Abstract
In this investigation, a simple, green and facile fluorescence mrtod using carbon dots (CDs) of hazelnut kohl is described for selective detection of dopamine (DA). The sensing system is based on hazelnut kohl (the black soot of kohl) which is used as a carbon source. Generally, kohl is a traditional eye cosmetic that used in different parts of the world and synthesized by the combustion process like burning natural materials. Here, it has been proven that black soot (kohl) obtained from hazelnut has a carbon dot structure and can be used for sensory applications. Some characterization methods are carried out to reveal the kohl structure. Also, the photoluminescence properties of the prepared CDs of kohl are investigated. It is found that the size of CDs is 2–4 nm. Besides, under the optimal conditions, the fluorescence of CDs is used for DA determination. CDs fluorescence intensity is decreased linearly with the increase of DA concentration. By using the fluorescence dependency toward the DA concentration, DA can be determined in the range 0.5–30 μM with the limit of detection of 0.30 μM. Finally, this method is successfully applied to discriminate the DA in the real samples (healthy human serum and cerebrospinal fluid (CSF)) which shows acceptable efficiently for diagnostic purposes.
Graphical abstract

The fluorescence of carbon dots, prepared from Hazelnut Kohl, is quenched in the presence of dopamine
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) is an essential catecholamine neurotransmitters in central nervous systems that has been discovered in mammalian brain in the 1950s [1]. Abnormal DA concentration in the brain may lead to some neurological system diseases like Parkinsonism, schizophrenia, Alzheimer and euphoria [2,3,4]. Hence, selective and precise measurement of DA is extremely important for clinical diagnosis. In the past few years, various analytical methods have been developed to determine DA effectively including high-performance liquid chromatography [5], electrochemical analysis [6], Electrogenerated chemiluminescence (ECL) [7], colorimetric [8] and fluorescence method [9]. Among these analytical methods, fluorescence spectroscopy utilizing semiconductor quantum dots (QDs) and carbon dots (CDs) have drawn attention for their attractive attributes. They possess some unique properties such as tunable photoluminescence (PL) properties, low toxicity, eco-friendliness and good biocompatibility [2, 10, 11]. The most attractive and practical feature of CDs is their fluorescence, which gives the high potential in biological labeling and bioimaging applications [12,13,14]. Additionally, fluorescence spectroscopy has outstanding features such as low cost, practicability, high sensitivity and good selectivity [15].
CDs as carbonaceous nanomaterial were first synthesized by Xu et al. in 2006 during separation and purification of single walled carbon nanotubes by the arc-discharge method [10, 16]. They are recently discovered as nanoparticles which are quasispherical with sizes below 10 nm [17]. Typically, sized below 10 nm, CDs exhibit unique and useful optical features such as high photostability, excitation wavelength-dependent fluorescence and tunable emission color which have not seen in their nanocarbon relatives (graphenes, fullerenes and carbon nanotubes) [18].
CDs can be synthesized using either top-down or bottom-up approaches. They are including arc-discharged soot [17], laser irradiation of carbon nanoparticles [19], combustion and thermal routes [20, 21] and thermal carbonization of molecular precursors [22]. Among all these synthetic methods, combusting organic material is an elegantly simple source of producing CDs [17]. Interestingly, through producing lab-made kohl (surmah) from hazelnut by the combustion process, we found that the kohl is containing CDs. Kohl is a traditional eye cosmetic which is wildly used in different parts of the world [23]. It’s an ophthalmology and ophthalmic product which is known as surmah in Iran, kohl or cohol in Arabic countries, kajal in India and tiro in Nigeria [24, 25]. They are mostly prepared using natural sources such as leaves, nuts (e.g., hazelnut), beef, oils and so on.
