Abstract
The present manuscript gives a detailed account of highly selective, validated and sensitive method for quantification of pirfenidone in its pharmaceutical dosage forms and spiked human urine. The developed method is relied on the systematic study of the fluorescence action of Pirfenidone in Tween – 80 micellar medium. The Pirfenidone exhibits strong fluorescence at λem 396 nm upon excitation at λex 318 nm in Tween −80 medium. The fluorescence - concentration plot was linear over concentration range of 0.5 – 5 μg/mL. There was greater extent (1.02 fold) of enhancement in fluorescence intensity in presence of tween – 80 with very low limit of detection and quantitation of 0.04 μg/mL and 0.11 μg/mL respectively. The application of developed methodology is successfully applied to content uniformity testing and spiked human urine. The proposed study was successfully applied for analysis of pirfenidone in commercially available pharmaceutical formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
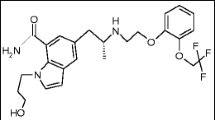
Pirfenidone (PIR) (Fig. 1) is chemically 5-Methyl-1-Phenyl-2-[1H]-Pyridone, a Pyridone analogue. It is a novel anti-fibriotic agent employed for treatment of Idiopathic Pulmonary Fibrosis (IPF) disorder. Pirfenidone has well established anti – fibriotic and anti – inflammatory properties in various invitro systems and animal models of fibrosis, [1] Literature survey reveals different analytical techniques for determination of pirfenidone in its bulk and pharmaceutical formulations including HPLC, [2, 3] LC, [4] Spectrophotometry and in combination with HPLC and HPTLC, [5,6,7] LC-MS-MS, [8] and HPTLC [9] methods. Spectrofluorimetric analysis stands first in place compared to other chromatographic or spectrophotometric techniques in terms of ease and selectivity. To the best of our knowledge till date no micelle enhanced spectrofluorimetric determination of pirfenidone has been reported. This manuscript presently describes the methodology adopted for determination of pirfenidone in available dosage forms and spiked human urine in presence of micellar medium. The proposed method is developed on the fact that Pirfenidone exhibits native fluorescence and further there was vast fluorescence enhancement in presence of tween −80 medium. This paved a way to develop a more sensitive micelle enhanced spectrofluorimetric technique for determination of pirfenidone which is more selective, reliable, economical, time saving without any derivatisation reactions. The proposed method was completely validated as per ICH guidelines. [10] The content uniformity test was performed by adapting USP guidelines. [11]
Experimental
Instrumentation
A HORIBA spectrofluorimeter model FLUOROMAX -4 with Xenon arc lamp was used for fluorescence measurements. A SPINIX vortex mixture MODEL No: 84798200 was used for uniform mixing of urine samples. A digital Ultra sonicator was used for sonication of standard and sample solutions. For weighing a SHIMADZU AUX – 220 analytical balance was used.
Materials and Reagents
A Standard sample of Pirfenidone (Batch No. ZB05L – BH) was procured from TCI Chemicals, Chennai, Tamil Nadu, India. Tween – 80 was supplied by Alfa Aesar, England. Sodium Dodecyl sulphate (SDS), Methyl β – Cyclodextrin (β –CD) and Cetrimide (CTAB) was supplied by Loba chemie, Mumbai, India. All solvents used in the developed methodology were of analytical reagent grade. Acetonitrile, Acetone and Dimethyl Sulfoxide (DMSO) were supplied by Emplura, Merck, Mumbai, India. Ethanol and methanol are supplied by Finar chemicals, Ahmedabad, Gujarat, India. Different brands of pirfenidone i.e. Pirfenex (B.No: BA55672), Pirfetab (B.No: BA55672), Fibridone (B.No: J501172) were procured from local pharmacy.
Preparation of Standard Solution
A stock solution of 100 μg/mL pirfenidone was prepared by dissolving 10 mg of standard drug in double distilled water and mixed uniformly with the help of ultra sonicator. Working standard solutions were then prepared by appropriate dilution of standard stock solution using double distilled water. The solutions are stored at 6 °C in a refrigerator and were found to be stable for ten days.
General Procedures
Construction of Calibration Curves
Native Pirfenidone Determination in Aqueous Medium
Aliquots of aqueous solutions were prepared by suitable dilution of stock standard solution of pirfenidone in double distilled water (DDW) in 10 mL calibrated flasks; so as to obtain concentrations in the range of (0.5 - 5 μg/mL). Fluorescence was measured at 398 nm using an excitation wavelength of 318 nm.
Pirfenidone Determination by Micelle – Enhanced Spectrofluorimetric Method
Aliquots of stock standard solution were transferred into a set of 10 mL calibrated flasks and 0.4 ml of Tween-80 was added to each flask and diluted upto mark using double distilled water so as to obtain concentration in the range of 0.5-5 μg/mL and measuring the fluorescence intensity at 396 nm after excitation at 318 nm.
