Abstract
A series of five new terbium(III) ion complexes with 4,4-difluoro-1-phenylbutane-1,3-dione (HDPBD) and anciliary ligands was synthesized. The composition and properties of complexes were analyzed by elemental analysis, IR, NMR, powder X-ray diffaraction, TG-DTG and photoluminescence spectroscopy. These complexes exhibited ligand sensitized green emission at 546 nm associated with 5D4 → 7F5 transitions of terbium ion in the emission spectra. The photoluminescence study manifested that the organic ligands act as antenna and facilitate the absorbed energy to emitting levels of Tb(III) ion efficiently. The enhanced luminescence intensity and decay time of ternary C2-C5 complexes observed due to synergistic effect of anciliary ligands. The CIE color coordinates of complexes came under the green region of chromaticity diagram. The mechanistic investigation of intramolecular energy transfer in the complexes was discussed in detail. These terbium(III) complexes can be thrivingly used as one of the green component in light emitting material and in display devices.

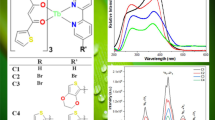
Illustrate the sensitization process of the Tb ion and intramolecular energy transfer process in the Tb3+ complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lanthanide complexes with various organic ligands have attracted intensive interest of researchers in last decades due to their unique and fascinating photophysical properties such as long life time, high color purity and large stokes shifts [1, 2]. These characteristic features make them an important and powerful tool for the diverse and enormous potencial applications in laser system [3], optical amplification [4], fluoroimmunoassay [5], luminescent probe in biological systems [6, 7] and emmiting materials in organic light emitting diodes [8]. The later demands especially RGB (red, green and blue) primary colors. The trivalent lanthanide ions show low absorption as well as weak emission intensity which is associated with the parity forbidden intra-configurational 4f-4f electronic and magnetic dipole transitions (laporte rule). In order to overcome the aforesaid problem, an effective strategy is applied in which an organic ligand of high absorption coefficient coordinated with the lanthanide ion. In this way, the organic ligand acts as sensitizer or antenna thereby transferring the absorbed energy to the cental metal ion efficiently through sensitization process [9, 10]. By keeping in view the vital importance of green color as one of the component of RGB primary color, a series of green light emitting terbium(III) complexes was synthesized [11].
Among the organic moieties, the β-diketone attracted the attention due to their excellent features such as high absorption in a broad range of wavelength, strong coordination ability and shielding the lanthanide ion from higher lattice vibrations. The goal of synthesis efficient luminescence material was accomplished by an appropriate selection of β-diketone ligand. Therefore, a β-diketone with C-F bonds selected as organic ligand in which low energy C-F oscillators could decrease the back energy loss by reducing the non-radiative quenching. These features of fluorinated β-diketone imparted intense luminescence properties in complexes [3, 12, 13]. The bidentate β-diketone ligands behaved as monoanion which not only engaged the six coordinative sites out of 8–12 possible sites by its three molecules but also compensated the charge of trivalent lanthanide ion. The rest of the coordinative sites were satisfied by water or solvent molecules that possessed stretching vibrational modes of C-H and O-H oscilattors, directing the decreased in photoluminescence intensity by vibronic quenching [14, 15] via back energy transfer to ligand-localized electronic level [16]. In account to evade vibronic quenching, the ternary lanthanide complexes chelated with the planar N,N-donor ancillary ligands such as 2,2′-biquinoline (biq), 1,10-phenanthroline (phen), neocuproine (neo) and 2,2′-bipyridyl (bipy) [17,18,19,20,21]. These chelations of anciliary ligands expelled the water molecules from the coordination sphere and also showed synergestic affect with main organic ligand in energy transfer mechnism resulting increased the luminescence intensity. The above mentioned features of β-diketone ligands and Tb(III) ion established a fascinating prospects for the synthesis of green emitting materilas which prompted us to produce a series of fluorinated β-diketonate based terbium(III) complexes.
