Abstract
Blue emitting 2-amino-4-(3, 4, 5-tri methoxyphenyl)-9-methoxy-5,6 dihydrobenzo[f]isoquinoline-1-carbonitrile (AMQC) dye was synthesized by one-pot multicomponent reactions (MCRs) of 3,4,5-trimethoxybenzaldehyd, malononitrile, 6-methoxy-1,2,3,4-tetrahydro-naphthalin-1-one and ammonium acetate. Results obtained from spectroscopic and elemental analysis of synthesized AMQC was in good agreement with their chemical structures. Fluorescence polarity study demonstrated that AMQC was sensitive to the polarity of the microenvironment provided by different solvents. In addition, spectroscopic and physicochemical parameters, including electronic absorption, excitation coefficient, stokes shift, oscillator strength, transition dipole moment and fluorescence quantum yield were investigated in order to explore the analytical potential of AMQC. Dye undergoes solubilization in different micelles and may be used as a quencher and a probe to determine the critical micelle concentration (CMC) of SDS and CTAB. Nonlinear optical parameters of AMQC dye shows relatively lower nonlinear refractive index and nonlinear absorption coefficient at the power levels. Variation of n2 with concentration is linear in the concentration range used in the present study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In linear optics, various phenomena, like polarizibilty, absorption coefficient, refractive index, refraction etc., depend upon the wavelength of the incident light only but are independent of its intensity. In nonlinear optics [1] the interaction of radiation with matter depends upon the intensity of incident radiation also. After the advent of laser, several experiments have been carried out to demonstrate the nonlinear optical behavior of optical media. It was then established that the refractive index also depends upon intensity, violation of superposition principle has been, frequency of light can be altered when it passes through a nonlinear optical medium. In fact nonlinearity exists in the medium itself, rather than the incident radiation. Nonlinearity has given birth to several new applications, like second and third order harmonic generation, Kerr effect, nonlinear refraction, and absorption etc. New devices have been prepared on the basis of the nonlinear optical properties of some materials.
Recently third order effects and nonlinear refraction in organic dyes were studied. These dyes are having very high nonlinear refractive index and can be used as power limiting devices and also as optical switches [2–7]. These studies also lead to the compilation of several databases listing the nonlinear optical properties of various known crystals, liquids, gasses, dyes [8].
Researchers have developed many techniques to measure the nonlinear refractive index and nonlinear absorption [9, 10] namely, phase distortion method [13] degenerate four wave mixing [11], nonlinear interferometery ellipse rotation [12]. Not only these techniques have very complicated experimental setups but also very expensive instruments have been used. Recently Z-scan technique [14, 15] has been introduced which is very simple, low cost as well as highly sensitive. It is a single beam method, where principles of spatial beam distortion have been used to measure both the sign and magnitude of the optical nonlinearity. Due to its simplicity and easy determination of nonlinear absorption and nonlinear refraction in solids, liquids, composite glasses and semiconductor material [16, 17], now a days this technique has been used by several researchers.
Nitrogen containing organic molecules such as pyrazolines pyrazoles, pyrimidines are important class of the heterocyclic compound in the field of medicinal chemistry. They are used as anti-bacterial [18], anti-tumor, anti-cancer [19], anti-viral agents [20]. During these days organic molecules are also applicable in the field of material sciences such as optical limiting [21], optical switching [22], electronic device [23], polymer coating [24], second order and third order non linear optical properties [25]. Due to numerous application of the nitrogen containing heterocyclic compounds, in this paper we prepared the new blue emitting 2-amino-4-(3, 4, 5-tri methoxyphenyl)-9-methoxy-5,6 dihydrobenzo[f]isoquinoline-1-carbonitrile (AMQC) dye and its physicochemical and non linear optical properties were investigated.
Experimental
Chemicals and Reagents
The 3,4,5-trimethoxybenzaldehyd, 6-methoxy-1,2,3,4-tetrahydro-naphthalin-1-one, malononitrile and ammonium acetate were purchased from Acros Organic. Other reagents and solvents (A.R.) were obtained commercially and used without further purification, except dimethylformamide (DMF), ethanol and methanol.
Apparatus
Melting points were recorded on a Thomas Hoover capillary melting apparatus without correction. FT-IR spectra were recorded on a Nicolet Magna 520 FT-IR spectrometer. 1 H-NMR and 13C-NMR experiments were performed in CDCl3 on a Brucker DPX 600 MHz spectrometer using tetramethyl silane (TMS) as internal standard at room temperature. UV-Vis electronic absorption spectra were acquired on a Shimadzu UV-1650 PC spectrophotometer. Absorption spectra were collected using a 1 cm quartz cell. Steady state fluorescence spectra were measured using Shimadzu RF 5301 PC spectrofluorphotometer with a rectangular quartz cell. Emission spectra were monitored at right angle. All fluorescence spectra were blank subtracted before proceeding in data analyses.
