Abstract
A fluorescent and colorimetric chemosensor for detection of cyanide ion based on a styryl quinoline derivative has been designed and synthesized. The chemosensor (E)-2-(4-mercaptostyryl)quinolin-8-ol L showed high selectivity for detection of cyanide over other anions such as F¯, Cl¯, Br¯, I¯, NO3¯, SCN¯, N3¯, ClO4¯, H2PO4¯, AcO¯, HCO3¯, SO4 2¯ and HSO4¯in aqueous solution. The chemosensor L displayed an immediate visible and fluorescence changes from nearly colorless to orange and greenish-blue to brick-red upon addition of cyanide ion respectively. It is more likely, these distinct changes can be attributed to hydrogen bonding interaction between phenol group and cyanide anion leading to a 1:1 binding stoichiometry following with deprotonation of phenol group. The detection limit for chemosensor L toward CN¯ was 2.73× 10−8 M. Thus, the chemosensor can be used efficiently and selectively for detection and monitoring of small amounts of cyanide ion in aqueous media.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to important role of anions in environmental, chemical, clinical, and biological processes [1–7], detection of anions such as cyanide has been of great interest in supramolecular chemistry. Cyanide is one of the most toxic anions because of its effects on numerous body functions such as inhibit oxygen utilization by cells and binding to the active site of cytochrome oxidase [8]. Therefore, central nervous systems and heart that requiring more oxygen compared with other body organs, are strictly affected by cyanide poisoning. Cyanide could be absorbed through the skin, gastrointestinal track, and the lungs and approximately 0.5–3.5 mg of cyanide per kg of body weight is fatal for human [9]. Despite its high toxicity, cyanide is used in many industrial processes (1.5 million tons per year), such as electroplating of metals, plastics manufacturing, in the recovery of gold and silver from ores, and metallurgy [10–12]. Thus, it is necessary to develop an efficient sensing system for selective detection of cyanide ion. Among the various approaches for the detection of cyanide such as chromatographic [13], flow injection [14, 15] and electrochemical analyses [16–19],fluorescence and colorimetric detections due to their high sensitivity, rapid response rate, easy detection, and low cost are the most convenient methods in this regard. To date, numerous fluorescent sensors for detection of cyanide ion have been developed based on various mechanisms including metal ion coordination of CN− [20–22], deprotonation reactions [23–26],nucleophilic addition reactions [27–31],and hydrogen bonding interactions [32–35]. Meanwhile, many of these sensors suffer from low selectivity and interference with other anions [36–39].
After our previous work in development of a fluorescence and colorimetric chemosensor for detection of Zn2+ and Sn2+ [40], herein, we report synthesis of a new simple styryl quinoline based chemosensor L and investigation of its application for highly selective detection of CN¯ which is one of the most important issues in the environmental chemistry.
Experimental Section
Materials and Instrumentations
All chemical reagents and solvents used were obtained commercially from Merck (Germany) at analytical grade and used without further purification. NMR spectra were recorded on Bruker Avance NMR spectrometer (Germany) at an operating frequency of 400 MHz for 1HNMR and 100 MHz for 13CNMR. UV/visible absorption spectra were recorded by using the Shimadzu UV-160 (Japan). Fluorescence spectra were measured on a Shimadzu RF-5000 Spectrofluorometer.
Synthesis of (E)-2-(4-Mercaptostyryl)Quinolin-8-Ol L
-
A)
In a 25 mL flask a mixture of 4-chlorobenzaldehyde (10 mmol, 1.41 g), 1,3-propanedithiol (11 mmol, 1.19 g), hexadecyltrimethylammonium bromide (HTAB, 20 mg), and NaI (20 mg) were dissolved in EtOH (10 mL) and refluxed for 5 h. Then, the reaction mixture was triturated in EtOAc-H2O (50:50, 30 mL) and the organic phase was separated and dried. After removal of the solvent, the residue was chromatographed on silica gel using EtOAc-hexane (1:5) as eluent to obtain pure 4-((3-mercaptopropyl)thio)benzaldehyde as a creamy-white solid having a characteristic odor (1.17 g, 55%).
