Abstract
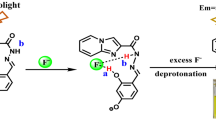
Novel anthraimidazoledione-based compounds (1–3) are synthesized as selective colorimetric and fluorescent sensors for fluoride ion. The binding properties of the probes (1–3) are studied with different anions in acetonitrile solvent. Spectral red shifts in the absorption spectra and ‘turn-off’ emission are observed when fluoride is added to 1–3. The striking green to orange color change in the ambient light is thought to be due to the deprotonation of the N–H proton of the imidazole moiety of the probes by the basic F− ion. Interestingly, in all three cases the nonfluorescent probe-F− solutions, on treatment with copper perchlorate, show distinct color change from orange to golden yellow with resumption of fluorescence intensity. Furthermore, the reversibility of sensors (1–3) for the detection of F− ion is tested for four cycles indicating that “ON-OFF-ON” mechanism is operative. Test strip based on sensor 2 acts as a reusable cost-effective F− sensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selective sensing of anions by artificial probes is an emerging field of supramolecular chemistry [1, 2]. Hence, design and development of new colorimetric and fluorimetric chemosensors for selective detection of anions have aroused significant interest among researchers [3, 4]. Fluoride, the smallest anion of halogen family, is gaining importance for its unique bifunctional activity towards mammals. A fluoride deficiency causes poor dental health and osteoporosis [5]. On the other hand, an excess of it can lead to fluorosis resulting in increased bone density [6, 7]. In drinking water, presence of this ion above 1.5 ppm is considered serious health risk as recommended by EPA [8]. So such a low level tolerance of fluoride ion deserves some very sensitive and selective transducer (signaling unit) to detect it.
Hydrogen bonding interactions and deprotonation of the N-H or O-H protons are the two well recognized strategies to couple a chromogenic or fluorogenic signaling unit to a receptor that can interact with fluoride ions [9]. A wide range of recognition subunits like thioureas, ureas, amides, sulphonamides, imidazolines or imidazoles, indoles, pyrroles, Schiff bases etc. have been reported in the literature for detection of fluoride ion colorimetrically and/or fluorimetrically [10, 11]. The biggest disadvantage of designing fluoride sensors are the interferences by acetate and/or phosphate ions of comparable basicity. Furthermore, most of these sensors act irreversibly for fluoride ion detection [12–14]. There are only a few fluoride sensors reported in the literature which can be used successfully as reversible and reusable systems [15–17]. So, development of new probes for reversible and selective “naked-eye” detection of fluoride in presence of other anions in an inexpensive and clear-cut method is a challenging task to the researchers.
From the last decades, considerable attention has been bestowed on imidazo-anthraquinone based chemosensors due to their versatility in structural features vis-á-vis photophysical properties [18–28]. These type of receptors generally show the charge transfer band which arise mainly from the πimidazole- πanthraquinone* transition. Here the anthraquinone part is electron deficient and serves as a magnificent acceptor and the imidazole fragment present in this system functions as an excellent hydrogen bond donor to the receptor anions. Moreover the acidity of the N-H proton can be tuned by substituting different functional groups at the 2-position of the imidazole ring that can be successfully exploited for anion detection [18–29]. Various derivatives of it are reported to act as colorimetric and/or fluorimetric sensors for F− and CN− ions [18–28]. Recently, some imidazo-anthraquinone scaffolds containing heteroaromatic ring at the 2-position of the aforesaid imidazole ring showed F− and CN− ion sensing properties [24, 25, 27]. Based on these observations, we have synthesized three receptors (1–3) in good to excellent yields in a slightly modified one-pot tandem protocol involving cyclocondensation cum oxidation procedure as reported earlier from our laboratory [30]. We have observed that all three probes (1–3) in acetonitrile medium are highly selective towards F− ions. Moreover they all act as dual channel sensors with high fluorescence “ON-OFF-ON” property and naked-eye detection (color changes from green to orange) of F− ions in acetonitrile solution at micromolar range. Moreover test strip can be prepared using sensor 2 which acts as a reusable F− sensor.
