Abstract
L-Ascorbic acid, α-tocopherol, procyanidin B3, β-carotene and astaxanthin are five classic dietary antioxidants. In this study, the interaction between the five antioxidants and ovalbumin was investigated by fluorescence spectroscopy, in combination with UV-vis absorption spectroscopy and circular dichroism (CD) spectroscopy. The quenching mechanism of ovalbumin by α-tocopherol is static quenching and the interaction between α-tocopherol and ovalbumin is synergistically driven by enthalpy and entropy. Electrostatic interactions and hydrophobic interactions play a major role in stabilizing the complex. For the other four antioxidants, the quenching mechanisms are all static quenching mechanisms at lower concentrations of antioxidants, but at higher concentrations of antioxidants, predominantly by the “sphere of action” quenching mechanisms. The binding processes of the other four antioxidants to ovalbumin are all entropy process and the major part of the action force is hydrophobic interactions. The binding constants of ovalbumin with the five antioxidants are in the following order as: astaxanthin > β-carotene > L-ascorbic acid > procyanidin B3 > α-tocopherol at 298 K. Synchronous fluorescence spectroscopy shows the interaction between L-ascorbic acid/β-carotene/astaxanthin and ovalbumin decreases the hydrophobicity of the microenvironment of tryptophan (Trp) and tyrosine (Tyr) residues. The hydrophobicity of Trp is increased while the hydrophility of Tyr is increased in the presence of α-tocopherol. However, the microenvironment of Trp and Tyr is not affected by procyanidin B3. The UV-vis absorption and CD spectra suggest that the interaction between the five antioxidants and ovalbumin leads to the loosening and unfolding of ovalbumin skeleton and exerts some influence on the natural secondary structure of ovalbumin. The study provides an accurate and full basic data for clarifying the binding mechanisms of L-ascorbic acid, α-tocopherol, procyanidin B3, β-carotene and astaxanthin interacting with ovalbumin and is helpful for understanding rational use of antioxidants as dietary supplements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
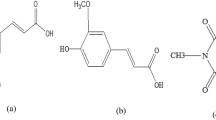
Antioxidants have attracted considerable attention from multiple areas of food chemistry or toxicology. In addition, growing concern has been expressed over the potentially mutagenic and carcinogenic effects of synthetic antioxidants from available literature [1]. L-Ascorbic acid, α-tocopherol, procyanidin B3, β-carotene and astaxanthin are five classic dietary antioxidants. L-Ascorbic acid (molecular structure: inset of Fig. 1a) is an essential component in the diet of humans, and also is a typical long used pharmaceutical agent [2]. α-Tocopherol (molecular structure: inset of Fig. 1b), the most biologically active form of vitamin E, has long been recognized as the most active naturally occurring, lipid-soluble chain-breaking antioxidant [3]. Proanthocyanidins are secondary metabolites of plants that are abundant in fruits and vegetable-based beverages like juices, beer, and wine [4]. One of the most widely studied proanthocyanidins is procyanidin B3 (catechin-(4β → 8)-catechin; molecular structure: inset of Fig. 1c) due to its high abundance in the human diet and relevant antioxidant activity [5]. β-Carotene (molecular structure: inset of Fig. 1d) consists of a polyene system with 11 conjugated double bonds and a β-ring at each end of the chain [6]. It has antioxidant activity and may consequently offer protection against cancer and cardiovascular diseases [7]. Astaxanthin (molecular structure: inset of Fig. 1e) is a carotenoid with great commercial potential in the pharmaceutical and food industries. The presence of the hydroxyl (OH) and keto (C = O) moieties on each ionone ring explains some of its unique features, namely, the ability to be esterified and a higher antioxidant activity and a more polar nature than other carotenoids [8]. People generally think that dietary antioxidants have little side effects. Therefore, the five antioxidans are widely used as food supplements.
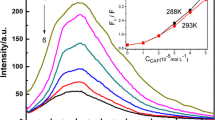
Emission spectra of ovalbumin in the presence of different concentrations of L-ascorbic acid (a), α-tocopherol (b), procyanidin B3 (c), β-carotene (d) and astaxanthin (e) at 298 K, pH 7.40 and λ ex = 280 nm. The inset corresponds to the molecular structure of L-ascorbic acid (a), α-tocopherol (b), procyanidin B3 (c), β-carotene (d) and astaxanthin (e), respectively
Egg white proteins (EWP) are extensively used in food industry due to their functional (foaming, gelling, emulsifying) and nutritional properties [9]. The main protein of egg white is ovalbumin (OVA) which represents 54 % of EWP [10]. Therefore, ovalbumin is widely used in the food industry and is also present in many biological systems [11]. It is a phosphorylated and glycosylated globular protein with a diameter 5.5 nm [12], an isoelectric point (pI) ~4.9 [13] and a molecular weight ~ 45 kDa [14]. This protein is composed by a single polypeptide chain of 385 amino acid, of which a half are hydrophobic and mainly buried into the protein structure, and a third are charged mainly located on protein surface in contact with aqueous medium [15, 16]. These amino acid residues fold into a globular conformation with a high secondary structure content (30.6 % α-helix and 31.4 % β-strand), and a flexible loop-helix-loop motif reactive center [17, 18]. Ovalbumin has one disulphide bond and four free sulfhydryl groups [19]. Furthermore, ovalbumin contains three tryptophan residues: Trp-148 in helix F, Trp-267 in helix H, and Trp-184 as the nearest neighbor residue of the carboxyl terminus of strand 3 A [18, 20, 21]. It has been demonstrated that ovalbumin has the ability to interact with many compounds, such as caffeine, theophylline, diprophylline [22], stearic acid [23], epigallo-catechin 3-gallate [24], gum arabic [25], tea polyphenol [26], quercetin [27], ruthenium(II)-bipyridine-tert-butylcalix [4]arene complexes [28], oxovanadium(IV)-salen complexes [29], etc. However, to our knowledge, an accurate and full basic data for clarifying the binding mechanisms of the five antioxidants to ovalbumin were not reported in the literature.