4-((3-mercaptopropyl)thio)benzaldehyde: mp: 35 °C (uncorrected); 1H NMR (400 MHz, DMSO-d 6 ) δ: 1.92 (qui, 2 H, J = 7.2 Hz), 2.61 (t, 2H, J = 7.2 Hz), 3.08 (t, 2H, J = 7.2 Hz), 7.30 (d, 2H, J = 8.4 Hz), 7.70 (d, 2H, J = 8.0 Hz); 13C NMR (100 MHz, CDCl3) δ: 27.1, 29.5, 30.0, 125.5, 127.2, 127.8, 129.0, 190.1; IR (KBr); 2835, 2736(=CH), 2566(S-H), 1694(C = O), 2918, 1590, 1489, 1088, 836.
-
B)
In a 10 mL round bottom flask equipped with a reflux condenser, a mixture of 4-((3-mercaptopropyl)thio)benzaldehyde (5 mmol, 1.06 g) and 8-hydroxyquinaldine (5 mmol, 0.80 g) were dissolved in acetic anhydride (4 mL) and heated under reflux condition for 6 h. After cooling, the reaction mixture was poured in a mixture of ice-water to deposit a crude gummy solid. Then the precipitated solid was added to a solution of NaOH in EtOH (2.5 M, 15 mL) and heated under reflux temperature for 2 h. The reaction mixture was cooled, neutralized with HCl (1 M), and extracted with EtOAc. Then, the organic phase was remove under reduced pressure and the residue was subjected to column chromatography using EtOAc-hexane (1:4) as eluent to afford the pure (E)-2-(4-mercaptostyryl)quinolin-8-ol L as a yellow powder (0.45 g, 32%).
(E)-2-(4-mercaptostyryl)quinolin-8-ol: mp: 87–89 °C (uncorrected);1H NMR (400 MHz, DMSO-d 6 ) δ: 7.09 (dd,J 1 = 7.2 Hz, J 2 = 2.0 Hz,1H), 7.35–7.41(m, 2H), 7.47–7.51(m, 3H), 7.72–7.77(m, 3H), 8.12 (d, J = 16 Hz, 1H), 8.29 (J = 8.8 Hz, 1H), 9.56 (s, 1H).13C NMR(100 MHz, DMSO-d 6 ) δ:110.3, 117.7, 120.4, 127.5, 128.4, 128.5, 129.1, 133.0, 134.5, 134.8, 136.6, 137.9, 152.0, 153.2; Anal. Calcd. for C17H13NOS: C, 73.09; H, 4.69; N, 5.01; S, 11.48 Found: C, 73.13; H, 4.67; N, 5.05; S, 11.45.
Results and Discussion
The chemosensor (E)-2-(4-mercaptostyryl)quinolin-8-ol L was synthesized in three simple steps starting from 4-chlorobenzaldehyde as a readily available compound. (Scheme 1). First, 4-chlorobenzaldehyde1 was reacted with 1,3-propanedithiol to produce 4-((3-mercaptopropyl)thio)benzaldehyde2. After that, compound 2 was condensed with 8-hydroxyquinaldine in the presence of acetic anhydride following with base catalyzed hydrolysis with ethanolic NaOH to afford (E)-2-(4-mercaptostyryl)quinolin-8-ol L as a yellow solid (scheme 1).
The anion sensing of L was first investigated by UV/visible absorption spectra. Figure 1 shows the absorbance change of L upon addition of various anions including F¯, Cl¯, Br¯, I¯, NO3¯, SCN¯, N3¯, ClO4¯, H2PO4¯, AcO¯, HCO3¯, SO4 2¯, HSO4¯ and CN¯ (in DMSO/H2O, 50/50 v/v). As shown in the Fig. 1, the chemosensor L displayed only a distinct response to CN¯ and other anions had no obvious effect in UV/visible absorption spectra. It was found that, bare chemosensor L showed intense absorption bands at 298 nm (ε = 23,314 M−1 cm−1) and 341 nm (ε = 13,514 M−1 cm−1). When CN¯ ion was added to the solution of L, intensity of signal at 298 nm was decreased with no shift and the intensity of signal at 341 nm was also suppressed alongside with a small blue-shift and subsequently a new broad peak was appeared in 460 nm. As shown in Fig. 1b, an emission band at 475 nm for the fluorescence spectra of the bare chemosensor L was observed and upon addition of CN¯ a big red-shift to 602 nm was detected and the fluorescence intensity was notably declined.
