Abstract
Four new Schiff base ligands carrying naphthalene groups were prepared from the reaction of 2,4-diamino-6-methyl-1,3,5-triazine and 2,4-diamino-6-undecyl-1,3,5-triazine with 2-hydroxy-1-naphthaldehyde. The influence of a series of metal ions including Cu2+, Co2+, Hg2+, Al3+, Cr3+, Fe3+, Pb2+, Ni2+, Cd2+, Zn2+, Mn2+, Ag+, Ba2+, Ca2+ and Mg2+ on the spectroscopic properties of the ligands was investigated by means of absorption and emission spectrometry. The results of spectrophotometric and spectrofluorimetric titrations disclosed the complexation stoichiometry and complex stability constant of the ligands with metal ions. A simple spectrofluorimetric method was developed using the Schiff base derived from 2,4-diamino-6-undecyl-1,3,5-triazine to determine Hg2+ ion. No cleanup or enrichment of the tap water sample was required. A modified standard addition method was used to eliminate matrix effect. The standard addition graph was linear between 0.2 and 2.6 mg/L in determination of Hg2+. Detection and quantification limits were 0.08 and 0.23 mg/L, respectively. The simple and cost-effective method can be applied to water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Schiff bases are versatile compounds prepared from a primary amine and an aldehyde or a ketone under certain conditions. These compounds are of great interest because of their biological activities, including antitumor, antioxidant, antibacterial, antifungal and antiviral properties [1–5]. Schiff bases, with an imine group as functional group, have been also used as analytical ligands to determine metal ions [6–9]. Reason for using this type of compound for many purposes is their easy synthesis with high yields. Especially, Schiff bases derived from aromatic primary amine compounds were synthesized with high yields under simple conditions [10, 11]. Generally, amine and carbonyl compounds react in alcoholic solution under room temperature or at boiling temperature [12, 13].
Recently, a number of approaches have been reported, including solvent-free microwave, infrared or ultrasound irradiation [14–19]. Among these approaches, microwave irradiation was more commonly used due to its simplicity and selectivity [14, 16]. Also, solid-state synthesis of Schiff bases was reported in the literature [20].
Triazine compounds having nitrogen donor atoms, as complexing moieties are interesting heterocyclic compounds. Especially 2,4-diamino-1,3,5-triazine can be used to prepare diimine compounds with suitable donor atom configurations to bind metal ions. However, limited number of studies about synthesis of Schiff bases derived from 2,4-diamino-1,3,5-triazine [21–24] are available in the literature. Recently, Singh et al. reported two diimine compounds derived from 6-methyl-1,3,5-triazine and 6-phenyl-1,3,5-triazine-2,4-diamine [24]. The authors showed their capability of providing the lubricity and anticorrosion properties by surface film formation in polyol being biolubricant reference fluid [24]. Naeimi reported only synthesis of some diimine compounds derived from 2,4-diamino-1,3,5-triazine [22]. However, there is no report about their metal complexes or usage as analytical ligands in the literature.
In the present study, we report four Schiff base ligands having naphthalene groups derived from substituted 2,4-diamino-1,3,5-triazine, and present the complexation properties of the ligands with a series of metal ions. Many Schiff base preparation methods were tested to obtain the target diimine compounds [21, 22, 25]. However, only solid-state synthesis was utilized to prepare the ligands. Diimine compounds have very low yield, while monoimine compounds have better yield. The complex stability constants and complex compositions of metal ions with the ligands were determined by spectrophotometric and spectrofluorimetric titrations in acetonitrile-water (1/1). A simple spectrofluorimetric method to determine mercury (II) in water samples was developed using the new diimine compound derived from 2,4-diamino-6-undecyl-1,3,5-triazine as the analytical ligand.
Experimental
Chemicals
Acetonitrile from Merck (spectrometric grade) was used as a solvent for absorption and fluorescence measurements. 2,4-diamino-6-metyl-1,3,5-triazine (Alfa Aesar), 2,4-diamino-6-undecyl-1,3,5-triazine (Alfa Aesar), 2-hydroxy-1-naphthaldehyde (Sigma-Aldrich) were purchased. 1000 mg/L of standard solutions of metal ions (Merck) were used to prepare working solutions by appropriate dilution of the standard solutions. The buffer solutions (AVS Titrinorm, Merck Certipur) were purchased from Merck.
Apparatus
1H NMR spectra were recorded on Bruker AVANCE III 400 MHz NMR spectrometer, using CDCl3 with TMS as the internal reference. IR spectra were recorded on a Perkin-Elmer 1600 FTlR spectrophotometer. The absorption spectra of the solutions were recorded using a Analytik Jena Specord 210 spectrophotometer. A Photon Technologies International Quanta Master Spectrofluorimeter (model QM-4/2006) was used for all fluorescence measurements.