Knowledge of interaction mechanism between antioxidant and ovalbumin is very important. First, ovalbumin can improve water solubility of hydrophobic compound such as α-tocopherol, β-carotene and astaxanthin allowing their incorporation in food systems, such as certain beverages [9]. Second, dietary antioxidant is considered to be a safe natural product. However, it may act as an antinutritional factor when ingested in excess. To minimize the antinutritional effects and make full use of antioxidant, knowledge of the interaction between antioxidant and ovalbumin is desirable. Third, the conformational changes of ovalbumin induced by its interaction with antioxidant may reduce the activity of ovalbumin. Ovalbumin represents the major allergen from avian egg white that causes IgE-mediated food allergic reactions particularly in children. The prevalence of ovalbumin allergy is estimated between 1.6 % and 3.2 % among children and 0.6 % among adults [30]. Ovalbumin conformation, digestibility and aggregation are important for biological activities of dietary proteins that elicit hypersensitivity reactions in humans [24].
In the present work, a comprehensive investigation was performed for the binding properties of the five antioxidants to ovalbumin under the physiological conditions. Using different spectroscopic methods, the binding information, including quenching mechanisms, binding parameters, thermodynamic parameters, binding modes and the natural secondary structure changes of ovalbumin was investigated.
Materials and Methods
Materials
Ovalbumin, L-ascorbic acid, α-tocopherol (density: 0.950 g mL−1 at 20 °C), procyanidin B3, β-carotene and astaxanthin were purchased from Sigma-Aldrich Chemical Company (St. Louis, USA). Ovalbumin was dissolved in a phosphate buffer solution of pH 7.40 (0.01 mol L−1 PBS), and it was stored at 0–4 °C in the dark. L-Ascorbic acid and procyanidin B3 were directly dissolved in phosphate buffer solution of pH 7.40 (0.01 mol L−1 PBS), and α-tocopherol was diluted with 99.5 % (v/v) ethanol before use. β-Carotene and astaxanthin were dissolved in 99.5 % acetone and then diluted with phosphate buffer solution of pH 7.40 (0.01 mol L−1PBS). The stock solutions of the five antioxidants were prepared and used immediately because of oxidation under light and air. Double distilled water was used to prepare solutions. The pH was determined using a PHS-2C pH-meter (Shanghai DaPu Instruments Co., Ltd., Shanghai, China) at ambient temperature. Sample masses were weighed accurately on a microbalance (Sartorius, BP211D) with a resolution of 0.01 mg. All other reagents were all of analytical reagent grade and were used as purchased without further purification.
Fluorescence Measurements
The fluorescence measurements were performed on Cary Eclipse fluorescence spectrophotometer (VARIAN, USA) equipped with a 1.0 cm quartz cell holder and a thermostat bath. Certain volume of the stock solution of ovalbumin and various volumes of the stock solution of antioxidants were transferred to 0.01 L volumetric flasks. The mixture was diluted to the experimental concentrations with phosphate buffer solution of pH 7.40 as the working solutions. Ovalbumin concentration was kept at 5 × 10−5 mol L−1. The excitation and emission slit widths were fixed at 5 nm. The excitation wavelength was set at 280 nm (where both Trp and Tyr are excited) and 295 nm (where only Trp is excited), and the emission spectra were read at 300–450 nm at a scan rate of 100 nm min−1. The experiment was measured at four temperatures (293, 298, 303 and 310 K) with recycle water keeping the temperature constant. The synchronous fluorescence spectra were scanned from 280 to 330 nm (Δλ = 15 nm) and from 310 to 400 nm (Δλ = 60 nm), respectively.
The fluorescence measurements are hindered by the inner-filter effect, which is that small ligands absorb the light at the excitation and emission wavelengths of proteins and leads to unreliable results. Thus it is very important to subtract such an effect from the raw quenching data. The extent of this effect can be roughly estimated with the following equation [31]:
where F cor and F obsd are the corrected and observed fluorescence intensities, respectively, whereas A ex and A em are the sum of the absorbance of protein and ligand at the excitation and emission wavelengths, respectively. The fluorescence intensity utilized in this study is the corrected intensity.