The present work deals with the synthesis of 4,4-difluoro-1-phenylbutane-1,3-dione (HDPBD) ligand and its corresponding five terbium(III) complexes. These materials characterized by FT-IR, CHN, NMR, TG-DTG, XRD, UV-visible and photoluminescence (PL) spectroscopy. To get better insight into photophysical properties, the excitation spectra and emission spectra as well as decay curves were investigated in detail. The transfer of energy from excited levels (singlet and triplet) of HDPBD and anciliary ligands to emitting levels of terbium ion (5D4) was examined through proposed energy transfer mechanism.
Experimental Section
Materials and Measurements
The metal nitrate of high purity [Tb(NO3)3.5H2O; 99.9%] and anciliary ligands like 2,2′-bipyridyl, 2,2′-biquinoline, neocuproine, 1,10-phenanthroline were purchased from Sigma Aldrich. The commercially available all starting materials and solvent used in this work were analytical grade and employed without any purification process. The synthesized HDPBD ligand recrystalized two times with ethanol solvent before the coordination process.
The complexometric titration was carried out with disodium ethylenediaminetetraacetate (EDTA) salt to enumerate the terbium(III) ion content. The carbon, hydrogen and nirogen elements were analyzed by Perkin Elmer 2400 CHN Elemental Analyzer. FTIR spectra were recorded on Perkin Elmer Spectrum 400 spectrometer using KBr pellets in the 4000–400 cm−1 region. The 1H-NMR spectra were executed by Bruker Avance II 400 NMR spectrometer. Powder X-ray diffraction (XRD) pattern were obtained from Rikagu Ultima IV diffractometer at 40 kV tube voltage and 40 mA tube current with CuKα radiation over 10–800 range of 2θ angle. The thermal behavior was examined by using SDT Q600 thermal analyzer under nitrogen atmosphere with a heating rate of 20 °C/min upto 800 °C temperature. The luminescent decay time, excitation and emission spectra were measured by Hitachi F-7000 fluorescence spectrophotometer at a 240 nm/min scanning rate with excitation source of xenon lamp. At the time of excitation and emission measurements, the slit widths were 2.5 nm.
Synthesis of Ligand HDPBD
Scheme 1 The ligand HDPBD was synthesized with the solution of acetophenone (0.42 mL, 3.60 mmol) in dry THF (120 mL) and then added sodium hydride (1.40 g, 5.80 mmol). After stirring for 15 min then added methyl-difluoroacetate (1.18 g, 10.8 mmol). Further, resulting solution was stirred for 12 h at room temperature. Finally, solid residue formed which was dissolved in dichloromethane. The mixture was acidified with 0.5 M hydrochloric acid and then washed with water. To obtained pure compound, the solid was recrystalized with ethanol. Finally, a dark brown solid was obtained with 62% yield having melting point 49 °C [22]. IR: cm−1 3430 (b), 3080 (m), 2998 (m), 2890 (w), 1635 (s), 1542 (s), 1480 (s), 1360 (s), 1270 (s), 785 (s), 705 (s); 1H-NMR: δ 15.01 (s, 1H, enolic OH), 7.45–7.22 (m, 5H, Ar-H), 6.65 (t, 1H, CHF2), 6.35 (s, 1H, enol CH), 4.22 (s, 2H, keto-CH2) ppm
Synthesis of Tb(III) Complexes
To an aqueous solution of terbium nitrate pentahydrate (1.0 mmol, 0.43 g) added an alcoholic solution of ligand HDPBD (3.2 mmol, 0.63 g) dropwise under vigorous stirring on magnetic stirrer. The pH of the resulting mixture was adjusted to 6.5 with 0.05 M sodium hydroxide solution, resulting in the formation of white precipitates after constant stirring for two hours at a temperature of 50-60 °C. The precipitates were filtered out and dried in hot air oven to obtained C1 complex.