Experimental
Reagents and Measurements
Solvents and reagents were purchased from commercial source and were used after purification whenever required. Solvent acetonitrile was used of HPLC grade. All anions, in the form of tetrabutylammonium salts, were purchased from Sigma-Aldrich. The melting points of molecules (1–3) were determined in open capillary tubes on Kofler block apparatus and are uncorrected. A Perkin- Elmer RXI FT-IR spectrophotometer was used to record IR spectra in KBr discs. NMR spectra (1H, 13C) were obtained in DMSO-d6 solution (chemical shifts in δ ppm and J in Hz) in 5 mm BBO probe fitted with a pulse field gradient and working with Topsin 1.3 programme in a Bruker AV-300 Supercon NMR spectrometer. Mass spectrometry was recorded on a Q-TOF Micromass Waters Limited mass spectrometer. Hitachi UV-VIS U-3501 spectrometer was used for recording of absorption spectra in acetonitrile solution at room temperature where the concentrations of the molecules (1–3) are in the order of 1.0 × 10−6 mol L−1. Prekin-Elmer LS-55 was used for recording fluorescence spectra of the probes. For spectrometric titrations, acetonitrile solutions of the three probes (1–3) were prepared (ca. 1.0 × 10−5 M) and tetrabutylammonium salts of the respective anions (tetrabutylammonium salts of F−, Cl−, Br−, I−, OAc−, HSO4 −, H2PO4 −, SCN−, ClO4 − and OH−) under study were prepared in the range of 1.0 × 10−3 M in acetonitrile. Absorption and emission titration spectra were measured at 298 K using 10 mm path length quartz cuvette of the sensors 1–3 after sequential addition of the aforesaid anions in ion equivalents. Chromatography columns were prepared from silica gel (100–200 mesh) and 4:1 mixture of petroleum ether (b.p. 60–80 °C)-ethyl acetate were used as eluant.
General Reaction Procedure for the Synthesis of Compounds 1–3
A mixture of respective aldehydes (1 mmol) and 1,2-diaminoanthraquinone (1 mmol) were stirred in 3 mL PEG-400 support at 80 °C in an oil bath. To this mixture phosphotungstic acid (10 mol%) was added and stirred for stipulated time (2 h). The progress of the reaction was monitored by TLC dissolving the aliquot in ethyl acetate. After completion of the reaction the mixture was cooled to room temperature and acetone was added to it. The precipitated catalyst (Phosphotungstic acid in PEG-400 support) was filtered by pump and washed several times with hot acetone. The combined filtrate were collected and concentrated in rotary evaporator and then washed with water followed by brine to remove PEG-400. The remaining organic phase was dried over Na2SO4, filtered, concentrated and chromatographed over silica gel column to obtain pure products.
2-(furan-2-yl)-1H-anthra[1,2-d]imidazole-6,11-dione (1)
Yield: 270 mg, 90%, brown solid; Mp 286–287 °C; 1H NMR (300 MHz, DMSO-d6) δ 6.74–6.76 (m, 1H), 7.83–7.84 (m, 1H), 7.86–7.89 (m, 2H), 7.90–7.91 (m, 1H), 7.99–8.04 (m, 2H), 8.16–8.20 (m, 2H), 13.26 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 112.7, 112.8, 122.0, 125.2, 126.3, 127.4, 128.4, 132.2, 132.9, 133.5, 133.8, 134.2, 144.3, 144.9, 148.3, 149.2, 182.3, 184.7; IR υmax (KBr) cm−1 3435, 2925, 2853, 1655, 1588, 1508; anal. Calcd for C19H10N2O3: C: 72.61, H: 3.21, N: 8.91%, found: C: 72.60, H: 3.22, N: 8.90%.
2-(thiophen-2-yl)-1H-anthra[1,2-d]imidazole-6,11-dione (2)
Yield: 290 mg, 94%, brown crystalline solid; Mp 282–283 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.25–7.26 (m, 1H), 7.82–7.83 (m, 1H), 7.89–7.91 (m, 2H), 8.03 (s, 2H), 8.16–8.21 (m, 2H), 8.51 (d, J = 3 Hz, 1H), 13.40 (s, 1H); 13C NMR (75 MHz, DMSO-d6) δ 118.9, 121.7, 124.9, 126.7, 127.3, 128.4, 129.2, 130.9, 131.5, 132.1, 132.8, 133.2, 133.6, 134.7, 134.9, 149.7, 153.6, 182.8, 183.7; IR υmax (KBr) cm−1 3539, 3390, 3075, 2925,1655, 1577, 1560; anal. Calcd for C19H10N2O2S: C: 69.08, H: 3.05, N: 8.48%, found: C: 69.06, H: 3.06, N: 8.45%.
