received from commercial sources. Sodium cyanide and iodine (I2) were purchased from Merck KGaA (Darmstadt, Germany) with no more purification. Bis (2, 4, 6-trichlorophenyl) oxalate(TCPO) was obtained from ACROS(New Jersy, Princeton, USA) and applied with no extra purification. Hydrogen peroxide (30% W/W) and imidazole were ordered from Fluka (Basel, Switzerland). All solvents were obtained from Romil (England). All measurements were done at ambient temperature (25 ± 1 C). A Cary-Eclipse Fluorescence-Spectrophotometer (Agilent, Springvale Rd, Australia) was applied for recording fluorescence spectra and steady-state Chemiluminescence with 5 nm spectral bandwidth. The Xenon lamp was turned off in the chemiluminescence made. The UV–Vis absorption spectra were taken with a T92 + spectrophotometer (PG instrument Ltd, Alma Park, UK). The FT-IR spectra were prepared with an ATR (Agilent, Cary 630, FTIR Spectrometer, Santa Clara, CA, USA) equipment in 4000 and 650 cm-1. 1H and 13C spectra were indicated on NMR, Bruker, Avance III 300 MHz (Amherst, Massachusetts, USA). Mass spectroscopy was employed by GC/5975 MSD detector, Agilent Technologies (Palo Alto, CA, USA) analysis.
General Procedure for the Synthesis of 6-chloro-2,3 Di(Pyridine-2yl) Quinoxaline
The benzoin condensation as a named reaction was carried out for the synthesis of the α-hydroxyl ketone from the related aldehyde (pyridine 2-carbaldehyde). 1 mmol pyridine 2-carbaldehyde (107 mg) in ethanol 80% (3 mL) was stirred at room temperature. Then, 0.01 mmol of sodium cyanide (0.049 mg) was dissolved in water (2 mL), and the stirring was continued. After 0.5 h, TLC analysis (chloroform/methanol, 9:1) showed that all pyridine 2-carbaldehyde was consumed; The resulting brown suspension was filtered with a Buchner funnel and washed with ethanol to yield 2-hydroxy-1,2-di(pyridine-2-yl)ethanone (95%), which was used without further purification. The appropriate oxidation process was performed to obtain the diketone. 1 mmol 2-Hydroxy-1-(pyridine-2-yl)ethanone (214 mg) was dissolved in ethanol 80% (5 mL) and added 2.2 mmol sodium acetate (182 mg) to this solution. The resulting suspension was added 1.1 mmol I2 (138.6 mg) over 2.5 h at 20 °C. The stirring was continued at 20 °C overnight. Then, 5% aqueous Na2S2O3 (5 mL) was added. The suspension was further stirred at 20 °C for an additional 1 h. The product was filtered off and washed with cold EtOH (2 × 5 L). The solid product was dried at room temperature as a white solid (Scheme 1). 1 mmol synthesized 1,2-bis(pyridine-2-yl)ethane-1,2-dione, was condensed with 1.1 mmol 4-chlorobenzene-1,2-diamine (156 mg) and 25% mmol iodine (31.5 mg) as a catalyst in acetonitrile as a solvent for synthesizing quinoxaline. The reaction was followed up by TLC (Scheme 1). After completion of the reaction, the mixture was transferred to crushed ice for 10–15 min. The isolated solid was filtered, washed with aqueous sodium thiosulfate solution 1% to remove by-products, and recrystallized from ethanol/water to obtain the pure 6-chloro-2,3 di(pyridine-2yl) quinoxaline.
2-hydroxy-1,2-di(pyridine2-yl)ethan-1-one 2 Mp. 115–120 °C;IR cm−1: 3100(C-H, pyridine cycle), 2600–3400(O–H), 1710 (C = O), 1589 (C = N); [M]+: 214; found: 214 [25].
1,2-bis(pyridine-2-yl)ethane-1,2-dione 3 Mp. 157–158 °C;IR cm−1: 3100 (C-H, pyridine cycle), 1680–1700 (C = O), 1591 (C = N) [M]+: 212; found: 212 [26].