Actually, cost and availability of precursor material are the prime concerns for production of CDs. So, a simple and economical manner for synthesizing of CDs has been attracting in recent years [26, 27]. In this study, soot-based hazelnut kohl is synthesized by the combustion process. It is demonstrated that it has a nanoparticle structure and can be used as an affordable source of CDs, which can be used in analytical chemistry. CDs with smaller sizes are synthesized by and easy-done and green method of kohl production, without using any chemical reagents. According to the best of our knowledge, there is no report on developing a facile, low cost and selective fluorescent sensor based on CDs of hazelnut kohl for detection of DA in alkaline solution. As it is discussed before, DA in alkaline environment has a quenching effect on the fluorescence of CDs [28,29,30]. The presented sensing system for DA is low cost, effortless, environments-friendly and less toxic which shows adequate performance for biological samples.
Experimental
Reagents
All reagents and chemicals used in this work were of analytical grade and utilized without further purification. Amino acids and DA were purchased from sigma. Calcium chloride (CaCl2), ascorbic acid, cysteine, sodium hydroxide (NaOH), hydrochloric acid (HCl), phosphoric acid (H3PO4), acetic acid (CH3COOH), sodium chloride (NaCl), urea (OC(NH2)2), maltose, ammonium chloride (NH4Cl), uric acid (C5H4H4O3), sodium nitrate (NaNO3), glucose and aniline were obtained from Merck Chemical Company. Boric acid (H3BO3) was purchased from BDH laboratory reagents. All solutions were provided by dissolving the reagents in deionized water. Aqueous universal buffer solutions (pH 2.0 to 12.0) were prepared by addition of appropriate amount of 0.2 M NaOH solution to the mixture of boric acid, acetic acid and phosphoric acid (all 0.1 M) [31].
Apparatus
Fluorescence spectra were recorded on a Perkin-Elmer LS50B fluorescence spectrophotometer with excitation slit of 10 nm. The fluorescence cell was water-thermostated at 25 °C using a PECO thermostat bath (Shiraz, Iran) equipped with a water circulator. The pH was measured using a Metrohm pH-meter model 827. The UV-VIS absorbance spectra were recorded using a BEL photonics, spectrum lab 53 (Italy), equipped with a quartz analytical cells of 1 cm path-length. Fourier transform infrared (FT-IR) spectra were recorded on a Perkin Elmer FT-IR spectrometer– spectrum RX1 equipped with a Perkin Elmer ZnSe ATR sampler accessory. High Resolution Transmission Electron Microscopy (HRTEM) images were taken on a JEOL instrument (JEM – 2100 F at 200 KV).
Synthesis of CDs from Hazelnut Kohl
Kohl is usually prepared by burning natural materials like hazelnut in the air. In a typical burning procedure, a medium sized hazelnut is burned in the air. To collect the soot, a glass plate is put 50 cm apart on the top of the flame. At the end of burning, the soot-based hazelnut kohl is scraped out with spatula and stored in sample tubes.
The soot-based kohl is then dispersed in alkaline solution to separate the CDs: a 0.12 g portion of kohl is added into 5.0 mL NaOH (1 M), and the mixture is sonicated for 30 min. The smaller particles are collected by removing the large particles through centrifugation at 12000 rpm for 30 min. Then, the solution is filtered with a 0.22 μM syringe filter and the resulting solution is kept in refrigerator for sensory applications.
Procedure for Sensing of DA
The typical procedure for detection of DA by kohl-based CDs was carried out as follows: 400.0 μL (0.002\( \frac{\ mg}{mL} \)) of CDs solution from its stock solution was diluted to 1.5 mL using universal buffer solution (0.1 M, pH 12.0) in the volumetric flask. Fluorescence data were recorded at 5 min after each addition of the DA (100.0 μL with desired concentrations) at the excitation wavelength of 260 nm, the scan speed of 500 nm min −1 and slit widths of 10 nm.
Results and Discussion
Characterization of Hazelnut CDs
High-resolution transmission electron microscopy (HREM) was the first characterization method that is used for kohl characterization. As shown in Fig. 1, hazelnut kohl contained CDs with a frequently microsphere structure. It was found that the spherical particles were dispersible in alkaline solution, and the size of CDs was 2–4 nm. One can find that the interlayer spacing of CDs is about 0.32 nm, which is close to that of (002) planes of graphite [32].