Analysis of Pirfenidone in Pharmaceutical Preparations
Ten tablets of each brand of pirfenidone i.e. Pirfenex tablets, Pirfitab tablets and Fibridone tablets were weighed individually and their average weight was calculated, then the contents were pulverised and mixed. A weight quantity of powdered tablets equivalent to 10 mg of pirfenidone was transferred into a 100 ml volumetric flask and about 70 ml of double distilled water was added and sonicated for uniform mixing and filtered using Whatmann No. 1 filter paper. Aliquots covering the range of working concentration i.e. 0.5-5 μg/mL were transferred into a series of 10 ml volumetric flasks and general procedure under construction of calibration curve was followed for quantification of pirfenidone. The amount of pirfenidone was estimated from corresponding regression equation.
Analysis of Pirfenidone in Spiked Human Urine
To a set of 5 mL tapered bottom centrifugation tubes 0.5 mL of drug free diluted urine sample was spiked with 1 ml of pirfenidone standard solution and then vortexed well for uniform mixing of samples for 5 min and the volume was made upto the mark with double distilled water. The method was carried out in triplicate using three varied concentrations within the working concentration range of pirfenidone for quality control or quality assurance purposes. Then procedure under construction of calibration curve was followed.
Content Uniformity Testing
The same method for analysis of tablets was followed by using one tablet as sample. Ten different tablets of each brand were analysed individually by applying official USP guidelines. [11] Individual contents of tablets were obtained from previously constructed calibration curve or corresponding regression equation.
Results and Discussions
The use of micellar medium generally enhances the fluorescence intensity vastly which paved the way to develop a spectrofluorimetric determination of pirfenidone in Tween – 80 micellar medium, pharmaceutical preparations and spiked human urine. Application of micellar medium in enhancing fluorescence intensity of target molecules helps in lowering the limit of detection (LOD). Usually water molecules get trapped into polyoxyethylene chains of non-ionic surfactants so the hydrogen bonds can be formed between ethylene oxide chains of non-ionic surfactants. When an electro-negative atom like oxygen is present in the target molecule a hydrogen bond can be formed with the hydrophilic head of Tween – 80. Several properties like reactivity, solubility and spectral characteristics undergo variable changes when a solute is allowed to pass from aqueous solution to micellar medium there by increasing the fluorescence intensity which is exceptionally good and beneficial. As a matter of fact the provision of rigid microenvironment by micellar medium limit the freedom of fluorophore units and thereby reducing the probabilities of non radiative process, which creates high viscous environment that can obstruct quenching due to molecular oxygen. These factors play a vital role in enhancing the fluorescence signals of host molecules. [12, 13]. More over formation of hydrogen bond between carbonyl group of pirfenidone and terminal hydroxyl group of tween – 80 is also a reason for enhancement in fluorescence intensity. The fluorescence properties of pirfenidone on different solvents and different micellar media has been studied and there was very good fluorescence enhancement in presence of Tween – 80 aqueous solution compared to others. The enhancement is due to provision of rigid microenvironment around pirfenidone and obstruction of free rotational motions which is characteristic of luminescent emission. [14] Hence Tween −80 has been selected as fluorescence enhancer for development of proposed spectrofluorimetric method.
Optimization of Experimental Conditions
Fluorescence Spectra of Pirfenidone in Aqueous Solution
The fluorescence spectra of pirfenidone in both aqueous and tween – 80 were studied Fig. 2. When compared to native fluorescence of pirfenidone there was nearly 1.02 fold enhancement in fluorescence intensity in presence of tween 80 medium.
Effect of Different Micellar Media
The fluorescence properties in different micellar mediums like non-ionic surfactant (tween – 80); anionic surfactant (SDS), cationic surfactant (CTAB) and macromolecule (β-CD) were studied. It was observed that CTAB, SDS and β-CD showed remarkable decrease in fluorescence intensity; Among all, Tween −80 showed a remarkable enhancement in Fluorescence intensity this may be attribute to formation of hydrogen bond with terminal hydroxyl group of tween – 80 with carbonyl oxygen of pirfenidone, which facilitates protection of lowest excited singlet state of target molecule in micellar microenvironment from non-radiative or possible quenching process which may readily occur in bulk aqueous solutions, causing substantial enhancement in fluorescence intensity [15] (Fig. 3).
Effect of Tween – 80 Concentration
The effect of Tween – 80 concentration on fluorescence intensity was studied. On increasing the tween 80 concentration there was a steady increase in fluorescence response and reached a stable level at 0.3% v/v Tween – 80. On substantial addition there was no increment in fluorescence intensity; so 0.4% v/v concentration was selected as suitable concentration (Fig. 4).