Tb(DPBD)3H2O (C1): white solid, yield 75%; IR: cm−1 3338 (b), 3070 (w), 2980 (w), 1609 (s), 1533 (s), 1467 (s), 1352 (s), 1270 (s), 1102 (s), 767 (m), 704 (m), 423 (m); 1H-NMR: δ 8.10–6.70 (m, 15H, Ar-H), 6.50 (t, 3H, CHF2), 3.50 (s, 3H, enol CH). Anal. Calcd for TbC30H25O8F6: C, 45.80; H, 3.18; Tb, 20.22; found: C, 45.38; H, 3.06; Tb, 19.98.
The complexes C2-C5 were synthesized using HDPBD (3.2 mmol, 0.63 g), biq (1.0 mmol, 0.25 g) and terbium nitrate (1.0 mmol, 0.43 g) for complex C2, HDPBD (3.2 mmol, 0.63 g), phen (1.0 mmol, 0.18 g) and terbium nitrate (1.0 mmol, 0.43 g) for complex C3, HDPBD (3.2 mmol, 0.63 g), neo (1.0 mmol, 0.20 g) and terbium nitrate (1.0 mmol, 0.43 g) for complex C4 and HDPBD (3.2 mmol, 0.63 g), bipy (1.0 mmol, 0.15 g) and terbium nitrate (1.0 mmol, 0.43 g) for complex C5 as the starting materials and then followed the same method as described for C1 complex.
Tb(DPBD)3biq (C2): white solid, yield 70%; IR: cm−1 3062 (m), 2978 (m), 1609 (s), 1560 (s), 1522 (s), 1470 (s), 1352 (s), 1270 (s), 1103 (s), 765 (s), 703 (s), 532 (s), 430 (m); 1H-NMR: δ 7.95 (s, 6H, Ar-H), 7.60–7.15 (m, 21H, Ar-H), 6.55 (t, 3H, CHF2), 3.0 (s, 3H, enol CH). Anal. Calcd for TbC48H33N2O6F6: C, 57.25; H, 3.28; N, 2.78; Tb, 15.80; found: C, 56.94; H, 3.18; N, 2.62; Tb, 15.32.
Tb(DPBD)3phen (C3): white solid, yield 72%; IR: cm−1 3066 (w), 2969 (w), 1609 (s), 1570 (s), 1522 (s), 1468 (s), 1350 (s), 1273 (s), 1103 (s), 769 (s), 714 (s), 524 (m), 424 (w); 1H-NMR: δ 8.70 (s, 2H, Ar-H), 7.90–6.65 (m, 21H, Ar-H), 6.35 (t, 3H, CHF2), 2.98 (s, 3H, enol CH). Anal. Calcd for TbC42H29N2O6F6: C, 54.19; H, 3.11; N, 3.01; Tb, 17.09; found: C, 54.07; H, 3.04; N, 2.97; Tb, 17.03.
Tb(DPBD)3neo (C4): white solid, yield 73%; IR: cm−1 3061 (s), 2973 (m), 2723, 1610 (s), 1570 (s), 1530 (s), 1467 (s), 1351 (s), 1268 (s), 1102 (s), 768 (s), 704 (s), 538 (m), 425 (w);; 1H-NMR: δ 7.85 (s, 6H, Ar-H), 7.50–6.60 (m, 15H, Ar-H), 6.45 (t, 3H, CHF2), 2.98 (s, 3H, enol CH), 2.80 (s, 6H, CH3). Anal. Calcd for TbC44H33N2O6F6: C, 55.11; H, 3.44; N, 2.92; Tb, 16.59; found: C, 54.96; H, 3.31; N, 2.87; Tb, 16.42.
Tb(DPBD)3bipy (C5): white solid, yield 70%; IR: cm−1 3066 (w), 2968 (w), 1606 (s), 1569 (s), 1527 (s), 1467 (s), 1352 (s), 1270 (s), 1105 (s), 767 (s), 704 (s), 524 (m), 423 (w); 1H-NMR: δ 7.90 (s, 8H, Ar-H) 7.60–7.15 (m, 15H, Ar-H), 6.40 (t, 3H, CHF2), 3.00 (s, 3H, enol CH). Anal. Calcd for TbC40H29N2O6F6: C, 52.98; H, 3.20; N, 3.09; Tb, 17.54; found: C, 52.83; H, 3.08; N, 3.02; Tb, 17.42.
