Abstract
Firstly, a novel pyrazole-pyrazoline fluorescent probe was developed and synthesized. The probe can be used to determine Fe3+ ions in a series of cations in tetrahydrofuran aqueous solution with high selectivity and high sensitivity. After the addition of iron ions, the fluorescence intensity is significantly reduced, Its structure was characterized by 1H NMR, 13C NMR and HR-ESI-MS. UV absorption spectra and Fluorescence spectroscopy were used to study the selective recognition of probe M on metal ions. The probe M can selectivity and sensitivity to distinguish the target ion from other ions through different fluorescence phenomena. In addition, the binding modes of M with Fe3+ were proved to be 1:1 stoichiometry in the complexes by Job’s plot, IR results. The combination of probe M and iron ions is 1:1, and the detection limit is 3.9 × 10−10 M. The binding mode and sensing mechanism of M with Fe3+ was verified by theoretical calculations using Gaussian 09 based on B3LYP/6-31G(d) basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal ion fluorescent probes have received extensive attention due to their wide application in environmental and biochemical fields [1]. Fe3+ ion is one of the essential ions for life. Many cell-level biochemical processes require the participation of iron ions [2, 3]. Therefore, the research on the detection of iron ions is of great significance in the environment and life sciences. The lack or excess of Fe3+ can induce many diseases, such as diabetes, hepatitis, anemia and Parkinson’s disease [4,5,6], so it is very important to detect Fe3+. Commonly used detection methods are colorimetry [7], atomic absorption spectrometry [8, 9], electrothermal atomic absorption spectrometry [10], cold vapor atomic absorption spectrometry [11], inductively coupled plasma emission spectrometry [12, 13], spectroscopy [14,15,16] and anodic stripping voltammetry [17], among them, fluorescence spectroscopy has the advantages of high sensitivity and simple operation, so it has attracted widespread attention, and many have been designed and synthesized probe [18].
Pyrazoline is an important nitrogen-containing five-membered heterocyclic compound. There are many methods to synthesize this compound [19,20,21] with many biological activities [22,23,24,25,26,27,28]. Pyrazoline has strong fluorescence properties, so it is also widely used in the field of optoelectronics. It is used as an optical brightener for textiles and papermaking materials. The pyrazoline fluorescent compound also has excellent stability, so it can be used in laser dyes, fluorescent dyes, for biological analysis, tracking detection, drug tracking and sun traps. Pyrazole is a colorless or white crystalline substance with high fluorescence quantum yield, high light stability, non-toxic and other optical properties, it is usually used as stabilizer, chelate, organic synthesis intermediate, etc [29,30,31]
Due to the excellent properties of pyrazole and pyrazoline, we designed and synthesized compound M that combines the advantages of both. This compound contains two fluorophores, a pyrazole unit and a pyrazoline unit. It has good selectivity and high-sensitivity fluorescence response to Fe3+. In order to clarify the complex relationship between the probe and Fe3+, theoretical calculations of density functional theory (DFT) were carried out. These results illustrate the reasonable design of fluorescent probes for Fe3+ detection. At the same time, the probe M has good yellow fluorescence, its emission wavelength is about 600 nm, the fluorescence quantum yield is high, the hole transport efficiency is high, it is easy to detect, and the color change can be directly observed with the naked eye. And it is also a good photoluminescent material. In addition, the resulting M-Fe3+ group reacts specifically with pyrophosphate (PPI) anion through fluorescence quenching.
Experimental Part
Materials and Characterization
FA1004 Electronic Balance (Changzhou Nuoji Instrument Co., Ltd.); SGWX-4 Micro-melting Point Instrument (Shanghai Precision Scientific Instrument Co., Ltd.); Bruker-AV400, 400 MHz Nuclear Magnetic Resonance Instrument; HPLC Q-Tof HR-MS Mass Spectrometer (Waters Micromass); Bruker DTX-400 carbon spectrum analyzer; UV-1800PC spectrometer; HITACHIF-4500 fluorescence spectrometer.
During the experiment, various metal ions correspond to chloride salts (Na+, K+, Ca2+, Cu2+, Zn2+, Ni2+, Pb2+, Mg2+, Al3+, Ba2+, Fe3+, Co2+, Mn2+) and nitrate (Ag+), Other reagents are commercially available analytical grade, and all samples are performed at room temperature.
