Abstract
Colorimetric fluorescent chemosensors 4 and 5 based on mono- and di- styryl borondipyrromethenes (BODIPY) linked methyl malonyl were designed for detection of hemoglobin (HgB). Their sensing behavior toward various analytes (Br−, EDTA, Glucose, CO3 2−, Fe2+, Fe3+, I−, NO3 −, PO4 3−, SO4 2−,Cl−, Urea, K+, Mg2+, Na+, NH4 +, Zn2+,Ca2+,Cd2+, Li+, Pb2+, Cs2+, Ag+, Mn2+, Cr2+, Ni2+, Hg2+, Al3+) were investigated by fluorescence spectroscopies. Addition of HgB to acetone: water (4:1) solutions of BODIPYs 4 and 5 solutions gave visual color changes, as well as significantly quenched fluorescence emissions, while other analytes induced no or much smaller spectral changes. The sensing method for both BODIPYs 4 and 5 was successfully applied to measure the HgB in human blood with satisfactory results. Spike and recovery tests in human blood samples exhibit good recovery rates for the spiked concentrations close to the limit of detection. It was found that BODIPYs 4 and 5 constituted the HgB selective fluorescent chemosensor (ON-OFF) and the detection limits were calculated to be 1.773 μg. mL−1 and 1. 295 μg.mL−1 respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selective detection of biomolecules plays an important role in many fundamental processes in biological systems [1]. Methods, allow selective sensing and specification of targeted proteins are useful in a wide range of area, including clinical diagnostics, therapeutic monitoring, and detection of organisms and toxins [2]. Although standard procedures meet the requirements for specificity, selectivity, and ease of use, these molecules still have several fundamental limitations, because production of suitable compounds are expensive, and time-consuming [3, 4].
The HgB molecule has a quaternary structure composed of four polypeptide chains and each chain attached to a heme group. These heme groups contain an iron atom chelated to a protoporphyrin ring [5]. HgB is responsible for carrying oxygen between respiratory organs (lungs or gills) to various parts of the body. The determination of the HgB concentration is greatly utilized in blood tests for the diagnosis of many diseases such as anemia, thalassemia, heart disease, leukemia, sickle cell disease and excessive loss of blood [6]. Also the release of HgB to urine commonly associated with chronic kidney diseases, infections and Malaria [7] Therefore, it is important to measure the content of HgB in urine/ blood for clinical diagnosis. Several methods have been used for the detection of HgB [8] such as electrochemistry [9], spectrophotometry [10, 11], high performance liquid chromatography [12], and chemiluminescence [13].
However, rapid, sensitive, and selective identification of HgB in urine/ blood for diagnosis is still a challenge because of the complicated sample treatment and the necessity of high cost devices. Therefore, designing a chemosensor with a simple and high yielded synthetic route to sense blood/ urine HgB is an attractive approach.
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacenes (BODIPYs) are a class of fluorescent dyes that are finding an increasing number of applications in both the materials and optical imaging fields [14, 15]. BODIPY dyes are used as fluorophores because of their many advantages, including high extinction coefficient, high quantum yield, narrow emission bandwidth, and chemical and photochemical stabilities in solution and in solid state [16, 17]. BODIPYs absorption/ emission wavelengths can be tuned by the modification of the core [18, 19]. Thus, visible and infrared regions of the spectrum can be spanned. These properties were used in applications of BODIPY dyes as labeling reagents [20], fluorescent switches [21, 22], chemosensors [23, 24], and laser dyes [25].
Herein, we reported the facile synthesis and characterizations of novel fluorescence chemosensors based on methyl malonyl – conjugates of mono- and di- stryl BODIPY dyes (4 and 5) for sensitive determination of HgB (Scheme 1.). HgB was monitored via fluorescence emission method by using its effect on optical properties of BODIPYs (4 and 5). BODIPYs (4 and 5) interact with HgB and undergo aggregation. Consequently, the change in optical characteristics of BODIPYs occurs. These optical changes in the emission intensities of BODIPYs are proportional to the concentration of HgB and were used as an analytical signal for quantitative determination of blood HgB concentration.
Experimental
Materials
The deuterated solvent (CDCl3) for NMR spectroscopy, silica gel, dichloromethane and metal chlorides were provided from Merck. Following chemicals were obtained from Sigma Aldrich; 2,4-dimethylpyrrole, trifluoroacetic acid, 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone, trimethylamine, Boron trifluoride diethyl etherate, glacial acetic acid, piperidine, benzene and 4-hydroxybenzaldehyde, Methyl malonyl chloride were purchased from Sigma Aldrich. All other chemicals used for the synthesis were reagent grade unless otherwise specified.