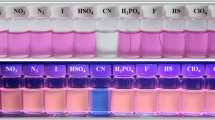
Fortunately, both colorimetric and the fluorescence response of L to CN¯ can be traced visually. During addition a 50 μM solution of CN¯ to also a 50 μM colorless solution of L a vivid orange color was appeared instantly while, the fluorescence emission was changed from a greenish-blue to a brick-red under illumination with a 365 nm UV lamp (Fig. 2). These clear changes indicate that compound L can be used as a selective, sensitive, and fast chemosensor for detection of cyanide anion.
The fluorescence intensity of L in the presence of various concentration of CN¯ in DMSO/H2O (50/50, v/v) is presented in Fig. 3. As it is obvious from Fig. 3a, the fluorescence intensity at 602 nm was increased with increasing the concentration of cyanide ion. The calibration curve of the fluorescence emission at 602 nm is shown in Fig. 3b which indicate that, there is a good linear relationship between cyanide concentration and the fluorescence intensity in the range of 0.1–1.2 μM (R = 0.993). Various solvents such as CH3CN, EtOH, dioxane, and DMF in different H2O percentages were examined for the evaluation of chemosensory role of L. We practically found that the optimal solvent mixture was DMSO/H2O (50/50, v/v). The detection limit of L for CN¯ in DMSO/H2O (50/50, v/v) was measured to be 2.73× 10−8 M which is far lower than the World Health Organization (WHO) guideline value for cyanide ion (1.9 μM).
To evaluate the selectivity of chemosensor L for detection of cyanide anion, competitive experiments were conducted in the presence of 100 equiv. of other anions in the solution of DMSO/H2O. Figure 4a indicates that, except CN¯, other anions have no observable effect on fluorescence emission of L at 602 nm. These findings confirm the high selectivity and anti-interference of chemosensor L for detection of cyanide anion.
The stoichiometric ratio between the L and CN¯ was determined by Job’s plots method. As shown in Fig. 4b, a 1:1 stoichiometry was observed for the interaction of L with CN¯ ion.
As well known, a long response time is a serious problem that chemosensors often encounter. In our study, the colorimetric and the fluorescence response time of chemosensor L to CN¯ was very fast and in the order of a few seconds (<5 s) and conservative (Fig. 4 in Supporting Information).
The interaction mode and the detection mechanism of sensor L for detection of CN¯ was further analyzed by 1HNMR spectroscopy through adding an equiv. amount of a solution NaCN in DMSO-d 6 to a solution of chemosensor L also in DMSO-d 6. Probably, upon addition of cyanide ion, a hydrogen bonding interaction between phenol group and cyanide ion is established and it is more likely following that, deprotonation of hydroxyl group is occurred. This fact was approved by disappearance of phenolic proton signal at 9.56 ppm and occurrence a noticeable downfield shift for H2O signal residue at 3.34 ppm. Furthermore, aromatic and vinylic protons were also displayed substantial upfield shifts as shown in the Fig. 5.
A comparison of other works with our study was shown in Table 1.
As shown in Table 1, high color and fluorescence change, short detection time, low detection limit, and no interference with other anions are the salient features of our chemosensor.
Conclusions
In summary, a new colorimetric and fluorescent chemosensor for rapid, highly selective and sensitive detection of cyanide anion was designed and synthesized. Upon addition of CN¯ to a solution of L, a significant color change was observed under visible light from colorless to orange and from greenish-blue to brick-red under UV illumination with a large red-shift. The detection mechanism was confirmed by 1HNMR and Job’s plot and indicated that, there was a 1:1 stoichiometric ratio between the L and CN¯ and following the establishment of a hydrogen bonding interaction between the CN¯ and phenolic group, deprotonation of hydroxyl group was occurred. The detection limit was measured to be 2.73× 10−8 M, which explicitly demonstrate that chemosensor L can be used as an efficient sensor for the rapid detection of CN¯ in aqueous media.