Z-Scan Measurement
The nonlinear refractive index n2 and nonlinear absorption coefficient β have been measured by Z-scan technique, proposed by Shiek Bahae et al. [14], which is a very simple, yet highly sensitive technique, based on the principle of spatial beam distortion. This technique provides the value of nonlinear refractive index and its sign, and the nonlinear absorption coefficient. From these, the real and imaginary parts of the third order nonlinear susceptibility χ(3) can easily be computed. An Ar ion laser Gaussian beam profile at 488 nm and power 75 mW is tightly focused by a convex lens having focal length of 50 mm. The sample is kept in 1 mm cuvette and is translated along the (Z-direction). The details of the experimental setup have been explained in our earlier papers [26, 27].
2-Amino-4-(3,4,5-tri Methoxyphenyl)-9-Methoxy-5,6 Dihydrobenzo[f]Isoquinoline-1-Carbonitrile (AMQC)
A one-pot mixture of the 3,4,5-trimethoxybenzaldehyd, (10 mmol), 6-methoxy-1,2,3,4-tetrahydro-naphthalin-1-one (1.46 g, 10 mmol), malononitrile (0.66 g, 10 mmol) and ammonium acetate (6.2 g, 80 mmol) in absolute ethanol (25 mL) was refluxed for 6 h [28]. The reaction mixture was allowed to cool, and the resulting precipitate was filtered, washed with water, dried and recrystallized from ethanol and chloroform. EI-MS m/z (rel. Int. %): 419 (62) [M + 1]+.; IR (KBr) v max cm−1: 3452 (NH2), 2954 (C-H), 2216 (CN),1565 (C = C), 1258 (N = C); 1 H NMR (600MXz CDCl3) δ: 8.14 (d, 1 H, CHAr, J = 9.6 Hz), 7.82 (s,1 H, CHAr), 6.96 (d, 1 H, CHAr, J = 2.4 Hz), 6.92 (s, 1 H, CHAr), 6.68 (s, 1 H, CHAr), 5.16 (s, 2 H, NH2) 3.98 (s, 3 H, OCH3), 3.94 (s, 3 H, OCH3), 3.86 (s, 3 H, OCH3), 3.82 (s, 3 H, OCH3), 2.78–2.74 (m, 2 H, C5), 2.68–2.56 (m, 2 H, C6); 13CNMR (CDCl3) δ: 162.56, 158.56, 157.58, 154.76, 152.72, 150.72, 149.16, 143.13, 141.86, 127.98 (Ar-C), 117.86 (CN), 115.72, 114.13, 113.12, 112.25, 108.36, 97.18, 95.24, 91.35, 56.70, 27.96 (C6), 26.12 (C5); Anal. calc.for C24H23N3O4: C, 69.05, H, 5.55, N, 10.07. Found: C, 68.88, H, 5.48, N, 9.82.
Result and Discussion
Chemistry
2-amino-4-(3, 4, 5-tri methoxyphenyl)-9-methoxy-5,6 dihydrobenzo [f] isoquinoline-1-carbonitrile (AMQC) was synthesized by one-pot multicomponent reactions (MCRs) of 3,4,5-trimethoxybenzaldehyd, malononitrile, 6-methoxy-1,2,3,4-tetrahydro-naphthalin-1-one and ammonium acetate (Scheme 1). The purified product was characterized by the FT-IR, 1 H-NMR, 13C-NMR and EI MS spectra. The IR spectrum of AMQC shows the characteristic band at 3452 cm−1 due to presence -NH2 group and at 2216 cm−1 attributed to the CN group. IR spectra shows sharp peek at 1258 cm−1 due presence of C = N stretch which conforms to formation of quinoline. 1 H-NMR spectra, which prove diagnostic tool for the positional elucidation of the proton. Assignments of the signals are based on chemical shift and intensity pattern. The 1 H-NMR spectra of AMQC measured at room temperature shows one singlet at 5.16 ppm for the NH2. The appearance of three singlets at δ 7.82, 6.92 and 6.68 and two doublet at δ 8.14 and 6.96 was due to aromatic protons and two multiplets at δ 2.78–2.74 and 2.68–2.56 ppm corresponding to the benzylic protons (C5-H and C6-H respectively). Moreover, 13C-NMR spectra showed signals at δ 27.96 ppm and at δ 26.12 ppm due to C6 and C5, respectively and the structure of the compounds was further confirmed due to presence of CN group at δ 117.86 ppm. Finally characteristic peaks were observed in the mass spectra of AMQC by the molecular ion peak. The mass spectrum of AMQC shows a molecular ion peak (M+.) m/z 419.