2,3-bis(pyridine-2-yl)-6-chloroquinoxaline 2-CPQ Mp. 134–138 °C; IR, cm−1: 3049 (C-H), 1585 (C-N), 1579 (C-N); 1H NMR (DMSO, 300 MHz), ppm: 8.38–8.43 (2H, m), 8.26 (1H, J = 2.4,d), 8.2–8.18 (2H, J = 9, d), 7.99 (2H, m), 7.85 (2H, m), 7.28, (2H, m); 13C NMR (DMSO, 80 MHz), ppm: 123.14, 123.23, 124.19, 124.25, 128.28, 130.60, 131.59, 136.32, 136.71, 136.74, 139.66, 141.41, 148.61, 148.64, 152.67, 153.31, 157.07, 157.013; [M]+: 318.5, found: 318.2 (Scheme 1).
General Procedure Chemiluminescence Measurement
The chemiluminescence procedure was carried out at ambient temperature 25 ℃ ± 1 in the following manner. The cuvette was filled with 500 µL TCPO (in ethyl acetate), 130 µL 2-CPQ (in DMF), 130 µL imidazole (in methanol). The samples were placed in a spectrofluorometer and continuously stirred up with a magnetic stirrer (400 rpm). After 30 s, the CL reaction was initiated by injecting 300 µL hydrogen peroxide (in acetonitrile) to the cuvette using a sampler.
Evaluation the Fluorescence Quantum Yield
Fluorescence quantum yield is determined by the following equations:
where, Q: Fluorescence quantum yield, m: Gradient of the plot of integrated fluorescence intensity against absorbance, n: Refractive index of the solvent, A: Absorbance of the solution, E: Integrated fluorescence intensity of the emitted light, and Subscripts ‘r’ and ‘s’ refer to the reference and new fluorophore respectively. The Rhodamine 6G (0.1 mM) was used as a standard fluorophore.
Results and Discussion
Synthesis and Characterization of 6-chloro-2,3 Di(Pyridine-2yl) Quinoxaline
New quinoxaline moiety was designed and prepared from classical 1,2-diketone and 1,2-diamine. The benzoin condensation was done in a typical process in which the catalytic conversion of 2-pyridinecarbaldehyde to 2-hydroxy-1,2-di(pyridine-2-yl)ethan-1-one 2 was performed by sodium cyanide as a catalyst. The synthesized 2-hydroxy-1,2-di(pyridine-2-yl)ethan-1-one 3 was oxidized to 1,2-bis(6-bromopyridine-2-yl)ethane-1,2-dione with considerable yield (97%). After optimizing various solvents for preparing the quinoxaline derivative, the reaction was carried out using acetonitrile as the solvent of choice. High efficiency with a lower reaction time is the advantage of the current condition. In general, the reaction in the polar solvents was done fastly, while it reacted in the nonpolar solvents slowly. The iodine-catalyzed method for the synthesis of quinoxalines was displayed very simple, efficient, and clean method with no extra by-products [25]. Then the nucleophilic addition of 1,2-diamine proceeded as shown in Scheme 1. The reaction of diamines with 1,2-diketone corresponding was accomplished using a catalytic amount of iodine (I2) in an acetonitrile medium [26]. The chemical structure of the products in each stage was characterized by FTIR, 1H-NMR, 13C-NMR, and Mass spectroscopy. Mass spectroscopy revealed oxidation of α-hydroxy ketone to 1, 2-diketone while mass units in molecular weight decreased, indicating loss of hydrogen (Fig. 1a and b).
The FTIR spectra of 3 are shown in (Fig. 2a). The stretching vibration peaks of C = O at 1710 cm−1 got stronger, indicating that the carbonyl group converts from α-hydroxyl group to a complete 1,2-diketone. Moreover, the stretching O–H bond at 2600 up to 3400 cm−1 completely disappeared during diketone synthesis. The IR absorption peaks of 2-CPQ at 1700 cm−1 were eliminated and replaced with 1585, 1579 cm−1 C-N stretching bonds. Figure 3 depicts 1HNMR spectra of synthesized 2-CPQ. The chemical shift at 8.404 ppm was the protons of the pyridine ring next to a heteroatom. The protons of benzene attaching to the pyrazine ring were detected at the range of 8.28 and 8.21 ppm as two doublet peaks indicating the protons at the position of 5 and 8, respectively. The chemical shifts at 8.02–7.28 ppm were ascribed to the aromatic protons of the pyridine ring, which was substituted on the pyrazine ring displayed in Fig. 3. The chemical shift of 7.81–7.79 ppm is related to the proton of benzene at the position of 7 [27, 28].