References
Xu Z, Kim SK, Yoon J (2010) Revisit to imidazolium receptors for the recognition of anions: highlighted research during 2006-2009. Chem Soc Rev 39:1457–1510
Kim SK, Kim HN, Xiaoru Z, Lee HN, Lee HN, Soh JH, Swamy KMK, Yoon J (2007) Recent development of anion selective fluorescent chemosensors. Supramol Chem 19:221–227
Zhang X, Yin J, Yoon J (2014) Recent advances in development of chiral fluorescent and colorimetric sensors. Chem Rev 114:4918–4959
Yoon JY, Kim SK, Singh NJ, Kim KS (2006) Imidazolium receptors for the recognition of anions. Chem Soc Rev 35:355–356
Kim SK, Kang BG, Koh HS, Yoon YJ, Jung SJ, Jeong B, Lee KD, Yoon J (2004) A new imidazolium cavitand for the recognition of dicarboxylates. Org Lett 6:4655–4658
Kim SK, Singh NJ, Kwon J, Hwang IC, Park SJ, Kim KS, Yoon J (2006) Fluorescent imidazolium receptors for the recognition of pyrophosphate. Tetrahedron 62:6065–6072
Song NR, Moon JH, Jun EJ, Choi J, Kim Y, Kim SJ, Lee JY, Yoon J (2013) Cyclic benzobisimidazolium derivative for the selective fluorescent recognition of HSO4 − in aqueous solution via C-H hydrogen bondings. Chem Sci 4:1765–1771
Kellin D (1929) Cytochrome and respiratory enzymes. Proc R Soc Lond B Biol Sci 104:206–252
Huang XH, Gu XG, Zhang GX, Zhang DQ (2012) A highly selective fluorescence turn-on detection of cyanide based on the aggregation of tetraphenylethylene molecules induced by chemical reaction. Chem Commun 48:12195–12197
Baskin SI, Brewer TG, Sidell F, Takafuji ET, Franz DR (1997) Medical aspects of chemical and biological warfare. TMM publications, Washington, pp 271–286
Sharma VK, Burnett CR, Yngard RA, Cabelli DE (2005) Iron (VI) and iron(V) oxidation of copper(I) cyanide. Environ Sci Technol 39:3849–3854
Ullmann’s Encyclopedia of Industrial Chemistry 1 edition (1999) Wiley-VCH, New York
Christison TT, Rohrer JS (2007) Direct determination of free cyanide in drinking water by ion chromatography with pulsed amperometric detection. J Chromatogr A 1155:31–39
Themelis DG, Karastogianni SC, Tzanavaras PD (2009) Selective determination of cyanides by gas diffusion-stopped flow-sequential injection analysis and an on-line standard addition approach. Anal Chim Acta 632:93–100
Surleva AR, Neshkova MT (2008) A new generation of cyanide ion-selective membranes for flow injection application: part III. A simple approach to the determination of toxic metal–cyanide complexes without preliminary separation. Talanta 76:914–921
Taheri A, Noroozifar M, Motlagh MK (2009) Investigation of a new electrochemical cyanide sensor based on Ag nanoparticles embedded in a three-dimensional sol-gel. J Electroanal Chem 628:48–54
Abbaspour A, Asadi M, Ghaffarinejad A, Safaei EA (2005) Selective modified carbon paste electrode for determination of cyanide using tetra-3,4-pyridinoporphyrazinatocobalt(II). Talanta 66:931–936
Lindsay AE, Hare DO (2006) The development of an electrochemical sensor for the determination of cyanide in physiological solutions. Anal Chim Acta 558:158–163
Timofeyenko YG, Rosentreter JJ, Mayo S (2007) Piezoelectric quartz crystal microbalance sensor for trace aqueous cyanide ion determination. Anal Chem 79:251–255
Park GJ, Hwang IH, Song EJ, Kim H, Kim C (2014) A colorimetric and fluorescent sensor for sequential detection of copper ion and cyanide. Tetrahedron 70:2822–2828
Shahid M, Razi SS, Srivastava P, Ali R, Maiti B, Misra A (2012) A useful scaffold based on acenaphthene exhibiting Cu2+ induced excimer fluorescence and sensing cyanide via Cu2+displacement approach. Tetrahedron 68:9076–9084
Jung HS, Han JH, Kim ZH, Kang C, Kim JS (2011) Coumarin-Cu(II) ensemble-based cyanide sensing Chemodosimeter. Org Lett 13:5056–50590