Equipment
Electronic absorption spectra were recorded with a Shimadzu 2101 UV spectrophotometer in the UV-visible region. Fluorescence excitation and emission spectra were recorded on a Varian Eclipse spectrofluorometer using 1 cm pathlength cuvettes at room temperature. The slit widths were set to 5 nm. The fluorescence lifetimes were obtained using Horiba- Jobin-Yvon-SPEX Fluorolog 3-2iHR instrument with Fluoro Hub-B Single Photon Counting Controller at an excitation wavelength of 390 nm and 674 nm. Signal acquisition was performed using a TCSPC module. Mass spectra were acquired Agilent QTOF/TOF mass spectrometer (G6230 A) with ion source AJS ESI. 1H and 13C NMR spectra were recorded in CDCl3 solutions on a Varian 500 MHz spectrometer. Analytical thin layer chromatography (TLC) was performed on silica gel plates (Merck, Kieselgel 60 Å, 0.25 mm thickness) with F254 indicator. Column chromatography was performed on silica gel (Merck, Kieselgel 60 Å, 230–400 mesh). Suction column chromatography was performed on silica gel (Merck, Kieselgel 60 Å, 70–230 mesh). Determinations of clinical HgB results were performed on mindray BC-2800 Auto Hematology Analyzer.
Parameters of Fluorescence Quantum Yields and Lifetimes
The fluorescence quantum yields (ΦF) of BODIPYs (4 and 5) were determined by the comparative method (Eq. (1)) [26].
where F and FStd are the areas under the fluorescence emission curves of the BODIPY 4 and 5 and the standard, respectively. A and AStd are the respective absorbances of BODIPY 4 and 5 and the standard at the excitation wavelengths, respectively. The refractive indices (n) of the solvents were employed in calculating the fluorescence quantum yields in different solvents. Rhodamine 6G (in water) (ΦF = 0.95) [27] and unsubstitued ZnPC (in DMSO) (ΦF = 0.18) [28] were employed as the standards. Fluorescence quenching experiments on the BODIPY 4 and 5 were carried out by the addition of different concentrations of HgB to a same concentration of the compounds. The fluorescence spectra of BODIPYs 4 and 5 at each HgB concentration were recorded, and the changes in fluorescence intensity related to HgB concentration by the Stern-Volmer (S-V) equation [29] (Eq. (2)):
where I0 and I are the fluorescence intensities of the BODIPY 4 and 5 in the absence and presence of quencher, respectively. [Q] is the concentration of the quencher. As expressed in Eq. (3) KSV is the Stern-Volmer constant, which is the product of the bimolecular quenching constant (Kq) and τ0. The ratios of Io/I were calculated and plotted against [HgB], according to Eq. (2), and KSV was determined from the slope.
Synthesis
Compound 1 was prepared with the same procedure described previously [30].
Synthesis of Compounds 2&3
Compounds 2 and 3 were prepared with a revision by the procedure described previously [31, 32].
A 100 mL round bottomed flask was charged with benzene (40 mL) and purged with argon for 15 min. To a solution of BODIPY 1 (100 mg, 0.31 mmol) and 4-hydroxybenzaldehyde (57.9 mg, 0.46 mmol) in benzene was added piperidine (0.4 mL) and glacial acetic acid (0.4 mL). The solution was refluxed in a Dean-Stark apparatus for 2 h. The resultant was concentrated by evaporation until 20 mL, and the mixture was extracted several times with dichloromethane/water. The combined extracts were dried over Na2SO4, evaporated under reduced pressure and then subjected to column chromatography using silicagel (100–200 mesh) as stationary phase and dichloromethane as mobile phase to get compound 2 as pink solid (yield: 32 %) and dichloromethane- methanol (3 %) to afford the compound 3 as blue solid, (yield: 47 %.
Spectral data of 2: (Found: C 72.94, H 5.43, N 6.49 %, C26H23BF2N2O (428) requires C, 72.91; H, 5.41; N, 6.54). 1H NMR (500 MHz, CDCl3) δ 7.56 (s, 1H), 7.53–7.46 (m, 6H), 7.32 (d, J = 4.5 Hz, 2H), 7.20 (d, J = 16.1 Hz, 1H), 6.86 (d, J = 7.6 Hz, 2H), 6.60 (s, 1H), 6.02 (s, 1H), 5.87 (s, 1H), 2.62 (s, 3H), 1.44 (s, 3H), 1.41 (s, 3H).13C NMR (126 MHz, CDCl3) 155.99, 155.45, 152.3, 144.12, 143.01, 141.72, 138.40, 137.3, 130.76, 130.31, 130.14, 129.94, 129.02, 122.23, 119.21, 117.49, 109.99, 31.59, 15.73, 15.39. MS (ESI-TOF) m/z (%): 428 [M]+, 409 [M-F]+.