Synthesis Route of Probe M
Scheme 1 shows the synthetic route of compound M.
The synthesis of probe M is as follows: o-hydroxyacetophenone was purchased from a commercial supplier. Compound a was prepared according to literature method [32] m.p.140–143 °C(lit [32]. m.p.144–146 °C), in 50 ml round-bottom flasks, add 0.210 g (1 mmol) of compound a, 0.136 g (1.0 mmol) of o-hydroxyacetophenone, 10 ml of ethanol and 1 ml of 10% NaOH at 75 °C was heated to reflux for 3 h. After the reaction was completed, it was cooled to room temperature to produce a bright yellow powder. The crude product was obtained by suction filtration. Recrystallized with V ethanol: V ethyl acetate (1:1) to obtain compound b. The rate is 88%. Dissolve 0.366 g (1.0 mmol) of compound b in 2 ml of acetic acid, completely dissolve it and transfer to an oil bath (120 °C), then add 1.0 ml of 80% hydrazine hydrate dropwise, heat for 4 h, and detect the completion of the reaction by TCL. After cooling to room temperature, white crystal M was produced with a yield of 78%, with a yield of 78%, mp: 230–233 °C The analysis data are as follows: 1H NMR (CDCl3, 400 MHz) δppm 2.39(s, 3H),3.21(dd, J = 4.1, 4.0 Hz, 1H),3.71(d, J = 4.0 Hz,2H),5.84(m, 1H),6.87(t, J = 4.0 Hz, 1H),7.01(s, 2H),7.25(s, 1H),7.33(d, J = 3.1 Hz, 2H),7.41(dd, J = 5.1, 1.2 Hz, 3H),7.69(dd, J = 4.0, 2.3 Hz, 4H),7.81(s, 1H), 10.18(s, 1H).13C NMR (DMSO-d6, 100 MHz):167.93,156.59,156.79,154.00,150.46,146.86,139.70,136.81,132.93,131.40,128.59,126.99,125.84,119.69,119.14,117.45,116.99,115.73,114.99,112.44,65.43,50.86,47.31,42.41,22.64,22.13. calculated for [M + H]+ 423.2411, found423.2497.
Spectral Test
The M mixed stock solution was prepared 1.0 × 10−3 M. The configuration of the cation stock solution is prepared from the corresponding chloride or nitrate. Make volume with soft water, the concentration is 5.0 × 10−3 M. Add 10 μL, 20 μL of Compound M solution (1 × 10−3 M) to the cell, with an optical path of 1 cm, which contains 2 mL of THF/water (1:1 (v/v) and HEPES buffer (0.02 mM pH = 7.4), then add a certain amount of cation, and measure its UV-visible absorption, fluorescence spectrum and infrared spectrum.
Results and Discussion
Identification of Metal Ions by Probes
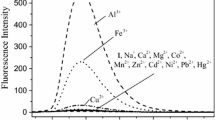
In the THT/water system, under the excitation wavelength of 280 nm, the probe showed strong fluorescence emission at 410 nm. The experimental results show that metal ions other than iron ions have little effect on the fluorescence of the system or have a very small fluorescence quenching effect (Fig. 1). This shows that probe M has a better response to iron ions.
Fluorometric Titration Test of Iron Ion by Probe
Add a different multiple of iron ions to the probe in a THF/water (1:1: v/v) solution containing HEPES buffer (0.02 mM, pH = 7.4), as shown in Fig. 2a, as the iron ion concentration as the factor increases, the fluorescence intensity of probe M decreases continuously until it reaches saturation. The quantitative response of probe M to Fe3+ has a good linear relationship. As shown in Fig. 2b, the linear regression equation is y = 640.19–350.28x, R2 = 0.9902. The Benesi-Hildebrand nonlinear curve fits a straight line, showing a linear fit, and confirms that the stoichiometric relationship between Fe3+ and probe M is 1:1. From Fig. 2c, we can conclude that the binding constant of the M-Fe3+ complex is 2.6 × 105 M−1. The detection limit calculated from the multiple of the fluorescence intensity of the probe M relative to the standard deviation is 3.9 × 10−10 M (when the probe concentration is 1.0 × 10−5 M). Table 1 lists several probes for Fe3+ detection. Compared with the LOD of other probes, M probe has a lower detection line and a higher Fe3+ detection sensitivity.