Saha S, Ghosh A, Mahato P, Mishra S, Mishra SK, Suresh E, Das S, Das A (2010) Specific recognition and sensing of CN¯ in sodium cyanide solution. Org Lett 12:3406–3409
Gimeno N, Li X, Durrant JR, Vilar R (2008) Cyanide sensing with organic dyes: studies in solution and on nanostructured Al2O3 surfaces. Chem Eur J 14:3006–3012
Kim S, Noh JY, Park SJ, Na YJ, Hwang IH, Min J, Kim C, Kim J (2014) Selective fluorescence assay of aluminum and cyanide ions using chemosensor containing naphthol. RSC Adv 4:18094–18099
Zhang C, Liu C, Li B, Chen J, Zhang H, Hu Z et al (2015) A new fluorescent turn-onchemodosimeter for cyanide based on dual reversible and irreversible deprotonation of NH and CH groups. New J Chem 39:1968–1973
Mouradzadegun A, Abadast F (2014) An improved organic/inorganic solid receptor for colorimetric cyanide-chemosensing in water: towards new mechanism aspects, simplistic use and portability. Chem Commun 50:15983–15986
Jayasudha P, Manivannan R, Elango KP (2015) Simple colorimetric chemodosimeters for selective sensing of cyanide ion in aqueous solution via termination of ICT transition by Michael addition. Sensors Actuators B Chem 221:1441–1448
Niamnonta N, Khumsrib A, Promchatb A, Tumcharernc G, Sukwattanasinittb M (2014) Novel salicylaldehyde derivatives as fluorescence turn-on sensors for cyanide ion. J Hazard Mater 280:458–463
Lin YD, Peng YS, Su W, Tu CH, Sun CH, Chow TJ (2012) Tetrahedron 68:2523–2526
Sun Y, Liu Y, Chen M, Guo W (2009) A novel fluorescent and chromogenic probe for cyanide detection in water based on the nucleophilic addition of cyanide to imine group. Talanta 80:996–1000
Zhang C, Ji K, Wang X, Wu H, Liu C (2015) A reversible and selective chemosensor based on intramolecular NH. . .NH(2) hydrogen bonding for cyanide and pH detection. Chem Commun 51:8173–8176
Gong WT, Zhang QL, Shang L, Gao B, Ning GL (2013) A new principle for selective sensing cyanide anions based on 2-hydroxy-naphthaldeazinecompound. Sens Actuators B Chem 177:322–326
You GR, Park GJ, Lee SA, Choi YW, Kim YS, Lee JJ, Kim C (2014) A single chemosensor for multiple target anions: the simultaneous detection of CN¯ and OAc¯ in aqueous media. Sensors Actuators B Chem 202:645–655
Miyaji H, Sessler JL (2001) Off-the-shelf colorimetric anion sensors. Angew Chem 40:154–157
Na SY, Kim JY, Kim HJ (2013) Colorimetric and fluorometric probe for the highly selective and sensitive detection of cyanide based on coumarinyl oxime. Sensors Actuators B Chem 188:1043–1047
Jia J, Xue P, Zhang Y, Xu Q, Zhang G, Huang T, Zhang H, Lu R (2014) Fluorescent sensor based on dimesityl boryl thiophene derivative for probing fluoride and cyanide. Tetrahedron 70:5499–5504
Ding Y, Li T, Zhu W, Xie Y (2012) Highly selective colorimetric sensing of cyanide based on formation of dipyrrin adducts. Org Biomol Chem 10:4201–4207
Anzenbacher P, Tyson DS, Jursikova K, Castellano FN (2002) Luminescence lifetime-based sensor for cyanide and related anions. J Am Chem Soc 124:6232–6232
Zareh Jonaghani M, Zali-Boeini H, Taheri R, Amiri Rudbari H, Askari B (2016) Naphthothiazole-based highly selective and sensitive fluorescent and colorimetric chemosensor for detection of pollutant metal ions. RSC Adv 6:34940–34945
Acknowledgements
We are grateful to the University of Isfahan research council for partial financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zali-Boeini, H., Zareh Jonaghani, M. New Fluorescent and Colorimetric Chemosensor for Detection of Cyanide with High Selectivity and Sensitivity in Aqueous Media. J Fluoresc 27, 1035–1040 (2017). https://doi.org/10.1007/s10895-017-2037-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2037-y