Results and Discussion
Elemental Analysis
The analytical data of C, H and N elements in HDPBD ligand and C1-C5 complexes are listed in Table 1. It can be seen that the calculated and experimental values are in close proximity which permit to set the stoichiometry of the complexes as Tb(DPBD)3H2O (C1), Tb(DPBD)3biq (C2), Tb(DPBD)3phen (C3), Tb(DPBD)3neo (C4) and Tb(DPBD)3bipy (C5). All the complexes were stable under atmospheric conditions and soluble in DMSO (dimethylsulphoxide), chloroform, acetone and in DMF (dimethylformamide).
Spectroscopic Studies
The binding mode of ligand analyzed by IR spectra and their data is listed in Table 2. All the complexes showed similar binding modes of ligands, therefore exhibited similar profile of spectra. Hence, the IR spectra of HDPBD ligand and one of the complex i.e. C5 is depicted in Fig. 1. The spectra of HDPBD ligand and C1 complex displayed a weak band at 3430 cm−1 and 3338 cm−1, attributed to the enolic O-H vibration in ligand and O-H vibration of water molecules in C1 respectively. This broad band was absent in spectra of C2-C5 complexes, indicating coordination of HDPBD ligand with Tb3+ ion occured through enolic O-H group and successful substitution of solvent molecules by ancillary ligands. The strong ›C=O and enolic ›C=C stretching vibrations of HDPBD ligand showed a red shift of 29–25 cm−1 and 20–9 cm−1 respectively in complexes, suggesting the involvement of ›C=O group of ligand in complexation and extended π-conjugation in complexes. The C=N stretching vibrations in 1560–1570 cm−1 region appeared in spectra of C2-C5 complexes only, which clearly revealed that the nitrogen atoms of ancillary ligand participate in complex formation. Furthermore, two bands in the region 538–524 cm−1 and 430–423 cm−1 noticed in spectra of complexes only, attributed to Tb-N and Tb-O stretching vibrations respectively [23].
The 1H-NMR spectra of complexes exhibited some apparent changes as compared to spectrum of free HDPBD ligand. The singlets of enolic –OH proton at 15.01 ppm and keto –CH2 protons at 4.22 ppm noticed in the spectrum of ligand which were not observed in spectra of complexes, suggesting that the ligand in enolic form coordinated to terbium ion through –OH group [24].
UV-Visible Spectra
Fig. 2 shows the UV-visible absorption spectra of HDPBD ligands and C1-C5 terbium(III) complexes in ethanol solution (1 × 10−5 mol/L) at room temperature. It is noticed that the absorption intensity of complexes increased as compared to ligand due to the expansion of π-conjugated system in complexes as the terbium ion coordinated. The broad absorption band centering around 282 nm of complexes assigned to the π-π* electronic transition of ligand. The absorption maxima of ligand and complexes located around 282 nm indicating the singlet excited state of ligand was not affected by the coordination of the terbium ion [25].
Thermal Analysis and Powder X-Ray Study
The thermal stability of terbium(III) complexes was examined by thermal gravimetric (TG) and differential thermal curves in a broad range of temperature from 35 °C to 800 °C. The thermal behavior of all complexes showed stability upto 190–200 °C temperature therefore, C5 is explained in detail as the representative of other complexes (Fig. 3). The first little mass loss of 1.12% coincided with the loss of water molecules from the complex. The second mass loss of 64.16% upto 322 °C temperature observed due to decomposition of three DPBD molecules. Further, mass loss of 13.92% upto 665 °C temperature obtained due to loss of bipy molecule and finally, the terbium oxides as the final residue observed.