Measurements
Absorption spectra of ligands in acetonitrile-water solution (1/1) containing 10 M equivalents of metal ion were measured using 1-cm long absorption cell. Fluorescence spectra of the ligand solutions were measured in 1-cm quartz cell. Excitation wavelengths were 365, 330, 340 and 320 nm for (3), (4), (6) and (7), respectively. Fluorescence emission spectra were recorded in the range of 330–600 nm with a slit width of 1.0 nm.
The stoichiometry of the complexes was determined by the molar-ratio method. The stability constants were calculated according to the known procedure [26].
Reaction of 2-Hydroxy-1-Naphthaldehyde with 2,4-Diamino-6-Methyl-1,3,5-Triazine
Solid starting materials, 2,4-diamino-6-methyl-1,3,5-triazine (1) (0.50 g, 4 mmol) and 2-hydroxy-1-naphthaldehyde (1.378 g, 8 mmol) were finely powdered in a mortar for one min and the mixture was kept at 160 °C for 5 h. Chloroform (15 mL) was added to the mixture. Unreacted amino compound was filtered. The filtrate was concentrated to 5 mL on an evaporator before purification by column chromatography on silica gel using chloroform as eluent to afford pure corresponding product monoimine (3) in 15 % yield, and diimine (4)s in 5 % yield. For (3); mp 217–218 °C. IR (cm−1): 3279–3115 (NH2), 3061 (Ar-H), 2925 (C-H), 1678 (C = O), 1624 (C = N); 1H-NMR (CDCl3): (δ) 13.62 (d, 1H, N-H), 9.40 (d, 1H, =CH), 7.93 (d, 1H, Ar-H), 7.62 (d, 1H, Ar-H), 7.48 (m, 2H, Ar-H), 7.29 (d, 1H, Ar-H), 6.66 (d, 1H, Ar-H), 5.37 (s, 2H, NH2), 2.46 (s, 3H, CH3); MS:m/z = 280.20 [M + 1]+. For (4); mp 209–210 °C. IR (cm−1):3444 (OH), 3077 (Ar-H), 2920–2849 (C-H); 1628 (C = N); 1H-NMR (CDCl3):(δ) 13.82 (d, 2H, NH), 9.40 (d, 2H, =CH), 7.99 (d, 2H, Ar-H), 7.66 (d, 2H, Ar-H), 7.50 (m, 4H, Ar-H), 7.33 (t, 2H, Ar-H), 6.66 (d, 2H, Ar-H), 2.61 (s, 3H, CH3); 13C-NMR (CDCl3): (δ) 25.55, 111.28, 119.85, 125.54, 127.09, 127.31, 129.16, 129.37, 129.68, 133.75, 141.50, 142.83, 187.02. MS:m/z = 434.55 [M]+.
Reaction of 2-Hydroxy-1-Naphthaldehyde with 2,4-Diamino-6-Undecyl-1,3,5-Triazine
Solid starting materials, 2,4-diamino-6-undecyl-1,3,5-triazine (5) (0.25 g, 0.94 mmol) and 2-hydroxy-1-naphthaldehyde (0.324 g, 1.88 mmol) were finely powdered in a mortar for one min and the mixture was kept at 160 °C for 1 h. Chloroform (15 mL) was added to the mixture and applied to column chromatography on silica gel using dichloromethane:ethylacetate (99:1) as eluent to afford pure corresponding product monoimine (6) in 25 % yield, and diimine (7) in 10 % yield. For (6); IR (cm−1): 3275–3108 cm−1 (NH2), 2950–2851 (C-H), 1678 (C = O), 1625 (C = N); 1H-NMR (CDCl3): (δ) 13.67 (d, 1H, N-H), 9.42 (d, 1H, =CH), 7.92 (d, 1H, Ar-H), 7.63 (d, 1H, Ar-H), 7.50 (m, 2H, Ar-H), 7.33 (d, 1H, Ar-H), 6.68 (d, 1H, Ar-H), 5.50 (s, 2H, NH2), 2.67 (t, 3H, CH3), 1.28–1.82 (m, 20H, CH2). For (7); IR (cm−1): 3060 (Ar-H), 2920–2850 (C-H); 1711 (C = O), 1630 (C = N); 1H-NMR (CDCl3):(δ) 13.84 (d, 2H, NH), 9.44 (d, 2H, =CH), 8.00 (d, 2H, Ar-H), 7.67 (d, 2H, Ar-H), 7.52 (m, 4H, Ar-H), 7.35 (t, 2H, Ar-H), 6.69 (d, 2H, Ar-H), 2.86 (s, 3H, CH3), 1.27–3.69 (m, 20H, CH2), 13C-NMR (CDCl3): (δ) 14.26, 22.70, 27.14, 29.79, 31.66, 38.53, 117.74, 120.07, 123.21, 124.58, 125.63, 126.91, 127.42, 129.56, 130.35, 133.23, 141.64, 142.72, 187.21. MS:m/z = 574.30 [M + 1]+.