Photophysical Properties of 6-chloro-2,3 Di(Pyridine-2yl) Quinoxaline
A problem encountered when using the peroxyoxalate chemiluminescence system is the selection of a suitable organic solvent. Esters and ethers are the most suitable solvents for TCPO. However, because ethers react with oxygen to peroxides, TCPO decomposes in ether solutions. Most common esters are not very miscible with water so that chemiluminescence analysis in aqueous solutions needs a third solvent to create a miscible solvent system. Following recommendations in the literature, ethyl acetate could be selected as a solvent for TCPO [29]. The spectral characteristics of the newly synthesized quinoxaline have been evaluated in various organic solvents. The results, including absorption (λabs) and fluorescence (λflu) maxima and fluorescence quantum yield (фF), are summarized in Table 1. For this purpose, fluorescence and spectroscopic studies were carried out using a freshly prepared solution containing 5 × 10−5 molL−1 2-CPQ in different solvents using a quartz cuvette. As seen, the maximum fluorescence yield was obtained in DMF as a polar aprotic solvent and hence was selected as a solvent within experiments. The UV–Vis absorption spectrum of 2-CPQ in DMF is shown in Fig. 4. There were three peaks, including a more significant peak at 451 and two weaker peaks at 518 and 556 nm.
The fluorescence characteristics of the fluorescence intensity and maximum emission bands of 2-CPQ are dependent on the groups bonded to the quinoxaline ring. Results show that by excitation wavelength 540 nm (as optimum wavelength), 2-CPQ has two peaks (Fig. 5). There is a more notable peak at 605 nm, while a weaker peak was observed at 653 due to π-π electron transient. We observed that the compound emitted strong luminescence in dilute solutions. The charming color of emission of 2-CPQ attracted our group to continue to utilize the new substance in cell imaging and the synthesis of new analogs in future works.
Chemiluminescence Characteristics
Since the first discovery of Chandross of observing a strong chemiluminescence light from the reaction between oxalyl chloride, hydroxide, and various fluorescent compounds, the so-called peroxyoxalate-chemiluminescence (PO-CL) has received increasing attention. In this type of Chemiluminescence, the reaction between hydrogen peroxide and an activated derivative of oxalic acid can produce chemiluminescent light in the presence of a fluorophore. This reaction has successfully been used as an empathetic detection technique in several procedures developed for low-level determinations of a wide variety of analytes, including a range of different fluorophores, hydrogen peroxide, fluorophore-labeled amino acids, and steroids as well as other quencher species [30].
Peroxyoxalate-chemiluminescence (PO-CL) is well-known as one of the most efficient non-biological light-producing systems. Like many chemiluminescence reactions, the POCL reaction can be presented in the following steps [31]. Within the experiments, an intense pink light would be seen by adding a few amounts of hydrogen peroxide to the cell, including TCPO, ImH, and 2-CPQ (Fig. 6a). The maximum emission wavelength in both spectrums of sensitized PO-CL of 2-CPQ and its fluorescence, recorded under comparable experimental conditions, were determined to be almost similar. It means that the singlet-excited state of the fluorescent types is created in the CL reaction and is responsible for the emission [21, 22].