Effect of Diluting Solvents
The effect of various diluting solvents on fluorescence intensity was investigated in presence of Tween – 80 micellar medium using double distilled water (DDW), dimethyl Sulfoxide (DMSO), acetonitrile, acetone, ethanol and methanol. The investigation showed that DDW is the best solvent for dilution since it gave highest fluorescence intensity and lowest blank reading. DMSO showed sharp decrease in response as it initiates inter system crossing which may be similar to that of heavy atom effect. [16] There was a remarkable decrease in fluorescence intensity in case of ethanol and methanol; this may be attributed to the fact that short chain alcohols get solubilised in aqueous phase there by disturbing the microenvironment of micellar medium which tends to change in solvent properties, size reduction and breakdown of micelles at high concentration. [17] Both acetone and acetonitrile also cause sharp decrease of fluorescence intensity due to their denaturing effect (Fig. 5).
Effect of Time
The effect of time on fluorescence intensity has been evaluated and it was found that the fluorescence intensity was instantaneously developed and was retained for about 3 h.
Effect of Temperature
The effect of temperature on fluorescence intensity has been studied by placing the standard solutions at wide range of temperature ranging from (50–95 °C) in a thermostatically controlled water bath. The results revealed that on increasing the temperature there was a rapid decrease in fluorescence response. This may be due to higher internal conversion at elevated temperature there by causing nonradiative deactivation of excited singlet state [18]. Therefore, the entire experiment has been performed at room temperature.
Method Validation
Linearity
Calibration plot for estimation of pirfenidone was plotted by taking concentration on X- axis and fluorescence intensity on Y- axis and were found to be linear over a concentration range of 0.5-5 μg/mL. Statistical analysis [19] of regression data gave reproducible results that are within limit. Table 1 represents the attributes of linearity parameters.
Accuracy and Precision
Statistical analysis [19] of results obtained by developed and comparison method by applying student’s t-test and variance F- ratio test showed no significant difference between two methods in terms of accuracy and precision. Inter-day and Intra-day precision are evaluated by analysing three concentrations on three different days and three successive occasions. Results indicate low percentage RSD values which are well within the limit show good repeatability and intermediate precision of developed method. The results of accuracy and precision are tabulated in Tables 2 and 3.
Robustness
The robustness of the method was evaluated by small and deliberate changes in volume of Tween – 80 (0.3 ± 0.2 mL). These minor changes did not show much effect on fluorescence intensity and it was constant with experimental conditions.
Selectivity
The selectivity of the method was assessed by interference from common excipients in tablets. Results showed good recovery and no interference from presence of these additives. Results are represented in Table 4.
Applications
Pharmaceutical Application
The commercially available tablets were analysed for determination of pirfenidone by developed method. Statistical analysis [19] was performed by employing student’s t-test and variance ratio F-test. Results are in good agreement with those obtained by comparison method [6]. Hence the developed method can be employed for the quality control analysis of OND in pharmaceutical preparations. Results are represented in Table 4.
Content Uniformity Test
The method is well employed in terms of sensitivity by measuring the fluorescence intensity of single tablet extract rapidly with sufficient accuracy it is ideal for performing content uniformity test. The test was performed as per the official guidelines framed by USP [13] and the acceptance value (AV) was found to be within the limit. The results are depicted in Table 5.
Application of the Proposed Method for Determination of Pirfenidone in Spiked Human Urine Samples
Pirfenidone is an orally administered drug. It follows hepatic metabolism. Nearly 50% of the drug is metabolized in liver enzymatically by CYP1A2 enzyme system to form 5-Carboxy Pirfenidone;(99.6%) an inactive metabolite. About 80% of the administered drug through oral route of administration is excreted in the Urine [20]. The high selectivity and sensitivity of the developed method allowed for quantification of pirfenidone in spiked human urine samples. Drug free human urine samples were obtained from healthy volunteers and were stored in poly tetra fluoro –ethylene (PTFE) flasks at −20 °C until further analysis. Urine samples were diluted 100 times with water before analysis. A 0.5 mL of urine was transferred into screw capped tubes; spiked with standard analyte at three different levels within the concentration range then the contents were subjected for uniform mixing using Vortex mixture for 3–5 min; and analysed in spectrofluorimeter. Their percentage recoveries were calculated which are found to be in the range of 99–102%. Similarly each aliquot of unspiked urine sample was analysed as method blank. The standard deviations of obtained recoveries are within the acceptable recovery showing that the developed method has good variability provoked from different matrix effects, instrumental and manual fluctuations. Results present in Table 6 shows the suitability of developed method for determination of pirfenidone in spiked human urine without any matrix related interferences.