Absorbance Measurements
UV-vis absorption spectra were recorded with a TU-1810 spectrophotometer (Puxi Analytic Instrument Ltd., Beijing, China) equipped with 1.0 cm quartz cells at 298 K. Ovalbumin concentration was kept at 5 × 10−6 mol L−1. Buffer (control) and samples were placed in the reference and sample cuvettes, respectively. The UV-vis absorption spectra of ovalbumin in the absence and presence of antioxidants were obtained by utilizing the mixture of antioxidants and phosphate buffer at the same concentration as the reference solution.
Circular Dichroism (CD) Measurements
The CD measurements were carried out on a Jasco J-715 spectropolarimeter under constant nitrogen flush. For measurements in the far-UV region, a quartz cell with a path length of 0.2 cm was used. Three scans were accumulated with continuous scan mode and a scan speed of 200 nm min−1 with data being collected at 0.2 nm and response time of 2 s. The sample temperature was maintained at 298 K. Ovalbumin concentration was fixed to 4 × 10−6 mol L−1 and the five antioxidants concentration used was 4 × 10−5 mol L−1. Corresponding blanks (without ovalbumin) were recorded and subtracted from the sample spectra, and results were taken as CD ellipticity in mdeg.
Results and Discussion
Effect of Antioxidants on Ovalbumin Fluorescence
Figure 1 shows the fluorescence emission spectra obtained for ovalbumin at pH 7.40 with the addition of the five antioxidants at λ ex = 280 nm. In all cases, the fluorescence intensity of ovalbumin decreases regularly with the increasing concentrations of antioxidants, indicating that the five antioxidants can bind to ovalbumin. Furthermore, a red shift is observed with increasing L-ascorbic acid/β-carotene/astaxanthin concentration, which suggests that the fluorophore of ovalbumin is placed in a more hydrophilic environment after the addition of L-ascorbic acid/β-carotene/astaxanthin [32]. The change in the emission peak of ovalbumin has a blue shift for α-tocopherol. The blue shift suggests a reduction in the polarity of the microenvironment around fluorophore [26]. Upon the gradual addition of procyanidin B3, the emission intensity of ovalbumin decreases without any obvious shift in the maximum emission wavelength. The result suggests that binding of procyanidin B3 to ovalbumin has little effect on the microenvironment around fluorophore.
Upon excitation at 280 nm, both Trp and Tyr are readily excited, but most of the fluorescence comes from Trp because of the efficient resonance energy transfer (RET) from Tyr to Trp, while at an excitation wavelength of 295 nm, only Trp emits fluorescence [33]. To determine whether both Trp and Tyr residues are involved in the interaction with an antioxidant molecule, the fluorescence of ovalbumin excited at 295 nm in the presence of the five antioxidants was also measured. The plots of F/F 0 against the concentration of antioxidants are shown in Fig. 2, where F 0 and F represent the fluorescence intensities before and after the addition of the antioxidants, respectively. For L-ascorbic acid, procyanidin B3, β-carotene and astaxanthin, significant difference is observed from the quenching of ovalbumin fluorescence after excitation at these two wavelengths. The results demonstrate that Trp and Tyr residues are both implicated in the fluorescence quenching. For α-tocopherol, no significant difference is observed at these two wavelengths. This demonstrates that only Trp is implicated in the fluorescence quenching and that Tyr residues do not participate in the molecular interaction between α-tocopherol and ovalbumin.
Fluorescence quenching of ovalbumin (5 × 10−5 mol L−1) at 298 K and pH 7.40, plotted as extinction of ovalbumin intrinsic fluorescence (F/F 0) against antioxidants concentration for L-ascorbic acid (a), α-tocopherol (b), procyanidin B3 (c), β-carotene (d) and astaxanthin (e). The fluorescence emission intensity was recorded at λ ex = 280 nm and 295 nm
Fluorescence Quenching Mechanisms
The different mechanisms of quenching are usually classified as either dynamic (a collisional process) or static (the formation of a complex between quencher and fluorophore). For fluorescence quenching, the decrease in intensity is usually described by the Stern-Volmer equation [34]:
where F 0 and F represent the steady-state fluorescence intensities at λ ex = 280 nm in the absence and presence of the antioxidants, respectively. k q is the bimolecular quenching constant, τ0 is the lifetime of the fluorescence in absence of the antioxidants. The lifetime of ovalbumin was used as 10 ns according to the reference [24]. [Q] is the concentration of the antioxidants, and K SV is the Stern-Volmer quenching constant.