In contrast, Samadi-Meybodi et al. reported two quinoxalines derivatives with beneficial fluorophore compounds containing aromatic functional groups with low-energy π → π* transition level (green light emission) [31]. In the other study, the naphthalene analogs of luminol were found to have chemiluminescent properties promising candidates for chemiluminescent probes. The extended aromaticity and the different substituents in the aromatic ring lead to green Chemiluminescence [32]. Chaichi and coworkers displayed that all coumarins derivatives act as blue fluorescent; among various coumarin derivatives they used, 7-amino-4-trifluoromethylcumarin discovered the most promising features as an efficient blue fluorescent emitter (λmax = 445). Several studies demonstrated the color of the CL emission could be influenced readily by introducing fluorescent additives with known emission characteristics. For example, a purple light can be achieved with 9,10-diphenylanthracene (λmax = 430 nm), blue with perylene (λmax = 471 nm), green with 9,10-diphenylanthracene (λmax = 513 nm) and orange with rubrene (λmax = 556 nm) [28]. In this way, a new quinoxaline derivative was designed and synthesized with novel fluorescence. Hence, 2-CPQ emitted pink color under the chemiluminescent system (λmax = 607 nm). To best our knowledge, no study has been reported quinoxaline-based pink fluorophore and more importantly, pink peroxyoxalate design.
Optimization of the CL System
Optimization of essential factors modifying CL intensity. Figure 7(a)-(d) displays the outcomes of the impact of the variables. The concentration of TCPO, hydrogen peroxide, imidazole, and fluorophore information optimization the POCL process. The TCPO concentration was evaluated at 2.5 × 10–4 to 5 × 10–3 molL−1. The results declared an increase in CL intensity as the concentration of TCPO extended to 2 × 10–3 molL−1 (Fig. 7a). This event is compatible with TCPO being the limiting reagent under this reaction condition [21, 22]. At a higher concentration, the CL signal reduced slowly due to the self-quenching of TCPO. Therefore, 2 × 10–3 molL−1 was selected for all later experiments. The concentration of oxidant was assessed from 4 × 10–4 to 1.8 × 10–2 molL − 1. It was observed that the reduction in CL signal at the concentration of H2O2 greater than 8 × 10 -3molL−1 might be a conclusion of the fast decay of oxalate ester (Fig. 7b). A concentration of 8 × 10-3molL−1 was thus chosen for the following experiments. Among catalysts presented for PO–CL reaction like sodium salicylate, triethylamine, and heterocyclic compounds, imidazole (ImH) has the most significant impact on reaction yield and kinetics [33]. The effect of imidazole concentration in the range of 8 × 10–5 to 2 × 10–3 molL−1 is shown in Fig. 7(c). As can be seen, the intensity was increased up to 8 × 10–4 molL−1. At higher concentrations, CL intensity is due to the quenching effect of the base at higher concentrations, which works to decompose the reactive intermediate duloxetine, consequently, decreases the PO-CL light [34]. The result shows that as the imidazole concentration increased, the intensity significantly increased. In contrast, the time to reach maximum intensity decreased, which vividly verified the catalytic role of imidazole in reaction. The studies of Irgum et al. and Birks and coworkers focused on the mechanism and kinetic pathway of PO–CL reaction in the presence of imidazole. According to their findings, imidazole as a nucleophile attacks one carbonyl group of oxalate ester, leading to generating a zwitterionic intermediate. Another imidazole molecule acts as a base catalyst and receives a proton from the acylimidazolium ion. The intermediate discharge of phenoxide in the same process is repeated at the other carbonyl of the oxalate to form 1,1-oxalydiimidazole Scheme 2 [35, 36].
Effect of Fluorophore (2-CPQ) on CL System
The peroxyoxalate system remains one of the most efficient chemiluminescent processes,2 exhibiting luminescence quantum yields (QCL). It consists of a base catalyzed reaction between activated oxalic phenyl esters and hydrogen peroxide in the presence of highly fluorescent aromatic hydrocarbons [37]. Figure 8 confirms steady-state chemiluminescence spectra with varying concentrations of 2-CPQ. As recorded without fluorophore, no reliable CL signal was observed, while by adding fluorophore, the intensive pink CL peak by 2 shoulders (607 and 654 nm) was assessed. The maximum emission wavelength in the sensitized PO-CL spectrum of the 2-CPQ and its fluorescence spectrum, registered under comparable experimental states, was determined to be nearly related, which shows that the singlet excited state of the fluorescent species is made in the CL reaction and is the emitting species. Figure 9 (Inset) shows the effect of concentration on the CL spectrum. As can be seen by raising the concentration of 2-CPQ, the CL peak constantly increased without any shift in wavelength. Furthermore, the kinetic profile of the CL reaction of TCPO–H2O2–ImH in the presence of different concentrations of 2-CPQ as a new fluorophore is presented in Fig. 8. The intensity of the peak rises immediately after mixing and reaches a maximum in a few seconds. The decrease of the light intensity from the maximum occurs for a much longer duration. The Table 2. Other quinoxaline core skeleton fluorophores are demonstrated that have been reported according to their light and emission wavelength.