Proposed Method for Hg2+ Determination
The proposed method is based on fluorescence enhancement of compound (7) with Hg2+ ion at 530 nm. A modified standard addition method, which has been recently reported in the literature [27], was used to determine Hg2+ ion. For this, an optimized amount of Hg2+ (0.5 mg/L) and 2 mL of ligand (1.3 × 10−5 M) were added to all tubes. Then, an aliquot sample solution was added to all tubes, except for the first tube. Increasing amounts of Hg2+ were added to the third and the rest of the tubes. pH of all solutions was set to 8 with citric acid buffer before the final volume was completed to 4 mL. Fluorescence intensity of all solutions was measured at 530 nm. From Eq. 1, Hg2+ concentration was calculated.
Where Cx is the Hg2+ concentration of the sample in the tubes. F0 and F1 are fluorescence intensities of the first and second tubes, respectively, and m is the slope of the standard addition graph. According to this type of standard addition procedure, the difference between F1 and F0 is related to the Hg2+ concentration of the sample in the tubes.
Results and Discussion
Synthesis and Characterization of the Ligands, (3) and (4)
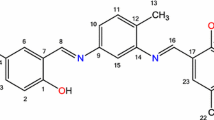
The synthetic pathway to new ligands, (3) and (4) is summarized in Fig. 1. Reaction between 2,4-diamino-6-methyl-1,3,5-triazine (1) and 2-hydroxy-1-naphthaldehyde (2) was carried out in solid state at 160 °C for 5 h. Compounds (3) and (4) were isolated from the reaction mixture by column chromatography on silica gel using chloroform with 15 % and 5 % yields, respectively.
The absence of the N-H stretching vibrations belonging to starting material (1) and the absence of the carbonyl stretching vibration belonging to compound (2) in the IR spectra has supported the structure of diimine compound (4). The stretching vibrations at 1628 cm−1 confirms the presence of C = N groups. In the 1H-NMR spectrum of (4), the absence of the singlet at δ = 5.03 ppm belonging to the primary amine groups of the starting material (1) supports the formation of the compound (4). There is a doublet (2H) at δ = 9.40 ppm (J = 10.9 Hz) for the N–CH protons indicating the coupling with a neighboring –NH hydrogen. Similarly, there is a doublet (2H) at δ = 13.82 ppm (J = 11.6 Hz) for the –NH protons indicating the coupling with a neighboring N–CH hydrogen. This type of splitting can be explained by the domination of the keto-amine form of the diimine compound (4) in CDCl3 (Scheme 1). It is well known that keto-amine form is dominant in naphthaldimine Schiff bases in solution [28–31]. Therefore, this result is in agreement with those of other naphthaldimine compounds in the literature. More detailed information about the structure of compound (4) is provided by its 13C-NMR spectrum. The carbon resonance at δ = 187.02 ppm belongs the corresponding carbonyl carbon of the keto-amine form (Scheme 1). On the other hand, the mass spectral analysis of (4), which shows a molecular ion peak at m/z = 434.55 [M]+, confirms the proposed structure.
In the IR spectra of monoimine compound (3), the stretching vibrations at 1624 cm−1 confirms the presence of C = N groups. However, there is a weak band at 1678 cm−1 assigned to the stretching vibrations of the carbonyl group. These results show that compound (3) exists in a tautomeric of the keto-amine and the phenol-imine form in solid state (Scheme 2). In the 1H-NMR spectrum of (3) in CDCl3, the doublets (1H each) belonging to NH proton of keto-amine form and N-CH proton are observed at δ = 13.62 ppm and δ = 9.40 ppm, respectively. The singlet (2H) at δ = 5.37 ppm belonging to NH2 protons disappeared with D2O exchange. The mass spectral analysis of (3), which shows a peak at m/z = 280.20 [M + 1]+, confirms the proposed structure.
Synthesis and Characterization of the Ligands, (6) and (7)
Figure 2 shows the synthetic pathway to obtain the ligands, (6) and (7). Reaction between 2,4-diamino-6-undecyl-1,3,5-triazine (5) and 2-hydroxy-1-naphthaldehyde (2) was carried out in solid state at 160 °C for 1 h. Compound (6) and (7) were isolated from the reaction mixture by column chromatography on silica gel using chloroform with 25 % and 10 % yields, respectively.