Figure 3 shows the Stern-Volmer plots for the ovalbumin fluorescence quenching by L-ascorbic acid, α-tocopherol, procyanidin B3, β-carotene and astaxanthin. A linear Stern-Volmer plot means that only one type of quenching mechanism occurs (dynamic or static). In the present case, a linear Stern-Volmer plot is observed for α-tocopherol (Fig. 3b), which means that only one type of quenching mechanism occurs (dynamic or static). The value of K SV obtained from the slope of the plot is listed in Table 1. The value of k q for α-tocopherol is greater than the limiting diffusion rate constant of the biomolecule (2 × 1010 L mol−1 s−1) [35], which suggests that the quenching mechanism of ovalbumin by α-tocopherol is not initiated by dynamic quenching but by static quenching. For L-ascorbic acid, procyanidin B3, β-carotene and astaxanthin, the Stern–Volmer plots are linear at lower quencher concentrations, while the plots exhibit an upward curvature, concave toward the y axis at high quencher concentrations. The results show that the quenching type is probably single quenching at lower antioxidants concentration. Therefore, to provide a semiempirical measure of the magnitude of the quenching in the four antioxidant-ovalbumin systems [36], we discussed the quenching in terms of K SV values computed by linear fits of the Stern-Volmer plots at low quencher concentrations where the plots are nearly linear. The values of K SV obtained from the slope of these plots are also listed in Table 1. From the results listed in Table 1, the values of k q for the four antioxidants are all larger than 2.0 × 1010 L mol−1 s−1, the maximum diffusion collision quenching rate constant of various quenchers with the biopolymer [35], which illustrates that the specific interactions between ovalbumin and the four antioxidants have occurred and their fluorescence quenching mechanisms are all mainly arisen from static quenching by complex formation in the linear range [37].
The positive deviation in the Stern-Volmer plots at high quencher concentrations can arise from a variety of processes, including combined static and dynamic quenching and the “sphere of action” model. Therefore, the upward-curving Stern-Volmer plots were at the beginning analyzed in terms of a combined static and dynamic quenching. Thus, the fluorescence data were analyzed by a modified Stern-Volmer equation [38]:
where [Q] is the concentration of the antioxidants, K S and K D are static and dynamic Stern-Volmer constants, respectively. Eq. 3 predicts that the plot of ((F 0/F)-1)/[Q] versus [Q] should be linear with an intercept of K D + K S and a slope of K D K S. The static and dynamic quenching constants can be obtained from the solutions of the quadratic equation. However, for the data plotted in Figure S1, (F 0/F-1)/[Q] versus [Q] is found to be highly nonlinear. The nonlinear nature of the plots indicates the involvement of other factors apart from static quenching and dynamic quenching. To explain the nonlinearity of the curve, these systems were treated in terms of the “sphere of action” model. In this situation, quenching occurs due to the quencher being adjacent to the fluorophore at the moment of excitation. These closely spaced fluorophore-quencher pairs are immediately quenched, but fluorophore and quencher do not actually form a ground-state complex. The modified form of the Stern-Volmer equation which describes this situation [39]:
in this equation, K app is the apparent static quenching constant. In the present case, the experimental results exhibit a very good linear relationship between ln (F 0/F) and [Q] (Figure S2). The results suggest that the quenching mechanisms of ovalbumin by L-ascorbic acid, procyanidin B3, β-carotene and astaxanthin are all static quenching at lower concentrations of antioxidants, but at higher concentrations of antioxidants, predominantly by the “sphere of action” quenching mechanisms.
Binding Parameters
When small molecules bind independently to a set of equivalent sites on a biomacromolecule, the binding constant (K a) and the number of binding sites (n) can be derived from the Eq. 5:
where F 0, F and [Q] are the same as in Eq. 2, K a is the binding constant and n is the number of binding sites per ovalbumin molecule. Figure 4 shows the plots of log (F 0-F)/F versus log [Q] for the five antioxidant-ovalbumin systems at different temperatures. The calculated binding constants (K a) and the number of binding sites (n) are presented in Table 2. For the five antioxidant-ovalbumin systems, the values of n approximately equals to 1, indicating that almost one molecular of antioxidant binds to one molecular of ovalbumin. The values of K a for α-tocopherol decreased with increasing temperature, indicating that the interaction between α-tocopherol and ovalbumin is getting weak when the temperature rose, resulting in the reduction of the stability of α-tocopherol-ovalbumin complex, and the binding reaction between α-tocopherol and ovalbumin is exothermic. The increasing temperature does not result in a reduction of the stability of the L-ascorbic acid/procyanidin B3/β-carotene/astaxanthin-ovalbumin complex, which may indicate forming four stable complexes and the bindings are all endothermic reactions. These results are validated by the enthalpy change, as shown in Table 3. The binding constants of ovalbumin with the five antioxidants are in the following order as: astaxanthin > β-carotene > L-ascorbic acid > procyanidin B3 > α-tocopherol at 298 K. The K a between ovalbumin and α-tocopherol is in the range of 103 L mol−1, which is similar to the binding affinity of tea polyphenol to ovalbumin at pH 7.50 [26]. The values of K a between ovalbumin and L-ascorbic acid/procyanidin B3 is range from 104 to 105 L mol−1 and is comparable with the purine alkaloids (caffeine, theophylline and diprophylline), epigallo-catechin 3-gallate and quercetin (104–105 L mol−1) with ovalbumin [22, 24, 27]. For β-carotene/astaxanthin-ovalbumin system, from these binding constant values (105–107 L mol−1), it is clear that the binding of β-carotene and astaxanthin with ovalbumin is very strong and comparable with ruthenium(II)-bipyridine-tert-butylcalix [4]arene complexes (105 L mol−1) and oxovanadium(IV)-salen complexes (104–107 L mol−1) with ovalbumin [28, 29]. In addition, astaxanthin binds ovalbumin in a more firmly way than the other four antioxidants.