Possible Mechanism
The mechanism of action for the CL reaction of TCPO–H2O2 has been examined before. The PO-CL reaction can be predicted in three main steps. First of all reaction, an aryl oxalate ester like TCPO reacts with H2O2 to create a chief chemical intermediate, C2O4, including the primary excitation energy. The second step includes the chemiexcitation of a fluorophore, similar to 2-CPQ, electronically excited states by the reactive intermediate by transforming the chemical energy into electronic excitation energy. The last step is the emission of light energy by exchanging the excited fluorophore molecule to its ground state [21]. It has been confirmed that H2O2 immediately decays to OH· radical via the catalytic behavior of imidazole [41]. Imidazole as a nucleophile attacks one carbonyl group of oxalate ester caused the generation of a zwitterionic tetrahedral intermediate. Another imidazole molecule operates as a base catalyst that receives a proton from the acylimidazolium ion. The intermediate release of phenoxide to form 1, 1-oxalydiimidazole. In the presence of imidazole, OH· radical is generated instantly in the process of reaction. Certainly, OH· radical is a very unstable reactive intermediate that could attack H2O2 to form a superoxide anion. Superoxide anion stimulates the production of intermediate 1,2‐dioxetanedione, which plays a vital role during the CL reaction of TCPO–H2O20 [42, 43]. The mechanism of 2-CPQ chemiluminescence activity from the reaction between TCPO, imidazole, and hydrogen peroxide is represented in Scheme 2.
Conclusion
Chemiluminescence has been absorbing humans ever since they first laid eyes on the natural luminescence. The substantial progress has been organized in considerate its nature and exploit its unique things in different scientific areas. The use of peroxyoxalate chemiluminescence in analytical methods has significantly enhanced the detection of even trace amounts of analytes in different media and CL is yet to be utilized to its full potential in the biological and medical fields. In the way of finding new fluorophores for the medical approach, we synthesized 6-chloro-2,3 di(pyridine-2yl) quinoxaline (2-CPQ) based fluorophores for the first time. The mentioned derivative of quinoxaline was found amongst several products synthesized using the current method. To do this, we conducted an adequate iodine catalyzed method to synthesize quinoxaline. The construction of the new quinoxaline-pyridine system went through two easy systems consisting of oxidation and cyclization procedures. The reaction provided a clean product with high yields. This approach has applications in the synthesis of biologically and medicinally important compounds.
Moreover, the compound was successfully applied as a fluorophore in the peroxyoxalate chemiluminescence system. Where the quinoxalines emit various colors such as green or blue, our synthetic approach (2-CPQ) could emit light pink underneath the peroxyoxalate system. In the recent years, numerous papers dealing with new chemiluminogenic compounds and their applications in immunoassays and biomedical research have been issued. We anticipate our synthesized fluorophores 2-CPQ will have great potential applications in imaging and medical markers.