2-(thiophen-3-yl)-1H-anthra[1,2-d]imidazole-6,11-dione (3)
Yield: 282 mg, 90%, yellowish brown solid; Mp 284–285 °C; 1H NMR (300 MHz, DMSO-d6) δ 7.72–7.70 (m, 1H), 7.88–7.86 (m, 2H), 7.97–7.96 (m, 1H), 8.01–8.00 (m, 2H), 8.17–8.13 (m, 2H), 8.85–8.84 (m, 1H), 13.13 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 118.7, 121.2, 124.9, 126.4, 126.9, 127.5, 127.7, 128.7, 128.9, 131.4, 132.8, 133.2, 133.4, 134.4, 134.7, 149.5, 154.2, 182.5, 183.4; IR υmax (KBr) cm−1 3433, 3079, 1667, 1584, 1561; anal. Calcd for C19H10N2O2S: C: 69.08, H: 3.05, N: 8.48%, found: C: 69.07, H: 3.04, N: 8.45%.
Results and Discussion
Synthesis and Characterization
Molecules 1–3 have been synthesized (Scheme 1) via condensation cum oxidation reaction of 1,2-diaminoanthraquinone (1 mmol) and the respective heteroaromatic aldehydes (1 mmol) stirring in PEG-400 with catalyst phosphotungstic acid (10 mol%). A sharp signal for NH proton in the downfield region of δ 13.13–13.40 were observed in the respective 1H–NMR spectrum of each of the molecules 1–3 indicating high acidity and strong hydrogen bonding capability of the NH group.
Naked-Eye Detection of Ions
Visual color change of the sensors 1–3 (1.0 × 10−6 M) were investigated in presence of different anions (F−, Cl−, Br−, I−, OAc−, HSO4 −, H2PO4 −, SCN−, ClO4 − and OH− ions) in acetonitrile solvent. Upon addition of F− ion (5 equiv) to a solution of probe 3 which was green in color, an orange color was developed detectable by naked-eye (Fig. 1). The other anions Cl−, Br−, I−, OAc−, HSO4 −, H2PO4 −, SCN−, ClO4 − (5 equiv) did not display any significant color change. Similar observations were obtained in case of sensors 1 and 2 also (Fig. S1 in the ESI). It was assumed that high negative charge density on F− ion experiencing strong hydrogen bonding interaction with -NH of the imidazole ring caused this color change. Addition of OH− ion to those sensors 1–3 in acetonitrile produced identical results (Fig. 1). Mechanistically, this could only be achieved through the abstraction of the designated -NH proton with the formation of the corresponding anions. Stabilization of the anions via delocalization of the negative charge over the extended aromatic framework would induce such red shift of color of the substrates.
Photophysical Study of 2-heteroannellated imidazo-anthraquinones 1-3
To explain the interactions between the sensors and the anions, the absorption and emission spectra of 2-heteroannellated imidazo-anthraquinones 1, 2 and 3 were recorded in acetonitrile solvent (ca. 20 × 10−6 M).
Spectrophotometric Titrations of Probes 1-3 with Anions in Acetonitrile Solution
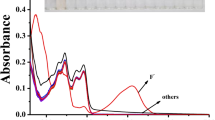
The anion binding abilities of the sensors (1–3) (20 × 10−6 M in CH3CN) with F−, Cl−, Br−, I−, OAc−, HSO4 −, H2PO4 −, SCN−, OH− and ClO4 − were studied through UV–VIS experiment. The anions tested were used as their tetrabutylammonium salts. In course of monitoring the effect of anions on the steady-state spectral properties of 1–3, the absorption spectra showed nominal or no decrement of absorbance in presence of different anions, excepting F− and OH− ions (Fig. 2). All the three probes exhibited their low energy ππ* transition at ~400 nm. Gradual addition of F− ion to acetonitrile solutions of the probes 1, 2 and 3 were found to accompany a red shift of the absorption signatures from 414, 413, 405 to 487, 489 and 483 nm respectively which was expected to be arising from the strongly hydrogen bonded clusters of the probes with F− ion. On the other hand, OH− ions induced similar red shifts for sensors 1, 2 and 3 to 477 nm, 479 nm and 472 nm respectively (Fig. 2). The above mentioned results clearly indicated the abstraction of the N-H proton of the imidazole ring by the basic F− and OH− ions.