Lower panel of Fig. 1 displays the powder XRD spectrum of CDs from soot-based kohl. It shows a sharp peak at around 2θ = 23°, corresponding to reflections of graphitic carbon, which indicates that the CDs of kohl are crystalline [32]. Another peak at 2θ= 44°, indexed to the reflections of the hexagonal graphite crystal structure [33]. The FTIR spectrum of CDs is also shown in lower panel of Fig. 1. The bond around at 3427 cm−1 is assigned to the O–H stretching vibration and the band at 2929 cm−1 is attributed to C–H stretching deformations. A weak absorption band at 3050 cm−1 corresponds to the C=C–H stretching vibration. It is accepted that some unsaturated groups would produce in the burning procedure of the flammable materials [34]. The absorption band at 1554 demonstrates that CDs contain some aromatic structures. The C–O stretching band at 1227 cm−1 indicates that CDs have oxygen-containing groups too. The peak at 919 cm−1 represents the aromatic hydrocarbons. Generally, the FTIR results showed that CDs are aromatic skeletons in the core and some oxygen-containing groups and alkyl groups at the surface [35].
The results of the optical investigations on the CDs are shown in Fig. 2. The colorless CDs solution emits a blue emission under UV irradiation (365 nm) (Fig. 2 (inset C)). According to the fluorescent spectra of CDs, the maximum excitation and emission wavelength of CDs are observed at 220 and 409 nm, respectively. It is observed from Fig. 2B that absorbance spectrum of CDs is associated with three absorption peaks. The intense peak located at 220 nm gives information about the physical size of the graphite structure [20, 35]. The two others, (one appeared as a shoulder at 270 nm and another as a weak peak at around 340 nm), which demonstrate respectively the π-π* and n-π* transitions [36], can be attributed to the presence of C=C, oxygen containing groups and aromatic structures in the hazelnut kohl CDs, as demonstrated by FTIR results. Addition of DA to CDs caused slight changes in the absorbances 270 nm and 340 nm peaks, suggesting interacting of the DA with the conjugated part (π system) of the CDs.
Sensing of DA by Kohl-Based CDs
As shown in Fig. 2A, the fluorescence intensity of CDs is quenched by addition of DA in the wavelength range of 350–500, whereas DA doesn’t have significant fluorescence characteristics compared to CDs in this wavelength range. So, decreasing in fluorescence intensity of CDs was used as a sensing criterion to detect and measure DA.
Optimization of the Experimental Condition
In the first step, some important parameters such as the amount of CDs, pH of buffer solution, temperature, incubation time and stability of the sensor response were optimized.
To decrease the consumption of the as prepared CDs solutions, the stock solution of CDs was diluted in various ratios, and the fluorescence intensities for each dilution were measured. As shown in Fig. 3, the highest fluorescent intensity was obtained when CDs stock solution was diluted 2, 3 and 4 times with water. Further dilution resulted in decreased fluorescence intensity. To use lower amount of CDs as much as possible and in the same time has the highest fluorescence intensity, fourfold dilution of CDs stock solution was selected as the optimum value for further experiments.
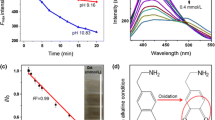
To investigate the effect of pH on the performance of the CDs sensing system, the universal buffer (0.1 M) was selected and the response of the sensor was evaluated in different pH values in the range of 7.0 to 13.0. In acidic pH conditions (PH< 7.0), the organic compounds such as carboxylic groups are more accessible on the surface of CDs. Carboxyl coated CDs absorb some emission signals while hydroxyl would advance fluorescence emission with strong electron donated ability. So, hydroxyls-coated CDs had better fluorescence emission ability than carboxyls-coated [37]. Thus, the fluorescence quenching in the alkaline solution is more efficient. In these conditions, DA was oxidized to dopamine-quinine [38]. Photoexcited electrons of CDs can transfer to dopamine–quinine and it might be the causes of fluorescence quenching [39]. As shown in Fig. 4a, the CDs represented the highest fluorescence intensity at pHs 11.0 and 12.0. However, the extent of quenching (as shown in Fig. 4b) is largest at pH 12.0. Consequently, next experiments were conducted in pH 12.0.