The absence of the N-H stretching vibrations belonging to starting material (5) and the absence of the carbonyl stretching vibration belonging to compound (2) in the IR spectra has supported the structure of diimine compound (7). The stretching vibrations at 1630 cm−1 confirms the presence of C = N groups. In the 1H-NMR spectrum of (7), there was a doublet (2H) at δ = 9.44 ppm (J = 10.9 Hz) for the N–CH protons indicating the coupling with a neighboring –NH hydrogen. Similarly, there was a doublet (2H) at δ = 13.84 ppm (J = 10.9 Hz) for the –NH protons indicating the coupling with a neighboring –CH hydrogen. This type of splitting can be explained by the domination of the keto-amine form of the diimine compound (7) in CDCl3 (Scheme 3). More detailed information about the structure of compound (7) is provided by its 13C-NMR spectrum. The carbon resonance at δ = 187.21 ppm belongs to the corresponding carbonyl carbon of the keto-amine form (Scheme 3). On the other hand, the mass spectral analysis of (7), which showed a molecular ion peak at m/z = 574.30 [M + 1]+, confirms the proposed structure.
In the IR spectra of monoimine compound (6), the stretching vibrations at 1625 cm−1 confirms the presence of C = N groups. However, there was a weak band at 1678 cm−1 assigned to the stretching vibrations of the carbonyl group. These results show that compound (6) exists in a tautomeric of the keto-amine and the phenol-imine form like compound (3) in solid state (Scheme 4). In the 1H-NMR spectrum of (6) in CDCl3, the doublets (1H each) belonging to NH proton of keto-amine form and N-CH proton were observed at δ = 13.67 ppm (J = 10.75 Hz) and δ = 9.42 ppm (J = 10.98 Hz), respectively. The singlet (2H) at δ = 5.50 ppm belonging to NH2 protons disappeared with D2O exchange.
Absorption and Emission Spectra of the Ligands
In UV-visible spectrum of Schiff bases derived from 2-hydroxy-1-naphthaldeyde, a band below 400 nm appears in the case of the enol-imine structure. However, two bands above 400 nm are observed for both keto and mixture of keto–enol forms, especially in polar solvents [29]. Diimine ligand (4) possesses two absorption bands above 400 nm in many organic solvents (Fig. S1). This result confirmed that the compound (4) achieved the keto-enol tautomeric equilibrium in all tested solvents and agreed especially with 1H-NMR data in CDCl3. Similar results were obtained for monoimine compounds, (3) and (6) and diimine compound (7) with the same solvents (Fig. S2).
The effect of solvents such as chloroform, dichloromethane, acetone, acetonitrile, DMF, DMSO, methanol and ethanol on the emission spectra of the ligands was investigated. Figure S3 shows the effect of the solvents on the emission spectra of diimine compound (4). Among the tested solvents, acetone gave the highest fluorescence intensity. When compound (4) was excited at 350 nm, the maximum fluorescence emission was observed at 390 nm. Similar investigation was carried out with compound (3). Figure S4 and S5 show the solvent effect on the emission spectra of monoimine compound (3) and diimine compound (7), respectively. As seen from Fig. S5, DMF and methanol provided high fluorescence intensities for diimine compound (7). Similar investigation was carried out with monoimine compound (6) (Fig. S6).
Metal-Ligand Interactions in Solution
The influence of metal ions including Cu2+, Co2+, Hg2+, Al3+, Cr3+, Fe3+, Pb2+, Ni2+, Cd2+, Zn2+, Mn2+, Ag+, Ba2+, Ca2+ and Mg2+ on the absorption and emission spectra of the ligands was investigated in solution. These ions did not cause any significant change on the absorption spectra of the ligands in some partially aqueous solutions, whereas pronounced changes were observed on the emission spectra of the ligands.
The presence of 10 equivalents of metal ions produced modest changes in the emission of the ligand (4), except for Fe3+, Pb2+, Ba2+ and Hg2+ in acetone-water (1:1) solution (Fig. S7), which caused decreased emission at 370 nm. On the other hand, there was a red shift at 415 nm with the fluorescence quenching for all metal ions. These results suggest that the investigated metal ions interacted with the ligand (4) in acetone-water media, with such interaction being more effective in the case of Fe3+, Pb2+ and Hg2+.
To investigate complexation with these ions, spectrofluorimetric titrations were carried out in acetone-water (1:1). Among Ba2+, Fe3+, Pb2+ and Hg2+ ions, a regular change in the emission spectra of ligand (4) was only observed with increasing concentrations of Pb2+ and Ba2+ ions. Therefore, the complex stoichiometry and the complex stability constant for these ions were calculated from titration data.