References
Pereira JA, Pessoa AM, Cordeiro MN, Fernandes R, Prudêncio C, Noronha JP, Vieira M (2015) Quinoxaline, its derivatives and applications: A State of the Art review. Eur J Med Chem 5:664–672. https://doi.org/10.1016/j.ejmech.2014.06.058
Sharma G, Abraham I, Tulsi P (2009) Synthesis of quinoxaline quinones and regioselectivity in their Diels-Alder cycloadditions. J Indian Chem 48:1590–1596
Nageswar YVD, Harsha Vardhan Reddy K, Ramesh K, Narayana Murthy S (2013) Recent developments in the synthesis of quinoxaline derivatives by green synthetic approaches. Org Prep Proced Int 45:1–27
Malik MA, Dar OA, Gull P, Wani MY, Hashmi AA (2018) Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. Med Chem Comm 9(3):409–436. https://doi.org/10.1039/C7MD00526A
Shamsi-Sani M, Shirini F, Abedini M, Seddighi M (2016) Synthesis of benzimidazole and quinoxaline derivatives using reusable sulfonated rice husk ash (RHA-SO3H) as a green and efficient solid acid catalyst. Res Chem Intermed 42:1091–1099. https://doi.org/10.1007/s11164-015-2075-5
Aparicio OA, Attanasi P, Filippone R, Ignacio S, Lillini F, Mantellini F, Palacios JM, Santos D (2006) Straightforward access to pyrazines, piperazinones, and quinoxalines by reactions of 1, 2-diaza-1, 3-butadienes with 1, 2-diamines under solution, solvent-free, or solid-phase conditions. J Org Chem 71(16):5897–5905. https://doi.org/10.1021/jo060450v
Kunkuma V, Bethala LAPD, Bhongiri Y, Rachapudi BNP, Potharaju SSP (2011) An efficient synthesis of quinoxalines catalyzed by monoammonium salt of 12-tungstophosphoric acid. Eur J Chem 2(4):495–498. https://doi.org/10.5155/eurjchem.2.4.495-498.413
Shi DQ, Ni SN, Shi JW, Dou GL, Li XY, Wang XS (2008) An efficient synthesis of polyhydroacridine derivatives by the three-component reaction of aldehydes, amines and dimedone in ionic liquid. J Heterocycl Chem 45(3):653–660. https://doi.org/10.1002/jhet.5570450303
Antoniotti S, Duñach E (2002) Direct and catalytic synthesis of quinoxaline derivatives from epoxides and ene-1, 2-diamines. Tetrahedron Lett 43(22):3971–3973
Abad N, Ramli Y, Ettahiri W, Ferfra S (2020) Quinoxaline derivatives: syntheses reactivities and biological properties. Moroccan J Heterocycl Chem. 19(2):1–62. https://doi.org/10.48369/IMIST.PRSM/jmch-v19i2.22352
Izadyar A, Omer KM, Liu Y, Chen S, Xu X, Bard AJ (2008) Electrochemistry and electrogenerated chemiluminescence of quinoxaline derivatives. J Phys Chem. 18;112 (50):20027–32. https://doi.org/10.1021/jp807202d
Qazvini NT, Zinatloo S (2011) Synthesis and characterization of gelatin nanoparticles using CDI/NHS as a non-toxic cross-linking system. J Mater Sci - Mater Med 22(1):63–6913. https://doi.org/10.1007/s10856-010-4178-2
Zinatloo AS, Taheri QN (2014) Inverse miniemulsion method for synthesis of gelatin nanoparticles in presence of CDI/NHS as a non-toxic cross-linking system. J Nanostruct 4(3):267–275. https://doi.org/10.1007/s10856-010-4178-2
Zinatloo-Ajabshir Z. Zinatloo-Ajabshir S (2019) Preparation and characterization of Curcumin niosomal nanoparticles via a simple and eco-friendly route. J Nanostruct 9(4):784–790. https://doi.org/10.22052/JNS.2019.04.020
Zinatloo-Ajabshir S, Mousavi-Kamazani M (2021) Recent advances in nanostructured Sn− Ln mixed-metal oxides as sunlight-activated nanophotocatalyst for high-efficient removal of environmental pollutants. Ceram Int 47:23702–23724. https://doi.org/10.1016/j.ceramint.2021.05.155
Zinatloo-Ajabshir S, Heidari-Asil SA, Salavati-Niasari M (2022) Rapid and green combustion synthesis of nanocomposites based on Zn–Co–O nanostructures as photocatalysts for enhanced degradation of acid brown 14 contaminant under sunlight. Sep Purif Technol 280:119841. https://doi.org/10.1016/j.seppur.2021.119841