Investigating the effect of temperature on the fluorescence quenching CDs after addition of DA (25.0 μM) shows that the performance of the sensing system is not significantly affected by temperature (Fig. S1). So, the ambient temperature was considered as the optimum temperature for subsequent experiments.
The stability of sensor was evaluated by time using two different methods. Firstly, the fluorescence characteristics of CDs in the absence of DA were monitored over time for 2 h in 10 min intervals (Fig. S2). A stable fluorescence was observed. In the second experiment, the fluorescence intensity of CDs after interaction with DA was investigated. This helps to find the optimum reaction time as well. As shown in Fig. S2, 5 min is required for completion of the reaction between CDs and DA. After that, the signal was stable for around 2 h.
Determination of DA
A gradual decrease in the fluorescence intensity of CDs was observed by increasing the concentration of DA (Fig. 5A). The calibration graph was constructed by plotting the relative decrease in fluorescence intensity (F0-F)/F0 (where F0 and F are respectively the fluorescence intensity of the system in the absence and presence of DA) versus the concentration of DA. A well-defined quadratic relationship was achieved between the relative fluorescence changes and DA concentration in the concentration range of 0.5–50 μM (Fig. 5B). As shown in part C of Fig. 5, the quadratic relationship can be transformed into linear calibration graph by double logarithm transformation. The limit of detection (LOD) was calculated using the equation suggested by IUPAC as LOD= (yb + 3 sb)/m, where yb, and sb stand for average and standard deviation of blank signal for 5 repeated measurements, and m denotes the slope of calibration curve [40]. Using this formula, a LOD of 0.30 μM was estimated. The relative standard deviation (RSD) was around 3% for all measurements.
(A) Changes in the fluorescence spectra (λexi 260 nm) of the kohl-made CDs in the presence of different amounts of DA (0.5, 1, 2.5, 5, 10, 25, 35, 40, 50 and 100 μM). (B) Relative changes in the fluorescence intensity at 490 nm as function of DA concentration, where the dotted line represents fitting to a quadratic regression model. (C) Double logarithm transformation of the plot shown in part B (in the concentration range of 0.5–50 μM), where the dotted line represents fitting to a linear regression model. Error bars represent the standard deviation of measurements (RSD ≅ 3.0%)
Table 1 compares analytical features of our proposed method with some typical methods used for DA detection. Our method represented comparable and in some instances, better results. A bold advantage that can be encountered for our method is simplicity of preparing the sensing regent.
Selectivity of the Method for DA Detection
To investigate the selective of methods toward DA, the fluorescent signals of CDs in the presence of some foreign substances of possible interferences such as glutathione, ammonium, aniline, arginine (arg), ascorbic acid, aspartic acid, calcium chloride (CaCl2), creatinine, cysteine (cys), galactose, glycine (gly), histidine (his), potassium chloride (KCl), lysine (lys), maltose, sodium chloride (NaCl), sodium nitrate (NaNO3), serine (ser), urea, uric acid and xylose were measured and compared with that of DA at the same concentration (25.0 μM). As shown in Fig. 6, only DA caused a dramatic decrease in the fluorescence intensity, while, no or small changes in the fluorescence intensity were observed in the presence of other species. The only possible interfering species is ascorbic acid.
Application to Real Sample Analysis
To determine the practicality of the designed probe, the sensor was used to assay DA in two different matrices (healthy human serum and cerebrospinal fluid (CSF)). The content of DA in plasma of the healthy people is in the range of 0.01–1 μM [41]. However, abnormal ranges higher than 16 μM have also been reported [2]. So, the plasma content of DA is in the working range of our method. The DA concentration in CSF is lower than the detection limit of our method. However, we applied our method to determination of DA in CSF as a proof of concept to show the suitability of our method for successful analysis of complex samples.