The plots of log (F 0-F)/F vs. log [Q] for the L-ascorbic acid-ovalbumin system (a), α-tocopherol-ovalbumin system (b), procyanidin B3-ovalbumin system (c), β-carotene-ovalbumin system (d) and astaxanthin-ovalbumin system (e) at four different temperatures and pH 7.40. The fluorescence emission intensity was recorded at λ ex = 280 nm
Thermodynamic Parameters and Binding Mode
In general, the essence of most protein–ligand recognition is non-covalent interactions, such as electrostatic interactions, multiple hydrogen bonds, van der Waals interactions, hydrophobic effect, etc. Intermolecular forces can be explained by calculating the thermodynamic parameters (free energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS)) of the binding reaction, which can be obtained based on the binding constants K a (Table 2) of different temperatures (293, 298, 303, and 310 K) using the van’t Hoff equation (Eq. 6) and Gibbs-Helmhotz equation (Eq. 7):
where K a is analogous to the binding constant at 293, 298, 303, and 310 K, and R is the gas constant. The enthalpy change (ΔH) and entropy change (ΔS) can be calculated from the slope and intercept of the linear van’t Hoff plot (Figure S3a) of lnK a versus 1/T based on Eq. 6. The free energy change (ΔG) is then estimated from Eq. 7. Figure S3b illustrates the values of the thermodynamic parameters for the interaction of the five antioxidants with ovalbumin at 298 K, while Table 3 shows these values at each studied temperature. The negative values of free energy (ΔG), as shown in Figure S3b and Table 3, support the assertion that the binding processes are all spontaneous. Ross and Subramanian have characterized the sign and magnitude of the thermodynamic parameter associated with various individual kinds of interaction which may take place in protein association process. If ΔH < 0, ΔS < 0, the main forces are van der Waals and hydrogen bond interactions; if ΔH < 0, ΔS > 0, electrostatic effect is dominant; if ΔH > 0, ΔS > 0, hydrophobic interactions play the main roles in the binding reaction [40]. So the results indicate that electrostatic force is the main binding force to stabilize the complex of α-tocopherol-ovalbumin. In addition, the positive entropy (ΔS) value is frequently taken as a typical evidence for hydrophobic interaction. Hence, the main intermolecular interactions may be electrostatic force and hydrophobic interactions in the binding process of α-tocopherol to ovalbumin. For L-ascorbic acid/procyanidin B3/β-carotene/astaxanthin-ovalbumin system, the positive values obtained for both ΔH and ΔS indicate that hydrophobic interactions is the major binding force. But besides that, in all cases, static interaction is along with covalent binding.
By the Eq. 7, the change of Gibbs free energy (ΔG) is the comprehensive embodiment of the changes of enthalpy (ΔH) and entropy (ΔS). The interaction for α-tocopherol and ovalbumin is synergistically driven by enthalpy and entropy, while the binding processes of L-ascorbic acid, procyanidin B3, β-carotene and astaxanthin to ovalbumin are all entropy process.
Investigation of Ovalbumin Conformation Changes
To explore the effect of the five antioxidants on the conformation changes of ovalbumin, synchronous fluorescence measurements, UV-Vis absorption and CD spectra were performed.
Synchronous fluorescence spectra can supply characteristic information about the molecular environment in the vicinity of fluorophore, such as Trp or Tyr [41]. The spectrum is obtained through the simultaneous scanning of the excitation and emission monochromators while maintaining a constant wavelength interval between them. When the wavelength intervals (Δλ) are stabilized at 15 or 60 nm, the synchronous fluorescence gives the characteristic information of Tyr or Trp, respectively [42, 43]. The effects of the five antioxidants on the synchronous fluorescence spectra of ovalbumin are shown in Fig. 5. The red shifts of the maximum emission wavelength of Trp and Tyr residues (Fig. 5a and b, Fig. 5g and h, Fig. 5i and j) suggest that the interaction of ovalbumin with L-ascorbic acid/β-carotene/astaxanthin results in a more polar environment for Trp and Tyr residues, the hydrophobicity is decreased [22]. With adding of α-tocopherol, the maximum emission wavelength of Trp is observed to have a blue shift along with a red shift of Tyr (Fig. 5c and d). This suggests that the hydrophobicity of Trp is increased while the hydrophility of Tyr is increased. For procyanidin B3 (Fig. 5e and f), the maximum emission wavelengths of Tyr and Trp residues are almost unchanged during the interaction, suggesting that the polarity around these residues is retained. In addition, there is a new emission at around 330 nm of α-tocopherol-ovalbumin system and procyanidin B3-ovalbumin system at the Δλ = 60 nm, respectively. The occurrence of this new band on the interaction of α-tocopherol/procyanidin B3 with ovalbumin is probably due to the efficient energy transfer from ovalbumin to α-tocopherol/procyanidin B3 [44].