Spectral data of 3: (Found: C 74.32, H 5.13, N 5.24 %, C33H27BF2N2O2 (532) requires C, 74.45; H, 5.11; N, 5.26). 1H NMR (500 MHz, CDCl3) δ 8.67 (s, 2H), 7.57 (d, J = 16.3 Hz, 1H), 7.52 (d, J = 7.4 Hz, 2H), 7.46 (d, J = 8.1 Hz, 4H), 7.40 (d, J = 6.4 Hz, 2H), 7.28 (d, J = 16.4 Hz, 1H), 6.79 (d, J = 8.2 Hz, 4H), 6.71 (s, 2H), 1.44 (s, 6H).13C NMR (126 MHz, CDCl3) δ 160.81, 154.76, 154.42, 142.78, 139.57, 137.65, 137.51, 134.70, 130.73, 130.62, 130.24, 118.99, 118.19, 117.45, 109.99, 15.67. MS (ESI-TOF) m/z (%): 532 [M]+, 513 [M-F] + .
Synthesis of Compound 4
A 100 mL round bottomed flask was charged with acetone (20 mL) and purged with argon for 15 min. To a solution of BODIPY 2 (30 mg, 0.06 mmol) in acetone, K2CO3 (9.4 mg, 0.07 mmol) and methyl malonyl chloride (9.6 mg, 0.07 mmol) were added. The solution was refluxed for 12 h. The resultant was concentrated by evaporation, and the mixture was then extracted several times with dichloromethane/water. The combined extracts were dried over Na2SO4, evaporated under reduced pressure and purified by silica gel column chromatography using dichloromethane as eluent to afford the compound 4 (yield: 92 %).
Spectral data of 4: (Found: C 68.26, H 5.21, N 5.24 %, C30H27BF2N2O4 (528) requires C, 68.20; H, 5.15; N, 5.30. 1H NMR (500 MHz, CDCl3) δ 7.66 (s, J = 16.05 Hz, 1H), 7.63 (d, J = 8.6 Hz, 2H), 7.55–7.48 (m, 4H), 7.33 (d, J = 7.7 Hz, 2H), 7.22 (d, J = 16.05 Hz, 1H), 7.16 (d, J = 8.6 Hz, 2H), 6.62 (s, 1H), 6.04 (s, 1H), 3.85 (s, 3H), 3.78 (s, 2H), 3.66 (s, 2H), 2.62 (s, 3H), 1.45 (s, 3H), 1.42 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 172.45, 169.04, 168.90, 166.23, 130.94, 130.92, 130.71, 130.11, 129.80, 129.78, 129.54, 123.63, 123.62, 123.59, 110.04, 109.99, 109.92, 109,43, 68.80, 31.51, 26.69, 16.34, 15.45, 14.06. MS (ESI-TOF) m/z (%): 551 [M + Na]+, 528 [M]+, 509 [M-F]+.
Synthesis of Compound 5
A 100 mL round bottomed flask was charged with acetone (20 mL) and purged with argon for 15 min. To a solution of BODIPY 3 (30 mg, 0.02 mmol) in acetone, K2CO3 (4.0 mg, 0.03 mmol) and methyl malonyl chloride (4.1 mg, 0.07 mmol) were added. The solution was refluxed for 16 h. The resultant was concentrated by evaporation, and the mixture was then extracted several times with dichloromethane/water. The combined extracts were dried over Na2SO4, evaporated under reduced pressure and purified by silica gel column chromatography using dichloromethane as eluent to afford the compound 5 (yield: 70 %).