CSF was taken from 41- year old man who had a stroke from Namazi-hospital Shiraz, and multi-control serum (5.0 mL) was obtained from Ziest Chem Diagnostics [1]. The 100-fold diluted samples were spiked with 7.0, 15.0 and 20.0 μM of DA and the concentration of DA in the samples was determined by the standard additions method. As shown in Table 2, satisfactory recoveries of DA in human serum and CSF samples were obtained. Thus, the proposed sensing system may hold potential application for DA detection in biological samples.
Conclusion
The results from the present study demonstrated an effective and selective fluorescence method for DA detection in alkaline solution. In this procedure, the fluorescent CDs, which are prepared by the combustion process, are used as probe. They were synthesized by burning the hazelnut to obtain black soot that is called kohl. Although the CDs preparation precursor is completely different from previous ones, the obtained CDs had similar HRTM images, sizes and aromatic compounds giving them similar UV-VIS absorption and photo-luminescence features. It should be noted that the proposed sensor based on kohl is simple, rapid and convenient, also all the materials used in the study are low toxic or even non-toxic that avoids environmental pollution. Eventually, the kohl based sensor has the potential for the determination of DA in biological samples with satisfactory results.
References
Foroughi F, Rahsepar M, Kim H (2018) A highly sensitive and selective biosensor based on nitrogen-doped graphene for non-enzymatic detection of uric acid and dopamine at biological pH value. Journal of Electroanalytical Chemistry 827:34–41
Zhu L, Xu G, Song Q, Tang T, Vang X, Wei F, Hu Q (2016) Highly sensitive determination of dopamine by a turn-on fluorescent biosensor based on aptamer labeled carbon dots and nano-graphite. Sensors and Actuators B: Chemical 231:506–512
Zhou X, Ma P, Wang A, Yu C, Qian T, Wu S, Shen J (2015) Dopamine fluorescent sensors based on polypyrrole/graphene quantum dots core/shell hybrids. Biosensors and Bioelectronics 64:404–410
Lewis MM, Huang X, Nichols DE, Mailman RB (2006) D1 and functionally selective dopamine agonists as neuroprotective agents in Parkinson’s disease. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 5(3):345–353
Wu D, Xie H, Lu H, Li W, Zhang Q (2016) Sensitive determination of norepinephrine, epinephrine, dopamine and 5-hydroxytryptamine by coupling HPLC with [Ag (HIO6) 2] 5–luminol chemiluminescence detection. Biomedical Chromatography 30(9):1458–1466
Zen JM, Chen PJ (1997) A selective voltammetric method for uric acid and dopamine detection using clay-modified electrodes. Analytical Chemistry 69(24):5087–5093
Li L, Liu H, Shen Y, Zhang J, Zhu JJ (2011) Electrogenerated chemiluminescence of Au nanoclusters for the detection of dopamine. Analytical Chemistry 83(3):661–665
Zheng V, Wang Y, Yang X (2011) Aptamer-based colorimetric biosensing of dopamine using unmodified gold nanoparticles. Sensors and Actuators B: Chemical 156(1):95–99
Halawa MI, Wu F, Fereja TH, Lu B, Zu G (2018) One-pot green synthesis of supramolecular β-cyclodextrin functionalized gold nanoclusters and their application for highly selective and sensitive fluorescent detection of dopamine. Sensors and Actuators B: Chemical 254:1017–1024
Das P, Ganguly S, Bose M, Mondal S, Dus AK, Banerjee S, Dus NC (2017) A simplistic approach to green future with eco-friendly luminescent carbon dots and their application to fluorescent nano-sensor ‘turn-off’ probe for selective sensing of copper ions. Materials Science and Engineering 75:1456–1464
Mao Y, Bao Y, Han D, Li F, Niu L (2012) Efficient one-pot synthesis of molecularly imprinted silica nanospheres embedded carbon dots for fluorescent dopamine optosensing. Biosensors and Bioelectronics 38(1):55–60
Sahu S, Behera B, Maiti TK, Mohapatra S (2012) Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chemical Communications 48(70):8835–8837
Li F, Liu C, Yang J, Vang Z, Liu W, Tian F (2014) Mg/N double doping strategy to fabricate extremely high luminescent carbon dots for bioimaging. RSC Advances 4(7):3201–3205
Liu Y, Liu C, Zhang Z (2012) Synthesis of highly luminescent graphitized carbon dots and the application in the Hg2+ detection. Applied Surface Science 263:481–485
He YS, Pan CG, Cao HX, Yoe MZ, Wang L, Liang GX (2018) Highly sensitive and selective dual-emission ratiometric fluorescence detection of dopamine based on carbon dots-gold nanoclusters hybrid. Sensors and Actuators B: Chemical 265:371–377
Zhang X, Zhang Y, Wang Y, Kalytchung S, Kershaw SV, Wang Y, Wang P, Zhang T, Zhao Y, Zhang H, Cui T, Wang Y, Zhao J, Yu WW, Rogach AL (2013) Color-switchable electroluminescence of carbon dot light-emitting diodes. ACS nano 7(12):11234–11,241.