Spectrophotometric titrations of probes 1–3 in acetonitrile solution (20 × 10−6 M) with F− and OH− ions were performed. Upon addition of increasing amount of F− ion to probe 1 (20 × 10−6 M) in acetonitrile solution, the absorbance of the bare sensor at 414 nm gradually decreased with the generation of a new red shifted broad peak at 487 nm (Δλ = 73 nm) having distinct isobestic points at 442 nm and 339 nm (Fig. 3a) which were in the visible region of spectrum associated with the color change from green to orange. In case of OH− titration of probe 1, the absorbance at 414 nm similarly decreased with the appearance of a red shifted band at 477 nm (Δλ = 63 nm) with isobestic points at 442 nm and 340 (Fig. 4a). Probe 2 exhibited similar tendency as probe 1 for F− ion, shifting the absorbance band from 413 nm to 489 nm (Δλ = 76 nm) having isobestic points at 443 nm and 346 nm (Fig. 3b). In case of OH− ion titration of the probe 2 absorbance band was similarly shifted to 479 nm (Δλ = 66 nm, isobestic points at 443 nm and 347 nm) (Fig. 4b). Probe 3 manifested the largest red shift among the three upon addition of F− ion from 405 nm to 483 nm (Δλ = 78 nm) with isobestic points at 435 nm and 339 nm (Fig. 3c). For OH− ion titration of the probe 3 following the same procedure, shifting of the absorbance band occurred at 472 nm (Δλ = 67 nm) with isobestic points at 436 nm and 338 nm (Fig. 4c). Similar types of red shifting of the absorbance bands by the basic F− and OH− ions in all cases suggested deprotonation of the -NH proton of the imidazole unit of the sensors justifying the visible color change.
Spectrophotometric titrations of imidazo-anthraquinones 1–3 with F− in acetonitrile ( [1–3] = 20 × 10−6 M, T = 298 K, 1-3exc = 414, 413 and 405 nm respectively). The insets represent the Benesi-Hildebrand (B-H) plots for the titration of 1–3 with F− ion at 487, 489 and 483 nm in acetonitrile respectively
From the UV-VIS titration data, the binding constants of the probes 1–3 with fluoride ion have been calculated. The binding constants, K of all the probes (1–3) and their interactions with F− have been determined with the help of Benesi-Hildebrand (B-H) relation [31, 32]. The binding constants (K) were calculated from the ratio of the intercept and slope of B-H plot of optical density (A). The B-H plots of 1/[A-A0] vs 1/[F−] for the titration of probes 1–3 and F− ion provided straight lines with association constants (k) 0.55 × 104 M−1, 4.69 × 104 M−1 and 1.69 × 104 M−1 respectively (Fig. 3 inset).
We have also calculated The Limit of Detection (LOD) of F− from UV-VIS spectrophotometric titrations for the three probes (1–3) at 487, 489 and 483 nm in acetonitrile (Fig. S2 in the ESI) which were found to be 0.9808 × 10−6 M, 0.5785 × 10−6 M and 0.7588 × 10−6 M respectively which are far below than the EPA recommendation [20, 27]. Thus the three probes can be considered as promising candidates for fluoride detection in acetonitrile media.
Spectrofluorimetric Titrations of Probes 1-3 with Anions in Acetonitrile Solution
It may be mentioned here that only F− and OH− ions imparted considerable quenching to the emission band intensities of the compounds 1–3, while others failed to convey any (Figs. 5, 6 and Fig. S3-S6, ESI). The emission intensity decrement of the studied probes in presence of increasing concentration of F− and OH− ions (along with a significant red shift) is presented in Fig. 5 (and Fig. S3, S4 and S7, ESI). The fluorescence spectrum of sensor 1 (20 μM) exhibited a strong emission band at 547 nm in MeCN (λex = 414 nm) (Fig. S3, ESI). Upon the addition of F−, Cl−, Br−, I−, OAc−, HSO4 −, H2PO4 −, SCN−, OH− and ClO4 − (5 equiv. each) in CH3CN, only F− and OH− quenched the emission band with a red shift to 575 nm and 607 nm respectively (Fig. S3, ESI). Fluorescence titration of probe 1 with various amounts of F− and OH− (0.0–5.0 equiv) in MeCN (λex = 414 nm) also supported the above observation (Fig. S3 and S7, ESI). Similar emission bands at 539 nm (λex = 413 nm) and 527 nm (λex = 405 nm) in acetonitrile were observed for sensors 2 and 3 also (Fig. S4 and Fig. 5). Upon the addition of various anions in CH3CN (5 equiv. each), only F− and OH− quenched the emission band with a red shift to 580 nm and 608 nm for sensor 2 and 537 nm and 598 nm for sensor 3 respectively (Fig. S4 and S7, ESI and Fig. 5). Fluorescence titration of sensors 2 and 3 with various amounts of F− and OH− (0–5 equiv) in MeCN (λex = 413 nm and 405 nm respectively) also corroborated these facts (Fig. S4 and S7, ESI and Fig. 5). Fig. 6 and also Fig. S5 and S6 presented comparative results of the fluorescence studies for the sensors 1–3 in presence of different anions (5 equiv) respectively.