Spectral data of 5: (Found: C 67.17, H 4.79, N 3.78 %, C41H35BF2N2O8 (732) requires C, 67.22; H, 4.82; N, 3.82;
1H NMR (500 MHz, CDCl3) δ 7.71 (d, J = 16.3 Hz, 2H), 7.65 (d, J = 8.5 Hz, 2H), 7.62 (d, J = 16.4 Hz, 2H), 7.56 (d, 3H), 7.19 (d, J = 8.5 Hz, 4H), 6.89 (d, J = 8.3 Hz, 4H), 6.64 (s, 2H), 3.86 (s, 6H), 3.67 (s, 4H), 1.59 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 166.20, 164.70, 161.22, 152.06, 150.88, 150.81, 136.76, 136.24, 136.05, 128.84, 128.25, 128.14, 128.08, 127.13, 114.90, 103.76, 103.74, 103.65, 102.66, 66.53, 33.42, 31.13, 28.56, 24.18, 21.80, 13.00. MS (ESI-TOF) m/z (%): 756 [M + Na]+, 734 [M+ 5H]+, 713 [M-F]+.
Results and Discussion
Synthesis and Structural Characterization
BODIPY derivatives are good candidates as fluorescent sensors in biological systems due to their high molar absorption coefficients and quantum yields, inertness under physiologically relevant conditions, and resistance to photobleaching [33, 34].
The synthetic strategy for the preparation of malonate-fused BODIPYs 4 and 5 initially involved the treatment of BODIPY 1 with 4-hydroxybenzaldehyde in a Dean–Stark apparatus using standard conditions of Knoevenagel condensation in the presence of piperidine and glacial acetic acid to obtain mono- and di- styryl BODIPYs 2 and 3 respectively (Scheme 1). Identifications of the BODIPYs 2 and 3 were performed through 1H and 13C NMR spectroscopy, mass spectrometry and elemental analysis and the results were consistent with the structures. Substitution reactions of the obtained BODIPYs 2 and 3 with methyl malonyl chloride led to the corresponding dipyrromethane derivatives 4 and 5 in good yields.
The structures of newly synthesized BODIPYs 4 and 5 were confirmed by 1H NMR, 13C NMR, ESI-TOF-MS and elemental analyses and the results were consistent with the assigned formulations. The mass spectra of compounds 4 and 5 were obtained by ESI-TOF-MS and the spectra revealed the peak groups of protonated molecular ions, molecular ion plus sodium and molecular ion rupture flours (Fig. 1). Well-resolved 1H NMR spectra of 4 and 5 showed sets of signals for benzene protons ~7–8 ppm region. The β- pyrrolic signals appeared as sharp singlets ~6.62 and ~6.04 ppm for mono-styryl compound 4 and ~6.64 ppm for di-styryl compound 5. In addition, the observed 16 Hz proton-proton coupling constants at ~7.66 and ~7.21 ppm for compound 4 and ~7.71 and ~7.62 ppm for compound 5 proved an E conformation of the double bonds as expected. The 1H NMR of compound 4 showed three sets of methyl protons of BODIPY and one maloyl unit appeared at 1.42, 1.45, 2.62 and 3.85 ppm, respectively. The methyl protons of compound 5 were observed at 1.59 ppm (BODIPY unit) and 3.86 ppm (malonyl unit) as singlets. Also the –CH2 protons of malonyl moieties of BODIPYs 4 and 5 were at 3.78 and 3.67 ppm.
Spectral Studies
The stock solution of BODIPYs 4 and 5 were prepared in acetone: water (4:1) and HgB solution were prepared in water. During the spectral measurements, appropriate amount of BODIPYs and HgB were added to UV or fluorescence cuvette by using a micropipet at 25 °C and final volume adjust to 2 mL. BODIPYs 4 and 5 exhibited absorption bands at ~558 and ~642 nm respectively characteristic for the mono- and di-styryl BODIPYs [18]. The fluorescence emission spectra of BODIPYs 4 and 5 were investigated with an excitation wavelength of 530 and 620 nm at room temperature (Fig. 2). Fluorescence emission peaks were observed at around 567 nm and 652 nm for BODIPYs 4 and 5 respectively with small stokes shifts 9 and 10 nm respectively. The absorption spectrum of HgB displayed band at 559 nm. The fluorescence spectra of HgB excited at the excitation points of BODIPYs 4 and 5 (530 and 620 nm) showed almost no emission. Fluorescence quantum yields were determined by a standard procedure using Rhodamine 6G (in water) (ΦF = 0.95) for BODIPY 4 and unsubstituted ZnPC (in DMSO) (ΦF = 0.18) for BODIPY 5. The quantum yields of BODIPYs 4 and 5 were calculated as 0.43 and 0.18 respectively.