To know the amorphous or crystalline nature and particle size of terbium complexes, the powder X-ray diffraction profile was obtained as depicted in Fig. 4. The XRD profile of C2-C5 complexes exhibited some characteristics peaks in 10–80° range at 2θ angle, indicating the crystallinity of the complexes. On the other hand, a regular profile obtained for C1 complex, pointing the amorphous nature of complex which may be the consequence of the presence of water molecules in coordination sphere. The particle size of complexes was estimated from the Scherrer’s eq. D = 0.941λ/β cos θ, here D is the average particle size, λ is X-ray wavelength, θ is diffraction angle and β is full width at half maxima (FWHM) of an observed peak in XRD profile. The calculated particle sizes were 51.73 nm for C2, 40.42 nm for C3, 44.92 nm for C4 and 78.54 nm for C5 which are appropriate for the materials to be used in display devices.
Luminescence Properties
The solid state photoluminescence excitation spectra of C1-C5 complexes measured by monitoring the 5D4 → 7F5 emission transition of Tb(III) ion at 546 nm and depicts in Fig. 5. The figure shows a broad excitation band in the range 200–500 nm, in which maxima (372 nm) associated with the π-π* transitions of HDPBD ligand.
Fig. 6 displays the emission spectra of complexes under UV excitation around 372 nm. The spectra dominated by characteristic emission peaks of 4f-4f transitions of terbium(III) ion at 491 nm, 546 nm, 584 nm and 616 nm assigned to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4 and 5D4 → 7F3 transitions of terbium ion respectively as listed in Table 3. It can be noticed that there was no emission peak of ligand in emission spectra, suggesting the complete transfer of absorbed energy of the ligand to the Tb(III) ion. The emission peaks associated with 5D4 → 7F4 and 5D4 → 7F3 transitions were of low intensity as they were forbidden in magnetic as well as electric dipole transitions, relative to allowed 5D4 → 7F6 magnetic dipole and 5D4 → 7F5 electric dipole transitions. The most intense 5D4 → 7F5 electric dipole transition was sensitive to the chemical environment associated with the ligand field around the Tb(III) ion [26,27,28]. It is worth mentioning that as the interactions of the terbium(III) ion with its chemical environment was stronger, an asymmetric environment around the ligand came into existence, which located the Tb(III) ion in asymmetric coordination site [29]. Hence, the peak at 546 nm of 5D4 → 7F5 transition became of highest intensity, which inferred intense green color to the terbium(III) complexes. Fig. 6 clearly revealed that the luminescence intensity of C2-C5 complexes was higher than C1 complex. This was due to the presence of water/solvent molecules in the coordination sphere of C1 complex which possessed high energy O-H oscillators that promoted non-radiative decay process, resulting to lower luminescence intensity. On the other hand, as the solvent molecules substituted by ancillary ligands in C2-C5 complexes, the decrease in non-radiative decay was observed. Hence, the higher luminescence intensity obtained [30]. In this way, these ancillary ligands also played an important role in the sensitization process which increased luminescence intensity upto great level.
The luminescence color of complexes was typically characterized in the form of Commission Internationale de ‘Eclairage (CIE) chromaticity color coordinates (x and y). On the basis of emission spectra, the CIE color coordinates of complexes estimated as (x = 0.2484, y = 0.5748) for C1, (x = 0.2476, y = 0.6062) for C2, (x = 0.2184, y = 0.3833) for C3, (x = 0.2232, y = 0.4460) for C4 and (x = 0.2320, y = 0.5120) for C5, and are listed in Table 3. The color coordinates of all complexes fall under green region of chromaticity diagram, as depicted in Fig. 7.
Fig. 8 shows the luminescence decay profiles of C1-C5 terbium(III) complexes in solid state corresponding to 5D4 → 7F5 transition of Tb(III) ion. The profiles can be fitted to single exponential function, represented as I = I0 exp. (−t/τ), here τ is radiative decay time, I and I0 are luminescence intensities at time t and 0 respectively. The average decay time was determined to be 0.20 ms, 0.46 ms, 0.64 ms, 0.82 ms and 1.05 ms corresponding to C1-C5 terbium(III) complexes respectively. The longer decay time of C2- C5 complexes as compared to C1 complex attributed to reduced non-radiative process due to the incorporation of ancillary ligands in coordination sphere.