Synchronous fluorescence spectra of ovalbumin in the presence of different concentrations of L-ascorbic acid (Δλ = 60 nm (a) and Δλ = 15 nm (b)), α-tocopherol (Δλ = 60 nm (c) and Δλ = 15 nm (d)), procyanidin B3 (Δλ = 60 nm (e) and Δλ = 15 nm (f)), β-carotene (Δλ = 60 nm (g) and Δλ = 15 nm (h)) and astaxanthin (Δλ = 60 nm (i) and Δλ = 15 nm (j)) at 298 K and pH 7.40
From Figure S4a, S4d and S4e, we can see that the curves of Δλ = 60 nm are all lower than the curves of Δλ = 15 nm, which leads to the conclusion that Trp plays an important role during fluorescence quenching of ovalbumin by L-ascorbic acid, β-carotene and astaxanthin. Possibly, during interactions L-ascorbic acid, β-carotene and astaxanthin are situated closer to Trp residues compared to Tyr residues [44]. For α-tocopherol and procyanidin B3 (Figure S4b and S4c), No significant difference is observed at Δλ = 60 nm and Δλ = 15 nm. Combining Fig. 2b and c, Conclusion can be drawn that only Trp is implicated in the fluorescence quenching for α-tocopherol, and the binding site of procyanidin B3 on ovalbumin is almost the same distance to Trp and Tyr residues.
UV-vis absorption technique can be used to explore the structural changes of protein. The UV-vis absorption spectra of ovalbumin in the absence and presence of antioxidants are shown in Fig. 6. Ovalbumin has two absorption peaks, the strong absorption peak at about 220 nm reflects the framework conformation of the protein, the weak absorption peak at about 280 nm appears to be due to the aromatic amino acids (Trp, Tyr, and Phe) [45]. With adding of L-ascorbic acid/α-tocopherol to ovalbumin solution, the intensity of the peak at 220 nm decreases and no obviously shift. The subtle variation might arise from the disturbance of the microenvironment around the polypeptide caused by the binding of L-ascorbic acid/α-tocopherol with ovalbumin. For L-ascorbic acid, the intensity of the peak at 280 nm increases (hyperchromic effect) obviously and has blue shift. The result indicates that the interaction between L-ascorbic acid and ovalbumin decreases the hydrophobicity of the microenvironment of the aromatic amino acid residues. With adding of procyanidin B/β-carotene/astaxanthin, the intensity peak of ovalbumin at 220 nm decreases with a red shift and the intensity of the peak at 280 nm has minimal changes. The results can be explained that the interaction between procyanidin B/β-carotene/astaxanthin and ovalbumin leads to the loosening and unfolding of the protein skeleton.
Circular dichroism (CD) is usually employed to monitor the conformational changes of protein because of its accuracy and sensitivity. The CD spectra of ovalbumin in the absence and presence of the five antioxidants are shown in Fig. 7. Free ovalbumin has a high percentage of α-helix structure which shows characteristic strong double minima signals with two negative bands in the UV range at 208 and 220 nm (Fig. 7). Both bands are due to n-π* transition of the carbonyl group of peptide [29]. The binding of the five antioxidants to ovalbumin causes only a decrease in negative ellipticity at all wavelengths of the far-UV CD without any significant shift of the peaks, which clearly indicates the changes in the protein secondary structure, and a decrease of the α-helix content in protein [29]. The percentage of α-helix can be calculated using the following equation [29]:
where MRE208 is the observed mean residue ellipticity (MRE) value at 208 nm, 4000 is the MRE of the β-form and random coil conformation cross at 208 nm, and 33,000 is the MRE value of the pure α-helix at 208 nm.
where Cp is the molar concentration of ovalbumin, n the number of amino acid residues (385 for ovalbumin) and l is the path length (0.2 cm). The α-helix content of protein was calculated from Eqs.8 and 9. It can be calculated that the native ovalbumin solution has 33.62 % of α-helix, while α-helix content of ovalbumin decreases to 31.90 %, 32.23 %, 31.34 %, 29.36%and 28.97 % with the addition of L-ascorbic acid, α-tocopherol, procyanidin B3, β-carotene and astaxanthin, respectively. These results indicate that the addition of the five antioxidants induces the changes of the natural secondary structure of ovalbumin, which may lead to changes of the physiological function of ovalbumin.