To explore the abilities of the probes (1–3) as colorimetric as well as fluorimetric sensors for F− ion in presence of other competing ions, experiments were performed in the presence of F− mixed with other anions for probes 1–3 as depicted in Fig. 7 and Fig. S8 and S9 (ESI). The competing anions had apparently no influence on the color change, UV-VIS spectral change as well as fluorescence intensities of the probes (1–3).
1H NMR Titration Spectra
The sensory behavior of the probes 1–3 identified from the spectrophotometric and spectrofluorimetric titrations with F− ion were also complimented by performing 1H NMR titration experiments in absence and in presence of F− ion (0–6 equiv) in DMSO-d6 at room temperature. The downfield signal attributed to the imidazo N-H proton discernible in the 1H–NMR spectrum for each of the probes 1–3 suggested high acidity and strong hydrogen-bonding ability of those N-H protons. In case of probe 3, the imidazo N-H proton resonating at δ 13.13 disappeared when 1.0 equiv. F− ions was added to the DMSO-d6 solution of the probe (1.0 × 10−2 M) at room temperature ((Fig. 8) with characteristic upfield shifts for the H5´ and other aromatic and heteroaromatic protons (~ δ 0.22). Successive addition of the F− ions (2 equiv., 4 equiv. and 6 equiv) to probe 3, observed an upfield shift of the H5´ and other protons (~ δ 0.22) and H5´ gradually divided into multiplets with shrinking size and finally it disappeared suggesting an extended π-conjugated system ((Fig. 8). However in case of probe 2 (1.0 × 10−2 M in DMSO-d6 solution), by addition of F− ions (6 equiv), H3´ proton reduced in size with an overall ~ δ 0.12–0.15 upfield shift of other protons (Fig. S10, ESI).
Reversibility, Reusability and Sensory Performance of Probes 1–3
The reversibility of a chemosensor is very beneficial and at the same instance the reusable nature of it is another essential aspect for sensory application. It is clear from the literature survey that except a few paradigms [15–17], most of the F− ion sensors are irreversible in character and of single use. It is advantageous that the 9,10-anthraquinone derivatives are able to coordinate to metal ions through the lone pairs of the oxygen of the carbonyl groups resulting in the formation of metal ion complexes [33, 34]. In addition, each of the deprotonated form of the probes 1–3 (in presence of 5 equiv. fluoride ion) offers an additional binding site i.e. the deprotonated N-atom of the imidazole ring. These deprotonated species of the probes 1–3 were non-emissive in nature and sensing behavior of these species were investigated in presence of perchlorates of three transition metal cations (Co2+, Cu2+ and Zn2+). In all cases, the absorption bands appearing at 483–489 nm (related to the deprotonated species of the probes 1–3) were blue-shifted to their original position at 408–416 nm suggesting the formation of metal complexes in solution. We carried out absorption and fluorescence experiment of the probe 3 by alternate addition of F− ion and copper perchlorates as a representative system to investigate the reversibility of the probes. The absorption spectra of deprotonated form of 3-F− (20 μM probe 3 with 5 equiv. of F− ions in acetonitrile solution) upon addition of 1 equiv. of copper perchlorate (20 μM in acetonitrile solution) resumed its original λmax at 408 nm (Fig. 9).