Mechanistic Investigation of Energy Transfer Process
The efficiency of intramolecular energy transfer is associated with two main processes. First is energy transfer from lowest triplet state (T1) of ligand to emitting levels of metal ion which deals with the Dexter luminescence theory [31]. According to this theory, the energy transfer rate constant (Ps) is determined the effectiveness of the first process by the overlap between triplet state of ligand and resonating levels of metal ion, which is given as
Here, Z2 is constant related with the distance between the metal ion and organic ligand; h is Planck’s constant; Fs (E) is emission spectrum of ligand (donor) and ξs (E) is absorption spectrum of metal ion (acceptor). The second process is the inverse energy transfer by thermal deexcitation process and their rate constant K(T) is expressed as below in Eq. 2:
Here, ∆E is energy difference between lowest excited triplet level of ligand and resonance energy level of the metal ion. On the basis of above discussion, too small or too large energy gap ∆E (T1-M3+) is not suitable for efficient energy transfer process. Latva’s et al. suggested that the lowest excited triplet state of ligand (T1) should be above the resonating level of the metal ion by 2000–5000 cm−1 value to result in efficient energy transfer [32]. To explicate the intramolecular energy transfer mechanism, the C5 complex is selected for detail discussion. The value of lowest singlet (S1, 32,154 cm−1) and triplet (T1, 25,125 cm−1) states of DPBD were calculated from referencing their edge wavelength of absorption spectra and lower wavelength of phosphorescence spectra (Fig. S1) respectively. Similarly, the lowest singlet and triplet states of bipy (S1, 29,900 cm−1; T1, 22,900 cm−1) ligand were also estimated which closely approach to the literature value [33, 34]. On the basis of above discussion, the proposed energy transfer mechanism in C5 complex is sketched as Fig. 9. It is worthy to mention that the ∆E (T1-M3+) value for DPBD and bipy observed to be 4759 cm−1 and 2534 cm−1 respectively which was appropriate according to Latva’s empirical rule. Hence, it can be infered that the triplet state (T1) of DBPD being higher energy, first transferred the absorbed energy to bipy ancillary ligand which subsequently transferred it to the trivalent terbium(III) ion. In this way, the ancillary ligands expressed their synergistic effect in energy transfer mechanism.
Conclusion
In summary, five new terbium(III) complexes were synthesized and characterized. Under UV irradiation these complexes emitted intense characteristic peak of terbium ion at 546 nm, accountable for green luminescent color. The highest intensity of electric dipole 5D4 → 7F5 transition indicated low symmetric coordination environment around the terbium(III) ion in complexes. It is noteworty that the emission spectra of ternary complexes (C2-C5) revealed the stronger luminescence intensity than the binary complex (C1). This enhancement in intensity attributed to the efficient intramolecular energy transfer from lowest triplet level of ligands to lowest excited level of terbium ion due to synergestic effect of anciliary ligands. Thus, the results of photophysical properties and high thermal stability demonstrated that these complexes can be employed as one of green component in light emitting materials and in display devices.