Competitive Testing
In order to further detect the actual application of the probe M for the recognition of Fe3+, an anti-interference experiment was performed on the probe M (Fig. 3a). Only adding iron ions to the probe will significantly reduce the fluorescence intensity, and then add other equal amounts of metal ions. The fluorescence intensity remains basically unchanged. The results prove that the probe M does not interfere with other ions during the recognition process of Fe3+, and has good anti-interference ability. In addition, in order to further explore the influence of anions on the identification process of iron ions in probe M, we configured iron sulfate ion, sulfate ion and chloride ion solutions in HEPES buffer (0.02 mM, pH = 7.4) THF /water Fluorescence spectroscopy tests were carried out in water (1:1: v/v) solution, as shown in Fig. 3b, the fluorescence response of the probe did not change significantly. The results show that in the process of identifying metal salt ions, anions will not affect the identification of metal ions.
a In the presence of Fe3+ and various other metal ions in a mixed solvent of THF/ water (1:1, v/v) containing HEPES buffer (0.02 mM, pH = 7.4) at room temperature Next, the fluorescence response of probe M. The black bar indicates the probe M plus other metal ion solutions. The gray bar indicates that the same amount of Fe3+ is subsequently added to the mixture solution. b represents the fluorescence spectrum of probe M in a mixed solvent THF/water (1:1, v/v) containing HEPES buffer (0.02 mM, pH = 7.4) and different iron salts at room temperature
Selective Features
In order to explore the practical application of probe M in a biological environment at room temperature, the absorption spectrum characteristics of probe L in a mixed solvent containing THF/water (1:1, v/v) for various metal ions were studied. As shown in Fig. 4a, without adding Fe3+ ions, the probe M showed a maximum absorption peak at 250 nm. Then, Fe3+ ions were added to the probe M. We found that it did cause a significant change in absorption, the absorbance at 310 nm increased, and a new band appeared at 310 nm. It can be clearly seen from Fig. 4b that when adding 1 eq Fe3+ and other metal ions (Ba2+, Ca2+, Mg2+, Na+, Al3+, Mn2+, Ni+, Pb2+, Cu2+, Co2+, K+, Zn2+, Ag+, M), only Fe3+ The absorption peak has obvious changes.
UV Absorption Spectrum of Fe3+ Recognized by Probe
In THF/water (1:1, v/v) buffer containing HEPES (0.02 mM, pH = 7.4), as shown in Fig. 5a, adding different concentrations of Fe3+ ions, the probe M starts to absorb at 350 nm. It shows that a new complex is formed between compound M and Fe3+. The UV-vis titration in Fig. 5b shows a good linear relationship between probe M and Fe3+. The linear regression equation is y = 0.022 + 0.8343x, R2 = 0.9946. The spectral fit of the absorption data obtained with Fe3+ titration probe M shows that the stoichiometric ratio between probe M and Fe3+ is 1:1.
Combination Ratio
In order to further explore the binding ratio of probe M to Fe3+, the molar ratio of probe M to Fe3+ (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9), to detect its fluorescence properties, and draw the curve shown in Fig. 6, the maximum molar fraction of probe M appears at 0.5, which supports the above 1:1 (M:M + Fe3+) In combination with stoichiometry.
Detection Limit
The detection limit is calculated by LOD = 3ơ/k, where & is the standard deviation of the relative fluorescence intensity of the 15 sets of probes at the position of their emission peaks. After probe M is combined with different concentrations of Fe3+, the emission peaks are relative to each other. The intensity is plotted against the corresponding concentration to obtain the slope k. Through calculation, the LOD of the probe M to Fe3+ is 3.9 × 10−10M. Compared with the fluorescent probe for detecting Fe3+ listed in Table 1, the fluorescent probe M made by us has a lower detection limit.
Optimized Calculation
To have a better understanding about the electronic structures of M and M + Fe3+, DFT calculations were performed at B3LYP/def2-SVP [39,40,41]. For the composite M+ Fe3+, HOMO and LUMO are different from M. Due to the addition of Fe3+ in pyrazoline, the HOMO of M + Fe3+ is limited to this fragment, and the CT from the electron donor to the electron acceptor group is interrupted in M + Fe3+, resulting in a decrease in fluorescence. Compared with M, the LUMO-HOMO orbital energy of M + Fe3+ is reduced (Fig. 7), and the acceptor system of the compound is destroyed. This is due to the hydrogen bonding interaction between C=N and C=O.