Baker SN, Baker GA (2010) Luminescent carbon nanodots, emergent nanolights. Angewandte Chemie International Edition 49(38):6726–6744
Essner JB, Kist JA, Parada L, Baker GA (2018) Artifacts and errors associated with the ubiquitous presence of fluorescent impurities in carbon nanodots. Chemistry of Materials 30(6):1878–1887
Li X, Wang H, Shimizu Y, Pyatenko A, Kawaguchi K, Koshizaki N (2010) Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents. Chemical Communications 47(3):932–934
Liu H, Ye T, Mao C (2007) Fluorescent carbon nanoparticles derived from candle soot. Angewandte Chemie 46(34):6473–6475
Tian L, Gosh D, Chen W, Pradhan S, Chang X, Chen S (2009) Nanosized carbon particles from natural gas soot. Chemistry of materials 21(13):2803–2809
Bourlinos AB, Stassinopoulos A, Anglos D, Zboril R, Geoegakilas V, Giannelis EP (2008) Photoluminescent carbogenic dots. Chemistry of Materials 20(14):4539–4541
Sharma V, Bhardwaj S, Kumar R (2019) On the spectroscopic investigation of Kohl stains via ATR-FTIR and multivariate analysis: Application in forensic trace evidence. Vibrational Spectroscopy 101:81–91
Ullah PH, Mahmood ZA, Sualeh M, Zoha SM (2010) Studies on the chemical composition of kohl stone by X-ray diffractometer. Pak J Pharm Sci 23:48–52
Said E, Shafey E, Al-Kitani BS (2017) Comparative chemical analysis of some traditional Omani-made kohl. Toxicological & Environmental Chemistry 99(2):233–251
Guo X, Wu F, Ni Y, Kokot S (2016) Synthesizing a nano-composite of BSA-capped Au nanoclusters/graphitic carbon nitride nanosheets as a new fluorescent probe for dopamine detection. Analytica chimica acta 942:112–120
Chibac AL, Melinte V, Buruiana T, Buruiana EC (2017) Fluorescent polymeric sensors containing boronic acid derivatives for sugars and dopamine detection Sensing characteristics enhancement by Au NPs. Sensors and Actuators B: Chemical 253:987–998
Medintz IL, Stewart MH, Trammell SA, Susumo K, Delehanty JB, Mei BC, Melinger JS, Belanco- Canosa JB, Dowson PE, Mattoussi H (2010) Quantum-dot/dopamine bioconjugates function as redox coupled assemblies for in vitro and intracellular pH sensing. Nature materials 9(8):676–684
Ji X, Palui G, Avellini T, Na HB, Yi C, Knappenberger KL, mattoussi H (2012) On the pH-dependent quenching of quantum dot photoluminescence by redox active dopamine. Journal of the American Chemical Society 134(13):6006–6017
Zhao D, Song H, Hao L, Liu X, Zhang L, Lev Y (2013) Luminescent ZnO quantum dots for sensitive and selective detection of dopamine. Talanta 107:133–139
Naghashian-Haghighi A, Hemmateenejad B, Shamsipur M (2018) Determination of enantiomeric excess of some amino acids by second-order calibration of kinetic-fluorescence data. Analytical biochemistry 550:15–26
Tripathi KM, Tran TS, Tung TT, Losic D, Kim TY (2017) Water soluble fluorescent carbon nanodots from biosource for cells imaging. Journal of Nanomaterials 2017:1–10
Zhuang Q, Cao W, Ni Y, Yang Y (2018) Synthesis-identification integration: One-pot hydrothermal preparation of fluorescent nitrogen-doped carbon nanodots for differentiating nucleobases with the aid of multivariate chemometrics analysis. Talanta 185:49–498