Effect of Solvent
Fluorescence spectra of 4 and 5 were collected in different solvents (Fig. 3). Changes in the fluorescence profile of the molecule in different solvents were more evident than the changes in absorbance spectra. In water and cyclohexane the fluorescence intensities were nearly zero because of solubility problem. In tetrahydrofuran, acetonitrile, methanol and dichloromethane BODIPYs 4 and 5 had similar intensities when BODIPY 5 emission band shifted 14 nm in THF. In acetone both BODIPYs (4 and 5) were highly fluorescent. To simulate biological environment acetone: water (1:1) and acetone: water (4:1) were employed in which best fluorescence response obtained in acetone: water (4:1) solutions for both BODIPYs (4 and 5). Thus we chose acetone: water (4:1) mixture as solvent.
Effect of pH
The investigation on the pH and its effect on chemical interactions, various strategies were applied, because of the importance of pH in biological systems [35, 36]. To investigate the effect of pH on sensing of HgB and establish an optimum pH, the HgB monitoring procedures were examined in various pH mediums in the ranges of 6–10 whereas the other experimental parameters were kept constant. The pH was adjusted to the desire value by the addition of 0.4 mL of Britton–Robinson buffer with pH values in the range of 6–10 to the solutions. The results of these investigations presented in Fig.4 and revealed that as the pH of the mediums were above 6, the analytical signals increased significantly. The analytical signals of BODIPYs 4 and 5 for determination of 12.5 and 5 μg.mL−1 of HgB respectively were highest at pH 7.5 and decreased after that. Hence, the pH values of 7.5 were chosen for further experiments for each BODIPYs (4 and 5). Also, while sensing system is designed, determination of proper buffer to adjust the pH of aqueous medium is important. The effects of various buffers like phosphate, carbonate and EDTA, besides Britton– Robinson with pH 6–10 on the emission intensities of these chemosensors (4 and 5) were analyzed. The results showed that the maximum and stable analytical signal occurred when the Britton–Robinson buffer was used. Thus, Britton– Robinson buffer was selected for pH studies.
Sensing Strategy
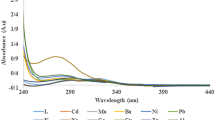
The effect of different kinds of possible interfering analytes on the fluorescence intensities of BODIPYs 4 and 5 were investigated for their potential usage in the detection of HgB. The analytes (Br−, EDTA, Glucose, CO3 2−, Fe2+, Fe3+, I−, NO3 −, PO43−, SO4 2−,Cl−, Urea, K+, Mg2+, Na+, NH4 +, Zn2+,Ca2+,Cd2+, Li+, Pb2+, Cs2+, Ag+, Mn2+, Cr2+, Ni2+, Hg2+, Al3+) (250 μg.mL−1) in aqueous solutions were mixed with the BODIPYs 4 and 5 (0.5 μM and 1 μM, respectively) in acetone: water (4:1) solution, followed by the recording of the fluorescence spectra of the resulting mixture within 1 min (Fig. 5). The addition of analytes only CO3 2− caused small amount of interference for both BODIPYs (4 and 5) and the others did not lead to measurable fluorescence decrease. The result showed that, of all the molecules tested, only the addition of Fe2+, Fe3+ and HgB generated quenching response in the fluorescence intensities of the sensors. The fluorescence intensities of the BODIPYs 4 (0.5 μM) and 5 (1 μM) solutions were quenched about 50 % and 47 % by adding HgB at the concentration of 250 μg. mL−1 and 100 μg. mL−1 respectively. According to the above results, the selectivity for HgB against these possible interfering molecules is high, and the property may therefore be utilized for the detection of trace HgB in biological samples.
As explained previously (Fig. 5), the color and intensity of emission changes for both 4 and 5 markedly only in the presence of HgB in a concentration dependent manner. To further study the interactions between BODIPYs (4 and 5) and HgB, effect of additions of Fe2+ and Fe3+ ions on the fluorescence signals of BODIPYs (4 and 5) were also investigated. As expected the coordination of BODIPYs (4 and 5) with Fe2+ and Fe3+ ions occurred and caused the decreasing on fluorescence intensities. These studies confirmed the formation of complexes between iron chelator BODIPYs (4 and 5) and hem group of HgB as iron source [37–39]. Job’s plots suggested that BODIPYs 4 and 5 formed 2: 1 and 1:1 (ligand: metal) complexes with Fe2+ ions individually (Fig. 6).