References
Kalyani NT, Doble SJ, Pode RB (2013) Synthesis and characterization of novel europium β-diketonate organic complexes for solid-state lighting. Luminescence 28:183–188
Yang CL, Xu J, Ma JY, Zhu DY, Zhang YF, Liang LY, Lu MG (2012) An efficient long fluorescence lifetime polymer-based sensor based on europium complex as chromophore for the specific detection of F−, CH3COO−, and H2PO4 −. Polym Chem 3:2640–2648
Binnemans K, in: Gschneidner Jr. KA, Bünzil, J-CG, Pecharsky VK (Eds.) (2005) Handbook on the physics and chemistry of rare earths, Elsevier, Amsterdam 35:107
Kido J, Okamoto Y, Organo (2002) Lanthanide Metal Complexes for Electroluminescent Materials. Chem Rev 102:2357–2368
Yuan J, Wang G (2005) Lanthanide Complex-Based Fluorescence Label for Time-Resolved Fluorescence Bioassay. J Fluoresc 15(4):559–568
Brunet E, Juanes O, Rodriguez JC (2007) Supramolecularly Organized Lanthanide Complexes for Efficient Metal Excitation and Luminescence as Sensors in Organic and Biological Applications. Curr Chem Biol 1:11–39
Yam VWW, Lo KKW (1999) Recent advances in utilization of transition metal complexes and lanthanides as diagnostic tools. Coord Chem Rev 184(1):157–240
Sabbatini N, Guardigli M, Lehn JM (1993) Luminescent lanthanide complexes as photochemical supramolecular devices. Coord Chem Rev 123(1):201–228
Bünzil J-CG, Piguet C (2005) Taking advantage of luminescent lanthanide ions. Chem Soc Rev 34:1048–1077
Fernandes M, Nobre SS, Goncalves MC, Charas A, Morgado J, RAS F, Carlos LD, Bermudez VZ (2009) Dual role of a di-urethanesil hybrid doped with europium β-diketonate complexes containing either water ligands or a bulky chelating ligand. J Mater Chem 19:733–742
Belousoff MJ, Ung P, Forsyth CM, Tor Y, Spiccia L, Ggraham B (2009) New Macrocyclic Terbium(III) Complex for Use in RNA Footprinting Experiments. J Am Chem Soc 131(3):1106–1114
Wang J, Wang R, Yang J, Zheng Z, Carducci MD, Cayon T, Peyghambarian N, Jabbour GE (2001) First Oxadiazole-Functionalized Terbium(III) β-Diketonate for Organic Electroluminescence. J Am Chem Soc 123(25):6179–6180
Bassett AP, Magennis SW, Glover PB, Lewis DJ, Spencer N, Parsons S, Williams RM, Cola LD, Pikramenou Z (2004) Highly Luminescent, Triple- and Quadruple-Stranded, Dinuclear Eu, Nd and Sm(III) Lanthanide Complexes Based on Bis-Diketonate Ligands. J Am Chem Soc 126(30):9413–9424
Whan RE, Crosby GA (1962) Luminescence studies of rare earth complexes: Benzoylacetonate and dibenzoylmethide chelates. J Mol Spectrosc 8:315–327
Sinha A, in: Rao C, Ferraro J Ed. (1971) Spectroscopy in Inorganic Chemistry, vol. 2, Academic Press, New York, London
Sato S, Wada M (1970) Relations between Intramolecular Energy Transfer Efficiencies and Triplet State Energies in Rare Earth β-diketone Chelates. Bull Chem Soc Jpn 43(7):1955–1962
Swavey S, Swavey R (2009) Dinuclear and polynuclear lanthanide coordination complexes containing polyazine ligands: Synthesis and luminescent properties. Coord Chem Rev 253(21):2627–2638
Ambili Raj DB, Biju S, Reddy MLP (2008) One-, Two-, and Three-Dimensional Arrays of Eu3+-4,4,5,5,5-pentafluoro-1-(naphthalen-2-yl)pentane-1,3-dione complexes: Synthesis, Crystal Structure and Photophysical Properties. Inorg Chem 47(18):8091–8100
Silva CRD, Maeyer JR, Wang R, Nichol GS, Zheng Z (2007) Adducts of europium β-diketonates with nitrogen p,p′-disubstituted bipyridine and phenanthroline ligands: Synthesis, structural characterization, and luminescence studies. Inorg Chim Acta 360(11):3543–3552