Similar observation obtained for probes 1 and 2 also (Fig. S11 and S12, ESI). Additionally, from the UV-VIS spectral analysis of the free probes (1–3), it was observed that they were insensitive towards these transition metal cations (Fig. S13, ESI)
The addition of F− ion to probes (1–3) in acetonitrile solution quenched its fluorescent nature and addition of copper perchlorate (1 equiv) reverted back its fluorescence character. This “ON-OFF-ON” process was detectable by naked eye as well as by UV lamp as in case of probe 3 (Fig. 10). During reversibility experiment the quenching of fluorescence (off) with F− ion and revert back of the fluorescence (on) by Cu2+ could be due to regeneration of bare molecule by formation of CuF2.
To investigate the practical application of the probes, we have prepared test strips using filter papers (Whatman filter paper) with probe 2 in acetonitrile solution (1.0 × 10−3 M) and then drying in air. The test strips were now utilised to sense F− ion. Upon addition of F− to the test strip, color changes from yellow to orange which can be detected by (a) naked eye and (b) under UV-lamp (Fig. 11). The strips can be reused after washing with copper perchlorate solution.
Conclusion
In summary, we have successfully synthesized three novel imidazoanthraquinone-based chromo−/fluorogenic dual signalling sensitive probes 1–3 for the selective detection of fluoride ion over various other anions in acetonitrile media. Similar types of red shifting of the absorbance bands by the basic F− and OH− ions for probes 1–3 suggested deprotonation of the -NH proton of the imidazole unit of the sensors justified the visible color change from green to orange. Addition of F− ions to acetonitrile solution of probes 1–3 led to a complete quenching of fluorescence of the three probes. Competitive binding experiments of the probes 1–3 in presence of various anions exhibited that they could specifically detect F− ions. The reversibility of the probes 1–3 were an added advantage of this system. Interestingly, alternate addition of F− and Cu2+ ions to the probes demonstrate an “ON-OFF-ON” mechanism. Paper strips made from probe 2 can be reused after washing with copper perchlorate solution.
References
Bell TW, Hext NM (2004) Supramolecular optical chemosensors for organic analytes. Chem Soc Rev 33:589–598
García JM, García FC, Serna F, De La Peňa JL (2011) Fluorogenic and chromogenic polymer chemosensors. Polymer Rev 51:341–390
Veale EB, Gunnlaugsson T (2010) Annual reports on the progress of chemistry, section B. RSC Publishing, Cambridge, UK 106:376–390
Gale PA, Caltagirone C (2015) Anion sensing by small molecules and molecular ensembles. Chem Soc Rev 44:4212–4227
Kirk KL (1991) Biochemistry of the halogens and inorganic halides. Plenum, New York
Bassin EB, Wypij DR, Davis B, Mittleman MA (2006) Age-specific fluoride exposure in drinking water and osteosarcoma (United States). Cancer Causes Control 17:421–428
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487
Kauffman JM (2005) Water fluoridation: a review of recent research and actions. Journal of American Physicians and Surgeons 10:38–44
Mondal D, Bar M, Maity D, Baitalik S (2015) Anthraimidazoledione-terpyridine-based optical chemosensor for anions and cations that works as molecular half-subtractor, key-pad lock, and memory device. J Phys Chem C 119:25429–25441
Zhou Y, Zhang JF, Yoon J (2014) Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem Rev 114:5511–5571
Jiao Y, Zhu B, Chen J, Duan X (2015) Fluorescent sensing of fluoride in cellular system. Theranostics 5:173–187
Lin C, Selvi S, Fang J, Chou P, Lai C, Cheng Y (2007) Pyreno[2,1-b]pyrrole and bis(pyreno[2,1-b]pyrrole) as selective chemosensors of fluoride ion: a mechanistic study. J Org Chem 72:3537–3542
Wang J, Yang L, Hou C, Cao H (2012) A new N-imidazolyl-1,8-naphyhalimide based fluorescence sensor for fluoride detection. Org Biomol Chem 10:6271–6274
Qu Y, Hua J, Tian H (2010) Colorimetric and ratiometric red fluorescent chemosensor for fluoride ion based on diketopyrrolopyrrole. Org Lett 12:3320–3323