Decreased emission intensity at 370 nm allowed determination of the complex stoichiometry of Pb2+-ligand (4). As seen from Fig. S8 (inset left), the inflection point was 1.0 ([M]/[L]). It can thus be concluded that the ligand (4) formed a stable 1:1 complex with Pb2+. To determine the complex stability constant, the ratio of Io/(Io-I) was plotted versus 1/[Pb2+], as in Fig. S8 (inset right), which gave a good straight line. Io and I are the emission intensities of free ligand and the solution containing Pb2+ ion, respectively. The complex stability constant was calculated from the ratio of intercept/slope [26]. The value of log K was 4.98 for Pb2+-complex.
Similar complex stoichiometry was determined for Ba2+ ion from the spectrofluorimetric titration data (Fig. S9). The value of log K for the Ba2+-(4) complex was calculated as 4.74. A stable complex formation could not be identified for other metal ions in acetone-water (1:1) media.
Both spectrophotometric and spectrofluorimetric titrations showed that compound (4) forms stable 1:1 complexes with many metal ions in acetonitrile-water (1:1) solution. Figure S10 shows spectrophotometric titration data for Ba2+ ion. Figure S10 (inset left) shows the molar ratio plot for this complex. The inflection point was 1.0 ([M]/[L]). Therefore, formation of a stable 1:1 (M:L) complex with Ba2+ from the absorbance changes at 440 nm was disclosed. Figure S10 (inset right) shows a plot of the ratio of Ao/(Ao-A) versus 1/[Ba2+] for calculation of the stability constant of this complex, where, Ao and A are the absorbance of free ligand and the solution containing Ba2+ ion, respectively. The complex stability constant was calculated from the ratio of intercept to slope. The value of log K was 5.01 for Ba2+-(4) complex.
Similar decrease in absorbance was observed in the absorption spectra of compound (4) in the case of titrations with Hg2+, Fe3+, Zn2+, Cd2+, Pb2+, Mn2+, Al3+, Co2+ and Mg2+ ions. Table 1 shows complex stability constants and complex stoichiometry of the ligand (4) with metal ions in acetonitrile-water (1:1). As seen from Table 1, the metal ions formed 1:1 complexes with ligand (4). The highest stability constant was obtained for Pb2+ ion with a log K value of 6.12, while Zn2+ formed the lowest stable complex (log K: 4.08 for Zn-(4) complex).
On the other hand, spectrofluorimetric titrations demonstrated that compound (4) formed stable 1:1 complexes with Cr3+ and Ni2+ ions. Figure S11 shows spectrofluorimetric titration data for Cr3+ with compound (4). From Fig. S11 (inset left), the 1:1 complex stoichiometry was determined because inflection point was 1.0 ([M]/[L]). Calculated complex stability constant was log K 4.61 from Fig. S11 (inset right).
Figure S12 shows variation of emission spectra of the ligand (4) with increasing concentrations of Ni2+. The variation was similar to that obtained for Cr3+ ion. There were regular decreases in emission at 416 nm, however, the complex stoichiometry of Ni2+ was disclosed from emission changes at 450 nm. Figure S12 (inset left) shows molar ratio plot for this complex. The inflection point was 1.0 ([M]/[L]), which indicates formation of a stable 1:1 (M:L) complex with Ni2+. Figure S12 (inset right) shows the plot used to calculate the stability constant of this complex. The value of log K was 4.93 for Cd2+-complex.
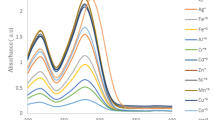
The effects of metal ions on the fluorescence spectra of diimine compound (7) was investigated in various solvents. Significant changes were observed in the emission spectra in the case of acetonitrile-water (1:1) media (Fig. 3).
Excitation at 320 nm of the ligand (7) gives emission bands of naphthalene at 340 and 352 nm. As seen from Fig. 3, fluorescence spectral behavior of ligand (7) did not show any emission band above 430 nm. The emission band intensities of the ligand at 340 and 352 nm were reduced in the presence of Pb2+, Ba2+, Ag+ and Hg2+ ions. The quenching was about 75 % with Hg2+. The new fluorescence band appeared in the presence of metal ions above 480 nm. The highest fluorescence enhancement belongs to Hg2+ ion at 530 nm. On the other hand, the quenching at 340 and 352 nm and fluorescence enhancement at 530 nm were ratiometric. It is interesting that a new emission band at 530 nm with many metal ions was not observed in case of diimine compound (4). These results suggest that the alkyl group is effective on fluorescence emission mechanism of the ligand in the presence of metal ions in acetonitrile-water solution.