Etemadi H, Afsharkia S, Zinatloo-Ajabshir S, Shokri E (2021) Effect of alumina nanoparticles on the antifouling properties of polycarbonate-polyurethane blend ultrafiltration membrane for water treatment. Polym Eng Sci 61(9):2364–2375
Kricka L (2000) Application of bioluminescence and chemiluminescence in biomedical sciences. Meth Enzymol 305:333–345. https://doi.org/10.1016/S0076-6879(00)05498-7Get
Yang L, Guan G, Wang S, Zhang Z (2012) Nano-anatase-enhanced peroxyoxalate chemiluminescence and its sensing application. J Phys Chem C 116(5):3356–3362. https://doi.org/10.1021/jp210316s
Kazemi SY, Abedirad SM, Vaezi Z, Ganjali MR (2012) A study of chemiluminescence characteristics of a novel peroxyoxalate system using berberine as the fluorophore. Dyes Pigm 95(3):751–756. https://doi.org/10.1016/j.dyepig.2012.05.022
Alvarez FJ, Parekh NJ, Matuszewski B, Givens RS, Higuchi T, Schowen R (1986) Multiple intermediates generate fluorophore-derived light in the oxalate/peroxide chemiluminescence system. J Am Chem Soc 108(20):6435–6437. https://doi.org/10.1021/ja00280a078
Stevani CV, Silva SM, Baader WJ (2000) Studies on the mechanism of the excitation step in peroxyoxalate Chemiluminescence. Eur J Org Chem 2000(24):4037–4046
Schuster GB (1979) Chemiluminescence of organic peroxides. Conversion of ground-state reactants to excited-state products by the chemically initiated electron-exchange luminescence mechanism. Acc Chem Res 12(10):366–373. https://doi.org/10.1021/ar50142a003
Ciscato LF, Bartoloni FH, Bastos EL, Baader WJ (2009) Direct kinetic observation of the chemiexcitation step in peroxyoxalate Chemiluminescence. J Org Chem 74(23):8974–8979. https://doi.org/10.1021/jo901402k
Shimakawa Y, Morikawa T, Sakaguchi S (2010) Facile route to benzils from aldehydes via NHC-catalyzed benzoin condensation under metal-free conditions. Tetrahedron Lett 51(13):1786–1789. https://doi.org/10.1016/j.tetlet.2010.01.103
18. Miyashita A, Suzuki Y, Iwamoto KI, Higashino T (1994) Catalytic action of azolium salts. VI. preparation of benzoins and acyloins by condensation of aldehydes catalyzed by azolium salts. Chem Pharm Bull 42(12):2633–2635. https://doi.org/10.1248/cpb.42.2633
Zhang Z, Xie C, Feng L, Ma C (2016) PTSA-catalyzed one-pot synthesis of quinoxalines using DMSO as the oxidant. Synth Commun 46(18):1507–1518. https://doi.org/10.1080/00397911.2016.1213297
Xie C, Zhang Z, Yang B, Song G, Gao H, Wen L, Ma C (2015) An efficient iodine–DMSO catalyzed synthesis of quinoxaline derivatives. Tetrahedron 71(12):1831–1837. https://doi.org/10.1016/j.tet.2015.02.003
De Jong GJ, Lammers N, Spruit FJ, Brinkman UT, Frei RW (1984) Optimization of a peroxyoxalate chemiluminescence detection system for the liquid chromatographic determination of fluorescent compounds. Chromatographia 18(3):129–133. https://doi.org/10.1007/BF02258768
Shamsipur M, Chaichi MJ, Karami AR (2003) A study of peroxyoxalate-chemiluminescence of acriflavine. Spectrochim Acta A Mol Biomol Spectrosc 59(3):511–517. https://doi.org/10.1016/S1386-1425(02)00188-9
Samadi-Maybodi A, Akhoondi R, Chaichi MJ (2010) Studies of New Peroxyoxalate-H 2 O 2 Chemiluminescence System Using Quinoxaline Derivatives as Green Fluorophores. J Fluoresc 20(3):671–679. https://doi.org/10.1007/s10895-010-0601-9
Martelo L, Periyasami G, Fedorov AA, Baleizao C, Berberan-Santos MN (2019) Chemiluminescence of naphthalene analogues of luminol in solution and micellar media. Dyes Pigm 168:341–346. https://doi.org/10.1016/j.dyepig.2019.05.005