Infrared Spectrum Test
Figure 8 shows the FT-IR spectra of probes M and M + Fe3+. The characteristic bands of -OH, pyrazoline C=O and C=N extend at 3430 cm−1, 1664 cm−1 and 1101 cm−1, respectively. After adding probe M, these three bands dropped from 3466 cm−1, 1652 cm−1 and 1287 cm−1 to 3428 cm−1, 1643 cm−1 and 1258 cm−1, which may be due to -OH, C=O and C=N The density of the group and Fe3+ coordination electron cloud decreases [41].
PPI Test Fluorescence Probe M Detection Fe3+ Reversibility Experiment
By adding the chelating agent PPI to the fluorescent probe M to recognize the fluorescence emission peak of Fe3+, we can gain a deeper understanding of the response of the fluorescent probe M to Fe3+, as shown in Fig. 9, when adding PPi to the mixed solution of M and Fe3+ At this time, the fluorescence intensity of the complex almost quenches to the intensity of the fluorescent probe M itself, which shows that the complexation between the fluorescent probe M and Fe3+ is reversible.
Recognition Mechanism of Fluorescent Probe M and Fe3+
The possible binding mechanism of M with Fe3+ induced the fluorescence changes is shown in Scheme 2. Based on the previously reported pyrazoline-based probes [42,43,44], we can speculate that Fe3+ may interact with C = N, C = O and -OH on the fluorescent probe M to undergo photoinduced electron transfer and chelation. The cooperation thus weakens the binding of fluorescence [45], but the chelation of probe M with Fe3+ makes the complex more rigid and limits the C = N isomerization [46, 47]. And because the binding ratio of probe M and Fe3+ ions is 1:1, the phenomenon of fluorescence quenching is further confirmed and their binding mechanism.
Conclusion
In summary, a novel fluorescent probe based on pyrazole-pyrazoline for Fe (III) ions recognition was designed and synthesized. By observing the ultraviolet absorption spectrum and fluorescence spectrum, it was found that tetrahydrofuran: water = 1:1 (v: v) in the presence of other metal ions, the probe M in the mixed solution has high selectivity, sensitivity and anti-interference ability for the detection of Fe3+, and the recognition process is reversible. The combination of probe M and iron ions is 1:1, and the detection limit is 3.9 × 10−10 M. The binding mode and sensing mechanism of M with Fe3+ was verified by theoretical calculations using Gaussian 09 based on B3LYP/6-31G(d) basis.
References
DeSilva AP, Gunaratne HQN, Gunnlaugsson T et al (1997) The stability of cyclodextrin complexes in solution. Chem Rev 97:1514–1566
Aisen P, Wessling-Resnick M, Leibold EA (1999) Iron metabolism Curr Opin. Chem Biol 3:200–206
Touati D (2000) Iron and oxidative stress in bacteria. Arch Biochem Biophys 373:1–6
Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK (2002) Glutathione iron and Parkinsons disease. Biochem Pharmacol 64(5):1037–1048
Honda K, Casadesus G, Petersen RB et al (2004) Oxidative stress and redox-active iron in alzheimers disease. Ann N Y Acad Sci 1012(1):179–182
Narayanaswanmy N, Govindaraju T (2012) Aldazine-based colorimetric sensors for Cu2+ and Fe3+. Chemical 161(1):304–310
Liang ZO, Wang CX, Yang JX, et.al. A highly selective colorimetric chemosensor for detecting the respective amounts of iron(I1) and iron (I1) irons in water. New J Chem 2007;31(6):906–910
Gonzáles APS, Firmino MA, Nomura CS, Rocha FRP, Oliveira PV, Gaubeur I (2009) Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry. Anal Chim Acta 636(2):198–194