Conclusions
The binding mechanisms of L-ascorbic acid, α-tocopherol, procyanidin B3, β-carotene and astaxanthin interacting with ovalbumin were investigated by fluorescence spectroscopy, UV-vis absorption spectroscopy and circular dichroism (CD) spectroscopy. Experimental results suggest that the five antioxidants can bind to ovalbumin and quench the fluorescence of ovalbumin. The quenching mechanism of ovalbumin by α-tocopherol is static quenching. For L-ascorbic acid, procyanidin B3, β-carotene and astaxanthin, the quenching mechanisms are all static quenching mechanisms at lower concentrations of antioxidants, but at higher concentrations of antioxidants, predominantly by the “sphere of action” quenching mechanisms. The binding constants of ovalbumin with the five antioxidants are in the following order as: astaxanthin > β-carotene > L-ascorbic acid > procyanidin B3 > α-tocopherol at 298 K. The number of binding sites (n) approximately equals to 1, suggesting that one molecule of antioxidant combines with one molecule of ovalbumin. By evaluating the thermodynamic parameters, it is found that the interaction for α-tocopherol and ovalbumin is synergistically driven by enthalpy and entropy, and electrostatic interactions and hydrophobic interactions play a major role in the reaction. The binding processes of L-ascorbic acid, procyanidin B3, β-carotene and astaxanthin to ovalbumin are all entropy process and the major part of the action force is hydrophobic interactions. Synchronous fluorescence spectroscopy shows the interaction between L-ascorbic acid/β-carotene/astaxanthin and ovalbumin decreases the hydrophobicity of the microenvironment of Trp and Tyr residues. However, the hydrophobicity of Trp is increased while the hydrophility of Tyr is increased in the presence of α-tocopherol. The polarity and hydrophobicity around Trp and Tyr microenvironment is not affected by procyanidin B3. In addition, the interaction of α-tocopherol or procyanidin B3 with ovalbumin has the efficient energy transfer from ovalbumin to α-tocopherol or procyanidin B3. The UV-vis absorption suggests that the interaction between the five antioxidants and ovalbumin leads to the loosening and unfolding of ovalbumin skeleton. The results of CD suggest that the five antioxidants indeed exert some influence on the natural secondary structure of ovalbumin and finally lead the reduction of the protein α-helix structure.
References
Wu D, Yan J, Tang P et al (2015) Binding properties and structure–affinity relationships of food antioxidant butylated hydroxyanisole and its metabolites with lysozyme. Food Chem 188:370–376
Lu XQ, Nan MN, Zhang HR et al (2007) Investigation of the antioxidant property of ascorbic acid. J Phys Chem C 111:14998–15002
Krumova K, Friedland S, Cosa G (2012) How lipid unsaturation, peroxyl radical partitioning, and chromanol lipophilic tail affect the antioxidant activity of α-tocopherol: direct visualization via high-throughput fluorescence studies conducted with fluorogenic α-tocopherol analogues. J Am Chem Soc 134:10102–10113
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073–2085
Gonçalves R, Mateus N, Freitas VD (2011) Influence of carbohydrates on the interaction of procyanidin B3 with trypsin. J Agric Food Chem 59:11794–11802
Britton G (1995) Structure and properties of carotenoids in relation to function. FASEB J 9:1551–1558
Knockaert G, Pulissery SK, Lemmens L et al (2012) Carrot β-carotene degradation and isomerization kinetics during thermal processing in the presence of oil. J Agric Food Chem 60:10312–10319
Hussein G, Sankawa U, Goto H et al (2006) Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod 69:443–449
Sponton OE, Perez AA, Carrara CR et al (2015) Linoleic acid binding properties of ovalbumin nanoparticles. Colloids Surf B 128:219–226
Weijers M, Sagis LMC, Veerman C et al (2002) Rheology and structure of ovalbumin gels at low pH and low ionic strength. Food Hydrocoll 16:269–276
Creamer LK, Jimenez-Flores R, Richardson T (1988) Genetic modification of food proteins. Trends Biotechnol 6:163–169
Venturoli D, Rippe B (2005) Ficoll and dextran vs. globular proteins as probes for testing glomerular permselectivity: effects of molecular size, shape, charge, and deformability. Am J Physiol Ren Physiol 288:F605–F613
Cannan RK, Kibrick A, Palmer AH (1941) The amphoteric properties of egg albumin. Ann N Y Acad Sci 41:243–266
Hu HY, Du HN (2000) Alpha to beta structural transformation of ovalbumin: heat and pH effects. J Protein Chem 19:177–183
Croguennec T, Renault A, Beaufils S et al (2007) Interfacial properties of heat-treated ovalbumin. J Colloid Interface Sci 315:627–636
Giosafatto CVL, Rigby NM, Wellner N et al (2012) Microbial transglutaminase-mediated modification of ovalbumin. Food Hydrocoll 26:261–267
Nisbet AD, Saundry RH, Moir AJG et al (1981) The complete amino-acid sequence of hen ovalbumin. Eur J Biochem 115:335–345
Stein PE, Leslie AG, Finch JT et al (1991) Crystal structure of uncleaved ovalbumin at 1.95 A resolution. J Mol Biol 221:941–959