Kazemi SY, Abedirad SM, Zali SH, Amiri M (2012) Hypericin from St. John’s Wort (hypericum perforatum) as a novel natural fluorophore for chemiluminescence reaction of bis (2, 4, 6-trichlorophenyl) oxalate–H2O2–imidazole and quenching effect of some natural lipophilic hydrogen peroxide scavengers. J Lumin 132(5):1226–1231. https://doi.org/10.1016/j.jlumin.2011.12.009
Givens RS, Schowen RL, Birks JW (1989) Chemiluminescence and photochemical reaction detection in chromatography. VCH, New York, USA ([Chapter 5])
Hadd AG, Seeber A, Birks JW (2000) Kinetics of two pathways in peroxyoxalate chemiluminescence. J Org Chem 65(9):2675–2683. https://doi.org/10.1021/jo9917487
Emteborg M, Pontén E, Irgum K (1997) Influence of imidazole and bis (trichlorophenyl) oxalate in the oxalyldiimidazole peroxyoxalate chemiluminescence reaction. Anal Chem 69(11):2109–2114. https://doi.org/10.1021/ac961225o
Stevani CV, de Arruda Campos IP, Baader WJ (1996) Synthesis and characterisation of an intermediate in the peroxyoxalate chemiluminescence: 4-chlorophenyl O, O-hydrogen monoperoxyoxalate. J Chem Soc Perkin Trans I 2(8):1645–1648. https://doi.org/10.1039/P29960001645
Dong J, Yang H, Li Y, Liu A, Wei W, Liu S (2020) Fluorescence sensor for organophosphorus pesticide detection based on the alkaline phosphatase-triggered reaction. Anal Chim Acta 1131:102–108. https://doi.org/10.1016/j.aca.2020.07.048
Wang L, Cui M, Tang H, Cao D (2018) Fluorescent nanoaggregates of quinoxaline derivatives for highly efficient and selective sensing of trace picric acid. Dyes Pigm 155:107–113. https://doi.org/10.1016/j.dyepig.2018.03.036
Yang W, Yang Y, Zhan L, Zheng K, Chen Z, Zeng X, Yang C (2020) Polymorphism-dependent thermally activated delayed fluorescence materials with diverse three dimensional supramolecular frameworks. Chem Eng J 390:124626. https://doi.org/10.1016/j.cej.2020.124626
Jonsson T, Irgum K (2000) New nucleophilic catalysts for bright and fast peroxyoxalate Chemiluminescence. Anal Chem 72(7):1373–1380. https://doi.org/10.1021/ac991339a
Emteborg M, Pontén E, Irgum K (1997) Influence of imidazole and bis (trichlorophenyl) oxalate in the oxalyldiimidazole peroxyoxalate chemiluminescence reaction. Anal Chem 558:69(11):2109–2114. https://doi.org/10.1021/ac961225o
Jonsson T, Emteborg M, Irgum K (1998) Heterocyclic compounds as catalysts in the peroxyoxalate chemiluminescence reaction of bis (2, 4, 6-trichlorophenyl) oxalate. Anal chim acta 361(3):205–215. https://doi.org/10.1016/S0003-2670(98)00029-4
Acknowledgements
This research was supported by a grant from the research council of Mazandaran University of Medical Sciences, Iran (No. 1161), this work dedicated to a part of Zahra Hashemi's post-doctoral project.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Zahra Hashemi was the first researcher, summarized the synthesis and characterization of 2-CPQ. Mohammad Ali Ebrahimzadeh was the principal investigator and designed the study. Pourya Biparva was the principal investigator and designed the analysis. Data analysis was performed by Seyed Mohammad Abedirad.
Corresponding authors
Ethics declarations
Conflicts of Interest
Not applicable.
Data Availability
The authors confirm that the findings and results of this study are available.
Code Availability
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashemi, Z., Ebrahimzadeh, M.A., Biparva, P. et al. Pyridine-2-yl Quinoxaline (2-CPQ) Derivative As a Novel Pink Fluorophore: Synthesis, and Chemiluminescence Characteristics. J Fluoresc 32, 723–736 (2022). https://doi.org/10.1007/s10895-022-02890-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-022-02890-w