Mashhadizadeh MH, Pesteh M, Talakesh M, Sheikhshoaie I, Ardakani MM, Karimi MA Solid phase extraction of copper (II) by sorption on octadecyl silica membrane disk modified with a new Schiff base and determination with atomic absorption spectrometry. Spectrochimica Acta Part B Atomic Spectroscopy 2008;63(8):885–8, 888
Liu J, Yu M, Wang XC, Zhang Z (2012) A highly selective colorimetric sensor for Hg2+ based on nitrophenyl-aminothiourea. Spectrochim Acta A 93(10):245–249
Gupta VK, Singh AK, Mergu N (2014) Antipyrine based schiff bases as turn-on fluorescent sensors for Al (III) ion. Electrochim Acta 117(4):405–412
Liu Y, Liang P, Guo L (2005) Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 68(1):25–30
Ferreira SLC, Queiroz AS, Fernandes MS, dos Santos HC (2002) Application of factorial designs and doehlert matrix in optimization of experimental variables associated with the preconcentration and determination of vanadium and copper in seawater by inductively coupled plasma optical emission spectrometry. Spectrochimica Acta Part B Atomic Spectroscopy 57(12):1939–1950
Tesfaldef ZO, Vanstaden JF, Stefan RI (2004) Sequential injecting spectrophotometric determination of irons as Fe(II) in multi-vitamin preparations using 1, 10-phenanthroline as complexing agent. Talanta 64(5):1189–1195
Gomes D, Segundo M, Lima J et al (2005) Spectrophotometric determination of iron and boron in soil extracts using a multi-syringe flow injecting system. Talanta 66(3):703–711
Lunvongsa S, Oshima M, Motomizu S (2006) Determination of total and dissolved amount of iron in water samples using catalytic spectrophotometric flow injecting analysis. Talanta 68(3):969–973
Mohadesi A, Taher MA. Voltammetric determination of cu(II) in naturalwaters and human hair at a meso-2,3-dimercaptosuccinic acid self-assembled gold electrode. Talanta 2007;72(1):95–0, 100
Sahoo SK, Sharma D, Bera RK et al (2012) Iron (III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 41(21):7195–7227
Levai A, Jeko J (2006) Synthesis of 1-Substituted 5-Aryl-3-styryl-2-pyrazolines and 3-Aryl-5-styryl-2-pyrazolines by the Reaction of Dibenzylideneacetones and E.E-Cinnamylideneacetophenones with Hydrazines. J Heterocyclic Chem 43:1303–1309
Wiley KH (1967) Pyrazles, Pyrazolines, Pyrazilidnes, Indazoles and Condensed rings. The Chemistry of Heterocyclic Compounds 20:180–188
Levai A (2002) Synthesis of 3-Aroyl-4-(3-chromony)-2-pyrazolines. J Heterocyclic Chem 39:1333–1336
Khali HZ, Yanni SA (1981) Synthesis of new Anilido-Pyrazoline and Isoxazoline Derivatines. J Indian Chem Soc 58:168–170
Rawal AA, Thakor VM, Shah NM (1963) Synthesis of some 1, 3,5-Triphenyipyrazolines and 3,5-Diphenyl-cyclohexen-1-ones. J Indian Chem Soc 40(4):323
Dhal PN, Acharya TE, Nayak A (1975) Studies on 1,3-Diaryl Pyrazolines and their derivatives. J Indian Chem Soc 52:1196–1198
Bhatia MS, Sood RK (1978) New heterocyclic ring containing phosphorus:synthesis of 4-Chloro-1,4-dihydro-2 H-naphth-[2,1-c][1,2] oxaphosphorin. Ind J Chem 16B:638–645
Turan-Zitouni G, Chevallet P, Kilic FS, Erol K (2000) Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur J Med Chem 35:635–641
Erhan R, Mutlu A, Tayfun U, Dilek E (2001) Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem 36:539–543
Husain MI, Shukla S (1986) Synthesis & biological activity of 4-(3-Aryl-4-exo-2-thioxothia-zolidin-5-ylimino)-3-methyl-1-(N,N-disubstituted amino-methyl)pyrazolin-5-ones. Ind J Chem 25B:983–985
Orrego-Hernández J, Portilla J (2017) Synthesis of dicyanovinyl-substituted 1-(2-pyridyl) pyrazoles: design of a fluorescent chemosensor for selective recognition of cyanide. J Org Chem 82(24):13376–13385