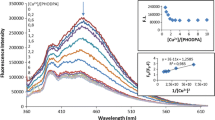
To investigate the complexation between compound (7) and the metal ions, spectrofluorimetric titrations were carried out with Pb2+, Ba2+, Ag+ and Hg2+. A stable complex was only identified for Hg2+ ion in acetonitrile-water (1:1) (see Fig. 4 for titration data for this ion).
Figure 4 (inset above) shows the molar ratio plot for Hg2+-(7) complex. The inflection point was 1.0 ([M]/[L]), which demonstrates formation of a stable 1:1 complex with Hg2+. Figure 4 (inset below) shows the plot used to calculate the stability constant of this complex. Calculated log K value was 5.96 for Hg2+-(7) complex.
Interaction of the monoamine compounds, (3) and (6) with metal ions in solution was also investigated by spectrofluorimetric measurements. Figure S13 shows the effects of metal ions on the emission spectra of compound (6) in THF-water (1:1).
Spectrofluorimetric titrations were carried out in THF-water (1:1) to predict complexation with Mn2+, Ag+, Fe3+ and Pd2+ ions. A stable complex was only identified for Fe3+ ion (see Fig. S14 for titration data for Fe3+ ion).
The complex stoichiometry and the complex stability constant for Fe3+ were calculated from titration data. Decreased emission intensity at 377 nm was used to determine the stoichiometry of Fe3+-(6) complex. As seen from Fig. S14 (inset above), the inflection point was 1.0 ([M]/[L]). It can thus be concluded that the ligand (6) formed a stable 1:1 complex with Fe3+. To determine the complex stability constant, the ratio of Io/(Io-I) was plotted versus 1/[Fe3+], as in Fig. S14 (inset below), which gave a good straight line. Io and I are the emission intensities of free ligand and the solution containing Fe3+ ion, respectively. The complex stability constant was calculated from the ratio of intercept/slope [26]. log K was 5.53 for the Fe3+-complex.
Method Optimization for Mercury (II) Determination
A regular emission intensity enhancement at 530 nm on the fluorescence spectra of compound (7) was observed with increasing Hg2+ concentration when the ligand was excited at 320 nm. This enhancement was used as an analytical response to determine Hg2+ ion. Method parameters such as solvent and concentration of ligand, solution pH, constant Hg2+ concentration were optimized. Water miscible solvent such as ethanol, methanol, acetone and acetonitrile was tested as ligand solvent. Acetonitrile-water (1:1) solution was found to be the most suitable media for determination of Hg2+ ion. The ligand concentration in the range of 1.0 × 10−5 - 1 × 10−6 M was tested to determine optimum ligand concentration. The values of fluorescence intensity at 530 nm were plotted against the Hg2+ concentration. The highest R2 value (0.9926) was obtained for a ligand concentration of 1.3 × 10−6 M (Fig. 5). Therefore, further studies were carried out at this concentration.
To find the pH for determination, the effects of pH on the fluorescence intensity of the solution containing equivalent ligand and Hg2+ (1.3 × 10−6 M) were investigated. Buffer solutions were used to set pH of the solutions. As seen from Fig. 6, the fluorescence intensity was unstable, while it was nearly the same between pH 8 and 9. Determination of mercury was tested with a variety of buffer solutions below pH 6. However, the lowest relative error % for the standard Hg2+ solution was obtained for the citric acid buffer solution at pH 8. Therefore, pH 8 was found to be the optimum value for determination.
The constant Hg2+ concentration used in the modified standard addition method was optimized in the range of 0.2 to 3.0 mg/L. Measurements were carried out in tap water sample for a Hg2+ concentration of 0.23 mg/L. The relative error was below 5 % for constant Hg2+ concentration of 0.5 mg/L. Therefore, this concentration was preferred as the constant Hg2+ concentration. Table 2 gives analytical performance data of the proposed method for Hg2+ determination. The correlation coefficient was 0.9926 indicating good linearity. The LOD (3xSd/m ) and LOQ (9xSd/m) were determined using the standard deviation of eleven measurements of the blank response (Sd) and the slope of the calibration line (m) according to the IUPAC recommendations.
Comparison with Other Methods
There have been spectrofluorometric and spectrophotometric methods in the literature for the determination of mercury. Some of those methods’ LOD values are given in the Table 3. As seen from the Table 3, the presented method gives lower LOD values than most of the others which shows higher sensitivity when compared to other optic methods in the literature. Moreover, being simple and time saving, the presented method proves to be advantageous.
Conclusions
Four novel Schiff base ligands carrying substituted 1,3,5-triazine group were synthesized from 2-hydroxy-1-naphthaldehyde in a simple solid-state reaction. The fluorescent diimine compound (7) showed selectivity for mercury (II) ion among a variety of metal ions so it was utilized to determine mercury (II) ion in a partially aqueous solution. The accuracy of the proposed method was verified by analyzing spiked tap water samples. Good recovery values were found. These results suggest that the novel diimine compound (7) can be used as a selective analytical ligand for the determination of Hg (II) in water samples. Compared to convenient atomic methods, the proposed method is relatively time-saving and simpler.