Our observations showed that the emission intensities of BODIPYs 4 and 5 were decreased after the addition of HgB accompanied by vanishing of the fluorescence colour. Figure 7 illustrates the fluorescence responses of BODIPYs 4 and 5 in the presence of various concentration of HgB. As shown in the Fig. 7, the emission intensities of the BODIPYs 4 and 5 were significantly decreased after additions of HgB. These decreases in emission intensities were proportional to the HgB concentrations and utilized as analytical signals for HgB measurements. For HgB complexes of BODIPYs 4 and 5, linear correlations could be constituted with coefficients of 0.989 and 0.992 between the logarithm of (I0/I) and the HgB concentrations throughout the entire dynamic working ranges from 0 to 250 and 0–100 μg L−1 which was described by the classical Stern-Volmer equations of (I0/I) − 1 = 4.3 × 103 × [Q] (for compound 4) and (I0/I) − 1 = 9.5 × 103 × [Q] (for compound 5) (Fig. 8) [40]. The limits of detection (LOD) were estimated to be 1.773 μg. mL−1 and 1.295 μg. mL−1 by using the curve fitting equation based on the definition of three times the deviation of the blank signal. The bimolecular quenching rate constants (Kq) of BODIPYs (4 and 5) were calculated as 1,10 × 1011 M−1. s−1 and 5,39 × 1010 M−1. s−1 respectively. The result showed that the method is sensitive and has potential for the detection of trace HgB. A.
Fluorescence titrations of BODIPYs (a) 4 (0.5 μM) in Acetone:Water (4:1) with various concentration of HgB (Excitation wavelength = 540 nm). Inset: Calibration curve of compound 4 (0.5 μM) in Acetone:Water (4:1) with various concentration of HgB. (b) 5 (1 μM) in Acetone:Water (4:1) with various concentration of HgB (Excitation wavelength = 620 nm). Inset: Calibration curve of compound 5 (1 μM) in Acetone:Water (4:1) with various concentration of HgB
To investigate the fluorescence dynamics of BODIPYs 4 and 5, fluorescence decay traces of 4 and 5 in acetone: water (4:1) were collected as functions of emissions wavelengths λem. The measurement of fluorescence lifetimes is a definitive method to distinguish static and dynamic quenching [41]. Time resolved fluorescence studies were revealed single exponential decays for BODIPYs (4 and 5), 4+ HgB and 5+ HgB emissions and the lifetimes were found to be 4.1145 ± 0.0005 ns, 3.7146 ± 0.0005 ns, 4.0566 ± 0.0006 ns and 3.5204 ± 0.0005 respectively. These only small changes in fluorescence lifetimes may indicate static quenching due to the formation of complexes in the ground state (Fig. 9). Since the complexed fluorophores are nonfluorescent and static quenching eliminate a fraction of the fluorophores from measurement, the only observed fluorescence should be from the non- complexed fluorophores. The non- complexed fraction is undisturbed, and hence the lifetime is τ0. Thus, for static quenching τ0/ τ should be equal to 1 [41]. The calculated τ0/τ for both BODIPYs (4/4+ HgB and 5/5+ HgB) are 1.01 and 1.05.
The photostability is preferred for many fluorescent chemosensor dyes. The photostabilities of BODIPYs 4 and 5 in the absence and presence of HgB were investigated for 30 min in acetone: water (4:1) solutions (Fig. 10). The fluorescence intensities of BODIPYs 4 and 5 and BODIPYs 4 + 250 μg. mL−1 HgB and BODIPY 5 + 100 μg. mL−1 HgB were remained the same as initial value. Consequently, it was found that BODIPY derivatives (4 and 5) and their HgB complexes showed excellent photostabilities.
Determination of Hgb Concentration in Clinical Blood
The clinical efficiency of the synthesized BODIPYs (4 and 5) for determination of HgB concentrations were validated by analyzing blood samples of 4 patients (volunteers). The blood samples were centrifuged for the settlement of red blood cells (RBCs) and the RBC phases were diluted by 200-fold with water. Then 8 mL of BODIPYs, 1.5 mL of Britton–Robinson buffer pH 7.5 and 0.5 mL of diluted blood sample were transferred into a 10 mL volumetric ask, made up to the mark with water and mixed thoroughly. Fluorescence signals of BODIPYs (4 and 5) were measured after 30 min when excited 540 nm and 620 nm, respectively. To evaluate accuracy of proposed methods, standard addition test and auto hematology analyzer as the reference method for clinical determination of HgB were carried out. Additionally, in order to determine the reproducibility or precision of the sensors, three measurements were carried out for each spike under the same experimental conditions. The relative standard deviations (RSD %) were also calculated. Thus, it may be said that the proposed sensors have high accuracy and precision The obtained results for standard addition tests and real sample applications were given at Table 1 and Table 2. The obtained results show the usability of proposed method for HgB measurement in clinical laboratories.