McEnally CS, Pfefferle LD, Atakan B, Kohse-Hoinghaus (2006) K Studies of aromatic hydrocarbon formation mechanisms in flames: Progress towards closing the fuel gap. Progress in Energy and Combustion Science 32(3):247–294
Zhang S, Zhang L, Huang L, Zheng G, Zhang P, Jin Y, Jiao Z, Sun X (2019) Study on the fluorescence properties of carbon dots prepared via combustion process. Journal of Luminescence 206:608–612
Alas MO, Genc R (2017) An investigation into the role of macromolecules of different polarity as passivating agent on the physical, chemical and structural properties of fluorescent carbon nanodots. Journal of Nanoparticle Research 19(5):185
LiQuin L, YuanFang L, Lei Z, Yue L, ChengZhi H (2011) One-step synthesis of fluorescent hydroxyls-coated carbon dots with hydrothermal reaction and its application to optical sensing of metal ions. Science China Chemistry 54(8):1342–1347
Klegeris A, Korkina LG, Greenfield SA (1995) Autoxidation of dopamine: a comparison of luminescent and spectrophotometric detection in basic solutions. Free radical biology and medicine 18(2):215–222
Zhao J, Zhao L, Lan C, Zhao S (2016) Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine. Sensors and Actuators B: Chemical 223:246–251
Massart D, Vandeginste B, Deming S, Michotte Y, Kaufman L (1998) Chemometrics: A textbook. Elsevier, Amsterdam, pp. 107–114.
Zhao C, Jiao Y, Hua J, Yang J, Yang Y (2018) Hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for the detection of dopamine. Journal of fluorescence 28(1):269–276
Mu Q, Xu H, Li Y, Ma S, Zhong X (2014) Adenosine capped QDs based fluorescent sensor for detection of dopamine with high selectivity and sensitivity. Analyst 139(1):93–98
Kim YR, Bong S, Kang YJ, Yang Y, Mahajan RK, Kim JS, Kim H (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosensors and Bioelectronics 25(10):2366–2369
Devi JA, Aswathy B, Asha S, George S (2017) Lactose tailored boronic acid conjugated fluorescent gold nanoclusters for turn-on sensing of dopamine. Analytical chemistry 72(4):445–459
Qu F, Huang W, You J (2018) A fluorescent sensor for detecting dopamine and tyrosinase activity by dual-emission carbon dots and gold nanoparticles. Colloids and Surfaces B: Biointerfaces 162:212–219
Niu S, Fang Y, Zhang K, Sun J, Wan J (2017) Determination of dopamine using the fluorescence quenching of 2, 3-diaminophenazine. Instrumentation Science & Technology 45(1):101–110
Availability of Data and Materials
Not applicable.
Funding
The work is funded by Shiraz University Research Council.
Author information
Authors and Affiliations
Contributions
Neda Chavoshi; design the work, acquiring data and preparing first draft.
Bahram Hemmteenejad; Conceptualization, supervision, validation and writing the final draft.
Corresponding author
Ethics declarations
Competing Interests
There are nothing to declare
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 232 kb)
Rights and permissions
About this article
Cite this article
Chavoshi, N., Hemmateenejad, B. Fluorescent Carbon Dots Prepared from Hazelnut Kohl as an Affordable Probe for Determination of Dopamine. J Fluoresc 31, 455–463 (2021). https://doi.org/10.1007/s10895-020-02668-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02668-y