Sponton OE, Perez AA, Carrara CR et al (2015) Impact of environment conditions on physicochemical characteristics of ovalbumin heat-induced nanoparticles and on their ability to bind PUFAs. Food Hydrocoll 48:165–173
Wright HT, Qian HX, Huber RJ (1990) Crystal structure of plakalbumin, a proteolytically nicked form of ovalbumin. Its relationship to the structure of cleaved alpha-1-proteinase inhibitor. J Mol Biol 213:513–528
Stein PE, Leslie AG, Finch JT et al (1990) Crystal structure of ovalbumin as a model for the reactive Centre of serpins. Nature 347:99–102
Wang RQ, Yin YJ, Li H et al (2013) Comparative study of the interactions between ovalbumin and three alkaloids by spectrofluorimetry. Mol Biol Rep 40:3409–3418
Kamilya T, Pal P, Talapatra GB (2007) Interaction and incorporation of ovalbumin with stearic acid monolayer: Langmuir–Blodgett film formation and deposition. Colloids Surf B 58:137–144
Ognjenović J, Stojadinović M, Milčić M et al (2014) Interactions of epigallo-catechin 3-gallate and ovalbumin, the major allergen of egg white. Food Chem 164:36–43
Niu F, Su Y, Liu Y et al (2014) Ovalbumin–gum arabic interactions: effect of pH, temperature, salt, biopolymers ratio and total concentration. Colloids Surf B 113:477–482
Shen F, Niu F, Li J et al (2014) Interactions between tea polyphenol and two kinds of typical egg white proteins-ovalbumin and lysozyme: effect on the gastrointestinal digestion of both proteins in vitro. Food Res Int 59:100–107
Lu Y, Wang YL, Gao SH et al (2009) Interaction of quercetin with ovalbumin: spectroscopic and molecular modeling studies. J Lumin 129:1048–1054
Mareeswaran PM, Maheshwaran D, Babu E et al (2012) Binding and fluorescence resonance energy transfer (FRET) of ruthenium(II)-bipyridine-calixarene system with proteins-experimental and docking studies. J Fluoresc 22:1345–1356
Mathavan A, Ramdass A, Rajagopal S (2015) A spectroscopy approach for the study of the interaction of oxovanadium(IV)-salen complexes with proteins. J Fluoresc 25:1141–1149
Sampson HA (2004) Update on food allergy. J Allergy Clin Immun 113:805–819
Sur SS, Rabbani LD, Libman L et al (1979) Fluorescence studies of native and modified neurophysins. Effects of peptides and pH. Biochemistry 18:1026–1036
Yuan T, Weljie AM, Vogel HJ (1998) Tryptophan fluorescence quenching by methionine and selenomethionine residues of calmodulin: orientation of peptide and protein binding. Biochemistry 37:3187–3195
Lakowicz JR (2006) Principles of fluorescence spectroscopy, third edn. Springer Science & Business Media, New York, p. 531
Lakowicz JR (2006) Principles of fluorescence spectroscopy, third edn. Springer Science & Business Media, New York, p. 278
Ware WR (1962) Oxygen quenching of fluorescence in solution: an experimental study of the diffusion process. J Phys Chem 66:455–458
Tan C, Atas E, Műller JG et al (2004) Amplified quenching of a conjugated polyelectrolyte by cyanine dyes. J Am Chem Soc 126:13685–13694
Lakowicz JR (2006) Principles of fluorescence spectroscopy, third edn. Springer Science & Business Media, New York, p. 281
Lakowicz JR (2006) Principles of fluorescence spectroscopy, third edn. Springer Science & Business Media, New York, p. 283
Soares S, Mateus N, Freitas VD (2007) Interaction of different polyphenols with bovine serum albumin (BSA) and human salivary a-amylase (HSA) by fluorescence quenching. J Agric Food Chem 55:6726–6735
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20:3096–3102
Vekshin NL (1996) Separation of the tyrosine and tryptophan components of fluorescence using synchronous scanning method. Biofizika 41:1176–1179
Brustein EA, Vedenkina NS, Ivkova MN (1973) Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol 18:263–279
Miller JN (1979) Recent advances in molecular luminescence analysis. Proc Anal Div Chem Soc 16:203–208
Mandal P, Ganguly T (2009) Fluorescence spectroscopic characterization of the interaction of human adult hemoglobin and two isatins, 1-methylisatin and 1-phenylisatin: a comparative study. J Phys Chem B 113:14904–14913
Glazer AN, Smith EL (1961) Studies on the ultraviolet different spectra of protein and polypeptides. J Biol Chem 236:2942–2947
Acknowledgments
This work was supported by the Key Research Project of Colleges and Universities of Henan Province (15 A150004), the Doctoral Startup Fund of Xinxiang Medical University (505078, 201509), the Foundation for Fostering of Xinxiang Medical University (2014QN122, 2014QN124) and the Fund of Fluorescence Probe and Biomedical Detection Research Team of Xinxiang City (CXTD16001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 634 kb)
Rights and permissions
About this article
Cite this article
Li, X., Yan, Y. Comparative Study of the Interactions between Ovalbumin and five Antioxidants by Spectroscopic Methods. J Fluoresc 27, 213–225 (2017). https://doi.org/10.1007/s10895-016-1948-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1948-3