Conclusion
In summary, highly sensitive and selective fluorescence sensors based on mono- and di-styrl BODIPYs 4 and 5 for the detection of trace HgB were developed. HgB produced selective quenching effects on the fluorescence of the BODIPYs 4 and 5, whereas the relevant biological molecules had no significant effect on the fluorescence. These methods offer an alternative approach to the classical colorimetric sensing of Hgb. The synthetic method to obtain chemo sensors is high- yielded, facile and cost- effective and also provide high sensitivity for HgB detection with a detection limit of 1.773 μg. mL−1 and 1.295 μg.mL−1 in acetone: water (4:1) for BODIPYs 4 and 5 respectively. Additionally, both BODIPYs (4 and 5) were successfully used for the determination of HgB in human blood samples and were validated by comparing the results with those obtained from a clinical auto hematology analyzer. The results of the present investigations approved the accuracy of the method and might be recommended to employ in clinical laboratories for HgB monitoring.
References
Yongqin L, Tianwei T, Frantisek S (2013) Molecular imprinting of proteins in polymers attached to the surface of nanomaterials for selective recognition of biomacromolecules. Biotechnol Adv 31:1172–1186
Whitcombe M (2011) Molecularly imprinted polymers: smart hydrogel crystal gardens. J Nature Chem 3:657–658
Thobhani S, Attree S, Boyd R, Kumarswami N, Noble J, Szymanski M (2010) Bioconjugation and characterisation of gold colloid-labelled proteins. J Immunol Methods 356:60–69
Yangs KG, Zhang LH, Liang Z, Zhang YK (2012) Protein-imprinted materials: rational design, application and challenges. Anal Bioanal Chem 403:2173–2183
Yang XF, Guo XQ, Li H (2003) Fluorimetric determination of hemoglobin using spiro form rhodamine B hydrazide in a micellar medium. Talanta 61:439–445
Kim DS, Choi JH, Nam MH, Yang JW, Pak JJ, Seo S (2011) LED and CMOS image sensor based hemoglobin concentration measurement technique. Sensors Actuators B 157:103–109
Houjuan Z, Suhua W (2013) Sensitive detection of trace hemoglobin using fluorescence method based on functionalized quantum dots. Anal Bioanal Chem 405:4989–4991
Hsieh MS, Wu TG, Su CS, Chen WJ, Ozbek N, Tsai KY, Lin CY (2011) Comparison of an electrochemical biosensor with optical devices for hemoglobin measurement in human whole blood samples. Clin Chim Acta 412:2150–2156
Tatikonda AK, Tkachev M, Naaman R (2013) A highly sensitive hybrid organic–inorganic sensor for continuous monitoring of hemoglobin. Biosens Bioelectron 45:201–205
Frenchik MD, McFaul SJ, Tsonev LI (2004) A microplate assay for the determination of hemoglobin concentration. Clin Chim Acta 339:199–201
Mieczkowska RK, Tymecki L (2011) Hemoglobin determination with paired emitter detector diode. Anal Bioanal Chem 399:3293–3297
Van Bommel MR, De Jong APJM, Tjaden UR, Irth H, Van der Greef J (2000) High-performance liquid chromatography coupled to enzyme-amplified biochemical detection for the analysis of hemoglobin after pre-column biotinylation. J Chromatogr A 886:19–29
Quickenden TI, Cooper PD (2001) Increasing the specificity of the forensic luminol test for blood. Luminescence 16:251–253
Benstead M, Mehl GH, Boyle RW (2011) 4, 4′-difluoro-4-bora-3a, 4a-diaza-s-indacenes (BODIPYs) as components of novel light active materials. Tetrahedron 67:3573–3601
Mohammed AH, Alamiry A, Mallon LJ, Ulrich G, Ziessel R (2008) Energy- and Charge-Transfer Processes in a Perylene–BODIPY–Pyridine Tripartite Array. Eur J Org Chem 16:2774–2782
Thoresen LH, Kim H, Welch MB, Burghart A, Burgess K (1998) Synthesis of 3, 5-Diaryl-4, 4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY®) dyes. Synlett 11:1276–1278
Rurack K, Kollmannsberger M, Daub J (2001) A highly efficient sensor molecule emitting in the near infrared (NIR): 3, 5-distyryl-8-(p-dimethylaminophenyl)difluoroboradiaza-s-indacene. New J Chem 25:289–292