References
Etaiw SEH, Abd El-Aziz DM, Abd El-Zaher EH, Ali EA (2011) Synthesis, spectral, antimicrobial and antitumor assessment of Schiff base derived from 2-aminobenzothiazole and its transition metal complexes. Spectrochim Acta - Part A Mol Biomol Spectrosc 79:1331–1337. doi:10.1016/j.saa.2011.04.064
Golcu A, Tumer M, Demirelli H, Wheatley RA (2005) Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: synthesis, characterization, properties and biological activity. Inorg Chim Acta 358:1785–1797. doi:10.1016/j.ica.2004.11.026
Jarrahpour A, Khalili D, De Clercq E, Salmi C, Brunel JM (2007) Synthesis, antibacterial, antifungal and antiviral activity evaluation of some new bis-Schiff bases of isatin and their derivatives. Molecules 12:1720–1730. doi:10.3390/12081720
Nawaz H, Akhter Z, Yameen S, Siddiqi HM, Mirza B, Rifat A (2009) Synthesis and biological evaluations of some Schiff-base esters of ferrocenyl aniline and simple aniline. J Organomet Chem 694:2198–2203. doi:10.1016/j.jorganchem.2009.02.032
Priya NP, Arunachalam S, Manimaran A, Muthupriya D, Jayabalakrishnan C (2009) Mononuclear Ru(III) Schiff base complexes: Synthesis, spectral, redox, catalytic and biological activity studies. Spectrochim Acta - Part A Mol Biomol Spectrosc 72:670–676. doi:10.1016/j.saa.2008.10.028
Afkhami A, Abbasi-Tarighat M, Khanmohammadi H (2009) Simultaneous determination of Co2+, Ni2+, Cu2+ and Zn2+ ions in foodstuffs and vegetables with a new Schiff base using artificial neural networks. Talanta 77:995–1001. doi:10.1016/j.talanta.2008.07.065
Aksuner N, Henden E, Yilmaz I, Cukurovali A (2009) A highly sensitive and selective fluorescent sensor for the determination of copper(II) based on a Schiff base. Dyes Pigments 83:211–217. doi:10.1016/j.dyepig.2009.04.012
Kara D, Fisher A, Hill SJ (2009) Determination of trace heavy metals in soil and sediments by atomic spectrometry following preconcentration with Schiff bases on Amberlite XAD-4. J Hazard Mater 165:1165–1169. doi:10.1016/j.jhazmat.2008.10.111
Marahel F, Ghaedi M, Montazerozohori M, Nejati Biyareh M, Nasiri Kokhdan S, Soylak M (2011) Solid-phase extraction and determination of trace amount of some metal ions on Duolite XAD 761 modified with a new Schiff base as chelating agent in some food samples. Food Chem Toxicol 49:208–214. doi:10.1016/j.fct.2010.10.018
Bhagat S, Sharma N, Chundawat TS (2013) Synthesis of some salicylaldehyde-based schiff bases in aqueous media. J Chem. Article ID 909217
Yang Z, Sun P (2006) Compare of three ways of synthesis of simple Schiff base. Molbank Article ID M514s
Alias M, Kassum H, Shakir C (2014) Synthesis, physical characterization and biological evaluation of Schiff base M(II) complexes. J Assoc Arab Uni basic. Appl Sci 15:28–34
Sabah HH (2014) Synthesis, spectroscopic characterization of schiff bases derived from 4,4'-methylen di aniline. Der Pharma Chemica 6:38–41
Kulshrestha A, Baluja S (2010) Microwave promoted synthesis of some Schiff bases. Arch Appl Sci Res 2:221–224
Shockravi A, Sadeghpour M, Olyaei (2010) A simple and efficient procedure for the synthesis of symmetrical bis-schiff bases of 5,5′-methylenebis(2-aminothiazole) under solvent-free conditions. Syn Commun 40:2531–2538
Mishra AP, Sharma N, Jain RK (2013) Microwave synthesis, spectral, thermal and antimicrobial studies of some Ni(II) and Cu(II) Schiff base complexes. Open J Synt. Theory Appl 2:56–62
Nikpassand M, Fekri LZ, Sharafi S (2013) An efficient and green synthesis of novel azo Schiff base and its complex under ultrasound irradiation. Orient J Chem 29:1041–1046
Vazquez MA, Landa M, Reyes L, Miranda R, Tamariz J, Delgado F (2004) Infrared irradiation: effective promoter in the formation of n-benzylideneanilines in the absence of solvent. Synth Commun 34:2705–2718