Madhu S, Ravikanth M (2014) Boron-dipyrromethene based reversible and reusable selective chemosensor for fluoride detection. Inorg Chem 53:1646–1653
Erdemir S, Kocyigit O (2015) Reversible “OFF-ON” fluorescent and colorimetric sensor based benzothiazole-bisphenol a for fluoride in MeCN. Sensors Actuators B 221:900–905
Wang L, Fang G, Cao D (2014) A reversible and reusable selective chemosensor for fluoride detection using a phenolic OH-containing BODIPY dye by both colorimetric ‘naked-eye’ and fluorometric modes. J Fluoresc 24:1757–1766
Peng X, Wu Y, Fan J, Tian M, Han K (2005) Colorimetric and ratiometric fluorescence sensing of fluoride: tuning selectivity in proton transfer. J Org Chem 70:10524–10531
Batista RMF, Oliveira E, Costa SPG, Lodeiro C, Raposo MMM (2007) Synthesis and ion sensing properties of new colorimetric and fluorimetric chemosensors based on bithienyl-imidazo-anthraquinone chromophores. Org Lett 9:3201–3204
Saha S, Ghosh A, Mahato P, Mishra S, Mishra SK, Suresh E, Das S, Das A (2010) Specific recognition and sensing of CN− in sodium cyanide solution. Org Let 12:3406–3409
Kumari N, Jha S, Bhattacharya S (2011) Colorimetric probes based on anthraimidazolediones for selective sensing of fluoride and cyanide ion via intramolecular charge transfer. J Org Chem 76:8215–8222
Luxami V, Kumar S (2012) A differential ICT based molecular probe for multi-ions and multifunction logic circuits. Dalton Trans 41:4588–4593
Batista RMF, Costa SPG, Raposo MMM (2013) Naphthyl-imidazo-anthraquinones as novel colorimetric and fluorimetric chemosensors for ion sensing. J Photochem Photobiol A Chemistry 259:33–40
Batista RMF, Oliveira E, Costa SPG, Lodeiro C, Raposo MMM (2014) Cyanide and fluoride colorimetric sensing by novel imidazo-anthraquinones functionalized with indole and carbazole. Supramol Chem 26:71–80
Batista RMF, Costa SPG, Raposo MMM (2014) Selective colorimetric and fluorimetric detection of cyanide in aqueous solution using novel heterocyclic imidazo-anthraquinones. Sensors Actuators B Chemical 191:791–799
Marin-Hernandez C, Santos-Figueroa LE, Moragues ME, Raposo MMM, Batista RMF, Costa SPG, Pardo T, Martinez-Manez R, Sancenon F (2014) Imidazoanthraquinone derivatives for the chromofluorogenic sensing of basic anions and trivalent metal cations. J Org Chem 79:10752–10761
Sarkar A, Bhattacharyya S, Mukherjee A (2016) Colorimetric detection of fluoride ions by anthraimidazoledione based sensors in the presence of Cu (II) ions. Dalton Trans 45:1166–1175
Suganya S, Park JS, Velmathi SJ (2016) Highly fluorescent imidazole probes for the pico molar detection of CN− ion and application in living cells. J Fluores 26:207–215
Li GY, Zhao GJ, Liu YH, Han KL, He GZ (2010) TD-DFT study on the sensing mechanism of a fluorescent chemosensor for fluoride: excited-state proton transfer. J Comput Chem 31:1759–1765
Bhattacharyya B, Kundu A, Das A, Dhara KP, Guchhait N (2016) One-pot protocol for J-aggregated anthraimidazolediones catalyzed by phosphotungstic acid in PEG-400 under aerobic condition. RSC Adv 6:21907–21916
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Kuntz ID, Gasparro FP, Johnston MD Jr, Taylor RP (1968) Molecular interactions and the Benesi-Hildebrand equation. J Am Chem Soc 90:4778–4781
Ranyuk E, Uglov A, Meyer M, Lemeune AB, Denat F, Averin A, Beletskaya I, Guilard R (2011) Rational design of aminoanthraquinones for colorimetric detection of heavy metal ions in aqueous solution. Dalton Trans 40:10491–10502
Huang MR, Huang SJ, Li XG (2011) Facile synthesis of polysulfoaminoanthraquinone nanosorbents for rapid removal and ultrasensitive fluorescent detection of heavy metal ions. J Phys Chem C 115:5301–5315
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 2646 kb)
Rights and permissions
About this article
Cite this article
Bhattacharyya, B., Kundu, A., Guchhait, N. et al. Anthraimidazoledione Based Reversible and Reusable Selective Chemosensors for Fluoride Ion: Naked-Eye, Colorimetric and Fluorescence “ON-OFF”. J Fluoresc 27, 1041–1049 (2017). https://doi.org/10.1007/s10895-017-2038-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2038-x