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 107:4891–4932
Sengul IF, Okutan E, Kandemir H, Astarcı E, Çoşut B (2015) Carbazole substituted BODIPY dyes: synthesis, photophysical properties and antitumor activity. Dyes Pigments 123:32–38
Yee MC, Fas SC, Stohlmeyer MM, Wandless TJ, Cimprich KA (2005) A cell-permeable, activity-based probe for protein and lipid kinases. J Biol Chem 280:29053–29059
Golovkova TA, Kozlov DV, Neckers DC (2005) Synthesis and properties of novel fluorescent switches. J Org Chem 70:5545–5549
Trieflinger C, Rurack K, Daub J (2005) Turn ON/OFF your LOV light”: Borondipyrromethene–Flavin dyads as biomimetic switches derived from the LOV domain. Angew Chem Int Ed 44:2288–2291
Turfan B, Akkaya EU (2002) Modulation of Boradiazaindacene emission by cation mediated oxidative PET. Org Lett 4:2857–2859
Rurack K, Kollmannsberger M, Resch-Genger U, Daub J (2000) A selective and sensitive Fluoroionophore for HgII, AgI, and CuII with virtually decoupled fluorophore and receptor units. J Am Chem Soc 122:968–969
Arbeloa TL, Arbeloa FL, Arbeloa IL, Garcia-Moreno I, Costela A, Sastre R, Amat-Guerri F (1999) Correlations between photophysics and lasing properties of dipyrromethene–BF2 dyes in solution. Chem Phys Lett 299:315–321
Fery-Forgues S, Lavabre D (1999) Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J Chem Educ 76:1260
Magde D, Rojas GE, Seybold P (1999) Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem Photobiol 70:737–744
Jacques P, Braun AM (1981) Laser flash photolysis of Phthalocyanines in solution and Microemulsion. Helv Chim Acta 64:1800–1806
Rose J (1964) Advanced Physico- chemical Experiments. Sir Isaac Pitman & Sons Ltd, London, p. 257
Tümay SO, Okutan E, Sengul IF, Özcan E, Kandemir H, Doruk T, Çetin M, Çoşut B (2016) Naked-eye fluorescent sensor for Cu (II) based on indole conjugate BODIPY dye. Polyhedron 117:161–171
Bartelmess J, Weare WW (2013) Preparation and characterization of multi-cationic BODIPYs and their synthetically versatile precursors. Dyes Pigments 97:1–8
Ali F, Anila HA, Taye N, Gonnade GR, Chattopadhyay S, Das A (2015) A fluorescent probe for specific detection of cysteine in the lipid dense region of cells. Chem Commun 51:16932–16935
Kobayashi T, Komatsu T, Kamiya M, Campos C, González-Gaitán M, Terai T, Hanaoka K, Nagano T, Urano Y (2012) Highly Activatable and environment-insensitive optical highlighters for selective spatiotemporal imaging of target proteins. J Am Chem Soc 134:11153–11160
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172
Maver U, Znidarsic A, Gaberscek M (2011) An attempt to use atomic force microscopy for determination of bond type in lithium battery electrodes. J Mater Chem 21:4071–4075
Pourreza N, Golmohammadi H (2015) Hemoglobin detection using curcumin nanoparticles as a colorimetric chemosensor. RSC Adv 5:1712–1717
Murale DP, Manjare ST, Lee YS, Churchill DG (2014) Fluorescence probing of the ferric Fenton reaction via novel chelation. Chem Commun 50:359–361
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114:4564–4601
Ulrich G, Ziessel R, Harriman A (2008) The chemistry of fluorescent Bodipy dyes: versatility unsurpassed. Angew Chem Int Ed 47:1184–1201
Shan H, Wang L, Huang C, Xie J, Su W, Sheng J, Xiao Q (2015) A carbon dots based fluorescent probe for selective and sensitive detection of hemoglobin. Sensors Actuators B 221:1215
Lakowicz JR (2006) Principles of Fluorescence. Spectroscopy, 3rd edn. Springer, Singapore, pp. 280–282
Acknowledgments
The authors wish to thank to M. HATİPOĞLU, MD, for providing blood samples and clinical results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okutan, E., Tümay, S.O. & Yeşilot, S. Colorimetric Fluorescent Sensors for Hemoglobin Based on BODIPY Dyes. J Fluoresc 26, 2333–2343 (2016). https://doi.org/10.1007/s10895-016-1929-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1929-6