Murhekar MM, Khadsan RE (2011) Synthesis of Schiff bases by organic free solvent method. J Chem Pharm Res 3:846–849
Schmeyers J, Toda F, Boy J, Kaupp G (1998) Quantitative solid–solid synthesis of azomethines. J Chem Soc Perkin Trans 2(4):989–994
Eissa HH (2013) Synthesis and characterization of new azo-schiff bases and study biological activity. J Curr Res Sci 1:96–103
Naeimi H, Nazifi ZS (2013) Convenient and mild synthesis and characterisation of some new. Bull Chem Soc Ethiop 27:143–149
Ali A, Abdullah N, Maah MJ (2013) Synthesis, characterization and antioxidant studies on 4-phenyl-1,3,5-triazine-2,6-diamine schiff bases and their nickel(II), copper(II) and zinc(II) complexes. Asian J Chem 25:3105–3108
Singh RK, Kukrety A, Saxena RC, Thakre GD, Atray N, Ray SS (2016) Novel Triazine Schiff Base-based cationic Gemini surfactants: synthesis and their evaluation as Antiwear, antifriction, and anticorrosive additives in polyol. Ind Eng Chem Res 55:2520–2526
Mobinikhaledi A, Steel PJ, Polson M (2009) Rapid and efficient synthesis of schiff bases catalyzed by copper nitrate. Syn React Inorg Metal-Org Nano-Met Chem 39:189–192
Bourson J, Valeur B (1989) Ion-responsive fluorescent compounds 2 Cation-steered intramolecular charge transfer in a crowned merocyanine. J Phys Chem 93:3871–3876
Başoğlu A, Tosun G, Ocak M, Alp H, Yaylı N, Ocak Ü (2015) Simple time-saving method for iron determination based on fluorescence quenching of an azaflavanon-3-ol compound. J Agric Food Chem 63:2654–2659
Issa RM, Khedr AM, Rizk HF (2008) 1H NMR, IR and UV/VIS spectroscopic studies of some schiff bases derived from 2-aminobenzothiazole and 2-amino-3-hydroxypyridine. J Chin Chem Soc 55:875–884
Salman SR, Kamounah FS (2003) Tautomerism in 1-hydroxy-2-naphthaldehyde Schiff bases: calculation of tautomeric isomers using carbon-13 NMR. Spectroscopy 17:747–752
Ünver H, Kabak M, Zengin DM, Durlu TN (2008) Crystal structure and tautomerism of 1-[N-(4-Iodophenyl)]aminomethylidene-2(1H)naphthalenone, Zeitschrift für Naturforschung B. 56:1003-1008
Hayvali Z, Yardimci D (2008) Synthesis and spectroscopic characterization of asymmetric Schiff bases derived from 4′-formylbenzo-15-crown-5 containing recognition sites for alkali and transition metal guest cations. Transit Met Chem 33:421–429
Sun Y, Wu AT (2010) Indole-based fluorescent sensor for selective detection of Hg(II. J Fluoresc 20:553–540
Li Y, Zhang X, Zhu B, Xue J (2011) A disulfide-linked naphthalimide dimer for Hg(II) detection in aqueous solution. J Fluoresc 21:1343–1348
Loo AYY, Lay YP, Kutty MG, Timpe O, Behrens M (2012) Spectrophotometric determination of mercury with iodide and rhodamine B. Sains Malaysiana 41(2):213–218
Hamzaa A, Bashammakhb AS, Al-Sibaai AA, Al-Saidi HM, El-Shahawi MS (2010) Part 1. Spectrophotometric determination of trace mercury (II) in dental-unit wastewater and fertilizer samples using the novel reagent 6-hydroxy-3-(2- oxoindolin-3-ylideneamino)-2-thioxo-2H-1,3-thiazin-4(3H)-one and the dual-wavelength β-correction spectrophotometry. J Hazard Mater 178:287–292
Wanga J, Huang L, Xue M, Liu L, Wanga Y, Gao L, Zhu J, Zou Z (2008) Developing a novel fluorescence chemosensor by self-assembly of bis-schiff base within the channel of mesoporous sba-15 for sensitive detecting of Hg2+ ions. Appl Surf Sci 254:5329–5335
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1253 kb)
Rights and permissions
About this article
Cite this article
Ocak, M., Ak, T., Aktaş, A. et al. Metal Complexation Properties of Schiff Bases Containing 1,3,5-Triazine Derived from 2-Hydroxy-1-Naphthaldehyde in Solution. A Simple Spectrofluorimetric Method to Determine Mercury (II). J Fluoresc 27, 59–68 (2017). https://doi.org/10.1007/s10895-016-1934-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1934-9