Kadjane P, Chabonniere L, Camerel F, Laine PP, Ziessel R (2008) Improving Visible Light Sensitization of Luminescent Europium Complexes. J Fluorosec 18(1):119–129
Ma Q, Zheng Y, Armaroli N, Bolognesi M, Accorsi G (2009) Synthesis and photoluminescence properties of asymmetrical europium(III) complexes involving carbazole, phenanthroline and bathophenanthroline units. Inorg Chim Acta 362(9):3181–3186
Räsänen M, Takalo H, Rosenberg J, Mäkelä J (2014) Study on photophysical properties of Eu(III) complexes with aromatic β-diketones- Role of charge transfer states in the energy migration. J Lumin 146:211–217
Hongfang J, Guode L, Zhijing Z, Yuehua F, Jianchao C, Tao F, Lixin Z (2011) Fluorescence enhancement of Tb(III) complex with a new β-diketone ligand by 1,10-phenanthroline. J Rare Earths 29(8):741–745
Luo Y, Li S, Li J, Chen X, Tang R (2010) Synthesis, fluorescence properties of Tb(III) complexes with novel mono-substituted β-diketone ligands. J Rare Earths 28(5):671–675
Pereira A, Gallardo H, Conte G, Quirino WG, Legnani C, Cremona M, Bechtold IH (2012) Investigation of the energy transfer mechanism in OLEDs based on a new terbium β-diketonate complex. Org Electron 13(1):90–97
Sun HJ, Fu XT, Chu HB, Du Y, Lin XM, Li X, Zhao YL (2011) Synthesis, characterization and luminescence property of ternary rare earth complexes with azatriphenylenes as highly efficient sensitizers. J Photochem Photobiol A 219:243–249
Xie H, Lu G (2014) Hydrothermal synthesis and luminescence of lanthanide complexes sensitized with dpphen. J Lumin 145:119–124
Safiullin GM, Nikiforov VG, Davydov NA, Mustafina AR, Soloveva SY, Lobkov VS, Salikhov KM, Konavalov AI (2015) Detailed mechanism of the ligand-to-metal energy transfer of silica-coated Tb(III) complex with p-sulfonatothiacalix[4]arene. J Lumin 157:158–162
Bala M, Kumar S, Taxak VB, Boora P, Khatkar SP (2016) Terbium(III) complexes sensitized with β-diketone and ancillary ligands: Synthesis, elucidation of photoluminescence properties and mechanism. J Mater Sci Mater Electron 27:9306–9313
Shi XY, Li WX, Qin CH, Guo L, Sun XJ, Sun XL, Geng GQ (2008) Synthesis and Characterization of Quaternary Complexes of Light Rare Earth Perchlorate with Diphenyl Sulfoxide,Benzoic Acid and Studies on Fluorescence of Eu3+ Complex. Chin J Lumin 29(5):772–778
Dexter DL (1953) A theory of sensitized luminescence in solids. Chem Phys 21(5):836–850
Latva M, Takalo H, Mukkala VM, Matachescu C, RodriguezUbis JC, Kankare J (1997) Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J Lumin 75(2):149–169
Biju S, Ambili Raj DB, Reddy MLP, Kariuki BM (2006) Synthesis, Crystal Structure, and Luminescent Properties of Novel Eu3+ Heterocyclic β-Diketonate Complexes with Bidentate Nitrogen Donors. Inorg Chem 45(26):10651–10,660
Yu X, Su Q (2003) Photoacoustic and luminescence properties study on energy transfer and relaxation processes of Tb(III) complexes with benzoic acid. J Photochem Photobiol A 155:73–78
Acknowledgements
The present work has been supported by Council of Science and Industrial Research (CSIR) of India [09/382(0155)/2012- EMR-I (Manju Bala)].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
(DOCX 90 kb)
Rights and permissions
About this article
Cite this article
Bala, M., Kumar, S., Devi, R. et al. Synthesis, Photoluminescence Behavior of Green Light Emitting Tb(III) Complexes and Mechanistic Investigation of Energy Transfer Process. J Fluoresc 28, 775–784 (2018). https://doi.org/10.1007/s10895-018-2239-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2239-y