Conclusion
A simple, reliable, sensitive and selective spectrofluorimetric method for determination of pirfenidone was developed. The developed method is fast, economical and least time consuming compared with chromatographic techniques. Further it does not involve any time consuming extraction and derivatisation reactions with ease of measuring enhanced fluorescence intensity that is developed immediately. In terms of simplicity, rapidity and high selectivity and sensitivity the proposed methodology could be easily employed in performing content uniformity test and determination of pirfenidone in spiked human urine samples without any pre-treatment steps. Out of all this method could serve as an alternative to the reported analytical techniques.
References
Schaefer CJ, Ruhrmund DW, Pan L, Seiwert SD, Kossen K (2011) Antifibrotic activities of Pirfenidone in animal models. Eur Respir Rev 20:85–97
Bodempudi SB, Babur R, Reddy KS (2015) Development and substantiation of a RP-HPLC method for monitoring of impurities in Pirfenidone drug substance. Am J Analyt Chem 6:1019–1029
Tamilselvi N, Kurian DS (2012) Bio analytical method development and validation of Pirfenidone by Rp-hplc method and its application to the determination of drug food interaction study in Wister rats. IJPBR 3:132–142
Shi S, Wu J, Shi S, Wu J, Zeng F (2008) Development and validation of an improved LC method for the simultaneous determination of Pirfenidone and its carboxylic acid metabolite in human plasma. Chromatographia 69:459–463
Ravi Sankar P, Anusha Rani K, Devadasu C, Srinivasa Babu P (2014) Development of a validated UV spectrophotometric method for the quantitative estimation of Pirfenidone in bulk drug and marketed tablet. IJPRD 6:025–031
Parmar VK, Desai SB, Vaja T (2014) RP-HPLC and UV spectrophotometric methods for estimation of Pirfenidone in pharmaceutical formulations. Indian J Pharm Sci 76:225–229
Thorat SG, Padamane SP, Tajne MR, Ittadwar AM (2016) Development and validation of simple, rapid and sensitive UV, HPLC and HPTLC method for the estimation of pirfenidone in tablet dosageform. J Chil Chem Soc 61:2978–2981
Tong S, Wang X, Jiang H, Xuegu X, Pan Y, etal KC (2010) Determination of pirfenidone in rat plasma by LC–MS-MS and its application to a pharmacokinetic study. Chromatographia 71:709–713
Thorat SG, Tajne MR, Padmane SP, Ittadwar AM (2015) A validated stability-indicating high-performance thin layer chromatographic method for estimation of pirfenidone in tablet formulation. JPC 28:398–404
ICH. Harmonized tripartite guideline, validation of analytical procedures: text and methodology, Q2 (r1), current step 4 version, parent guidelines on Methodology Dated November 6 1996, Incorporated in November 2005. http://www.ich.org/LOB/media/MEDIA417.pdf. (Accessed 15 February 2014)
US Pharmacopoeial Convention (2007) The United States Pharmacopeia 30 The National Formulary 25. Rockville, MD, USA: US Pharmacopeial Convention, electronic version
Hinze WL, Singh HN, Baba Y, Harvey NG (1984) Micellar enhanced analytical fluorimetry. TrAC Trends Anal Chem 3:193–199
McIntire GL (1990) Micelles in analytical chemistry. Crit Rev Anal Chem 21:257–278
Wang CC, Masi AN, Fernandez L (2008) On-line micellar-enhanced spectrofluorimetric determination of rhodamine dye in cosmetics. Talanta 75:135–140
Mohamed A-MI, Omar MA, Hammad MA, Mohamed AA (2016) Development and validation of highly sensitive stability indicating Spectrofluorimetric method for determination of amlodipine in pharmaceutical preparations and human plasma. J Fluoresc 26:2141–2149
Skoog DA, Holler FJ, Crouch SR (2007) Principles of instrumental analysis. 6th ed. Belmont: Thomson, 406
Leung R, Shah DO (1986) Dynamic properties of micellar solutions: I effects of short-chain alcohols and polymers on micellar stability. Colloid Interface Sci 113:484–499
Skoog DA, West DM, Holler FJ, Crouch SR. editor#. Fundamentals of analytical chemistry. 8th ed. Saunders College Publishing: Philadelphia, 2004 1003–6
Miller JN, Miller JC (2005) Statistics and chemo metrics for analytical chemistry. 5th ed. Harlow: Pearson Education, 39–73, 107–149, 256
Esbriet 267 mg hard capsules Summary of product characteristics Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_product_Information/human/002154/WC500103049.pdf
Acknowledgements
The authors (V.M.Biju and S Naga Gayatri) thank MHRD and DST – FIST, New Delhi for their Financial Support for the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The Authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sambhani, N.G., Biju, V.M.N. A Micelle-Enhanced Spectrofluorimetric Determination of Pirfenidone: Application to Content Uniformity Testing and Human Urine. J Fluoresc 28, 951–957 (2018). https://doi.org/10.1007/s10895-018-2258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2258-8