Ibnaouf KH, Elzupir AO, Ibrahem MA, Ali MKM, Al-Muhanna MK (2019) Optical characteristics and structural properties of 3-(p-Nitrophenyl)-5-phenyl-1H-pyrazole. J Electron Mater 48(2):861–866
Maliyappa MR, Keshavayya J, Mahanthappa M, Shivaraj Y, Basavarajappa KV (2020) 6-substituted benzothiazole based dispersed azo dyes having pyrazole moiety: synthesis, characterization, electrochemical and DFT studies. J Mol Struct 1199:126959
Orrego-Hernandez J, Portilla J (2017) Synthesis of Dicyanovinyl-substituted 1-(2-Pyridyl)pyrazoles: Design of a Fluorescent Chemosensor for selective recognition of cyanide. J Org Chem 82:13376–13385
Wang CC, Zhang D, Huang XY, Ding P, Wang Z, Zhao Y, Ye Y (2014) A fluorescence ratiometric chemosensor for Fe3+ based on TBET and its application in living cells. Talanta 128:69–74
Jin XD, Wang SF, Yin WZ, Xu T, Jiang Y, Liao Q, Xia X, Liu J (2017) A highly sensitive and selective fluorescence chemosensor for Fe3+ based on rhodamine and its application in vivo imagine. Sensors Actuators B 247:461–468
Bozkurt E, Arik M, Onagner Y (2015) A novel system for Fe3+ ion detection based on fluorescence resonance energy transfer. Sensors Actuators B 221:136–147
Zhao B, Liu T, Fang T et al (2017) A new selective chemosensors based on phenanthro[9,10-d] imidazole-coumarin with sequential“on-off-on”fluorescence response to Fe3+ and phosphate anions and its application in living cells. Sensors Actuators B 246:370–379
Li P, Zhang M, Sun XK, Guan S, Zhang G, Baumgarten M, Müllen K (2016) A dendrimer-based highly sensitive and selective fluorescence-quenching sensor for Fe3+ both in solution and as film. Biosens Bioelectron 85:785–791
Sen S, Sarkar S, Chattopadhyay B, Moirangthem A, Basu A, Dhara K, Chattopadhyay P (2012) A ratiometric fluorescence chemosensor for iron: discrimination of Fe2+and Fe3+ and living cell application. Analyst 137:3335–3342
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behaviour. J Phys Rev A 38:3098–3000
Becke AD (1993) Density-functional thermochemistry III the role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a function of the electron density. Phys Rev B 37:785–789
Zhang TT, Wang FW, Li MM, Liu JT, Miao JY, Zhao BX (2013) A simple pyrazoline-based fluorescent probe for Zn2+ in aqueous solution and imaging in living neuron cells. Sensors Actuators B 186:755–760
Kasirajan G, Krishnaswamy V, Raju N, Mahalingam M, Sadasivam M, Palathurai Subramaniam M, Ramasamy S (2017) New pyrazolo-quinoline scaffold as a reversible colorimetric fluorescent probe for selective detection of Zn2+ ions and its imaging in live cells. Journal of Photochemistry and Photobiology A 341:136–145
Li MM, Wang FW, Wang XY, Zhang TT, Xu Y, Xiao Y, Miao JY, Zhao BX (2014) A new turn-on fluorescence probe for Zn2+ in aqueous solution and imaging application in living cells. Anal Chim Acta 826:77–83
Mikata Y, Yamashita A, Kawamura A et al (2009) Bisquinoline-based fluorescent zinc sensors. Dalton Trans 19:3800–3806
Maity D, Govindaraju T (2011) Naphthaldehyde-urea/thiourea conjugates as turn-on fluorescent probes for Al3+ based on restricted C=N isomerization. Eur J Inorg Chem 36:5479–5485
Velmurugan K, Raman A, Easwaramoorthi S, Nandhakumar R (2014) Pyrene pyridine-conjugate as Ag selective fluorescent chemosensor. RSC Adv 4:35284–35289
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 462 kb)
Rights and permissions
About this article
Cite this article
Zhang, YP., Li, XF., Yang, YS. et al. A Novel Fluorescent Probe Based on Pyrazole-Pyrazoline for Fe (III) Ions Recognition. J Fluoresc 31, 29–38 (2021). https://doi.org/10.1007/s10895-020-02632-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02632-w