Spectral Behavior of 2-Amino-4-(3, 4, 5-tri Methoxyphenyl)-9-Methoxy-5,6 Dihydrobenzo[f]Isoquinoline-1-Carbonitrile (AMQC)
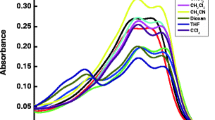
Absorption and emission spectra of 1 × 10−5 mol dm−3 AMQC dye in various non-polar, polar aprotic and protic solvents were studied (Fig 1 and Fig 2). Calculated physicochemical parameters obtained from steady state absorption and fluorescence spectra are tabulated in Table 1. As seen in Fig 1 polarity of solvent has an effect on the absorption maxima. AMQC show a broad absorption band at 368–384 nm region with a red shift of 16 nm on going from n-hexane to DMSO indicating that the allowed transition is π-π* with charge transfer character. On excitation at 360 nm, the emission spectrum of AMQC shows a large red shift of 32 nm on changing the solvent polarity from n-hexane (400 nm) to DMSO (432 nm). This indicates that photoinduced intramolecular charge transfer (ICT) occurs in the singlet excited state from the electron donating substituent O-CH3 group to the electron acceptor CN group of the molecule and therefore the polarity of AMQC increases on excitation. Further, a considerable difference in the magnitude of Stokes shift was observed from 2173 to 2893 cm−1 on changing the solvent polarity from non polar to polar, indicating that the excited state is different from the ground state. As seen in Fig. 1 and Fig. 2 absorption spectra show little sensitivity to change in solvent polarity, but emission spectra show a large red shift with increasing solvent polarity, confirming the presence of π-π* transitions in AMQC and stabilization of highly dipolar excited state in polar solvents.
The energy of absorption (Ea) and emission (Ef) spectra of the AMQC in different solvents correlated with the empirical Dimroth polarity parameter ET (30) [29] of the solvent (Fig. 3). A linear correlation between the energy of absorption and emission versius polarity of solvents was obtained (Eq. 1 and 2), implying potential application of these parameters to probe the microenvironment of AMQC.
Determination of Oscillator Strength and Transition Dipole Moment
The solvatochromic performance of AMQC allows to establish the difference in the dipole moment between the excited singlet and the ground state (∆μ = μe – μg). This variation can be obtained using the simplified Lippert- Mataga equation as follows [29, 30]:
where \( \varDelta {\overline{\nu}}_{st} \) is known as Stokes–shift which decreases with decrease in the solvent polarity indicating to week stabilization of the excited state in non polar solvents [29]. ∆f is the orientation polarizability of the solvent, μe and μg are the dipole moments in the excited and ground state, respectively which measures both electron mobility and dipole moment of the solvent molecule. c is the speed of light in vacuum, a is the Onsager cavity radius and h is Planck’s constant, n and ε are the refractive index and dielectric constant of the solvent in eq. 3 respectively. The Onsager cavity radius was chosen to be 4.2 Å because this value is comparable to the radius of a typical aromatic fluorophore [31].
\( \varDelta {\overline{\nu}}_{ss} \) is the Stokes shifts of the AMQC in different solvents were deliberate, as shown in Table 1, using the following the equation [32]:
where \( \varDelta {\overline{\nu}}_{ss} \) is the difference between λmax of the \( {\overline{\nu}}_{ab} \) and \( {\overline{\nu}}_{em} \)indicate the wavenumbers of absorption and emission maxima (cm−1) respectively.
The change in dipole moments (Δμ) between the excited singlet and ground state were calculated from the slope of plot of Stokes shifts (\( \varDelta {\overline{\nu}}_{ss} \)) and orientation polarizability of the solvent (Δf) as 4.95 Debye for AMQC respectively Fig. 4, positive value indicating that the excited sate is more polar than the ground state.
The change in transition dipole moments (Δμ12) between the excited singlet and ground state of AMQC in various solvents were calculated as in Table 1, using the eq. 6 [33].
where Emax is the maximum energy of absorption in cm−1and f is the oscillator strength.
The oscillator strength (f), can be calculated using the following equation:
where \( \overset{\_}{v} \) represents the numerical value of wavenumber (cm−1) and ε is the extinction coefficient (Lmol−1 cm−1). Oscillator strength values of AMQC in various solvents were calculated from the equation no. 7 and reported in Table 1, [34].
ET (30) and \( {E}_T^N \) is the empirical Dimroth polarity parameter of AMQC was also premeditated according to the following equation [35].
where λ max corresponds to the peak wavelength (nm) in the red region of the intramolecular charge transfer absorption of the bitain dye. AMQC has bathochromic when solvent polarity increase from n-hexane to DMSO indicates that the polarity of AMQC and photoinduced intramolecular charge transfer (ICT) occurs in the singlet excited state, therefore increasing the excitation.
Fluorescence Quantum Yield of AMQC
The fluorescence quantum yield (ϕf) was measured using the optically diluted solution to avoid reabsorption effect (absorbance at excition wavelength); relative method with solution of 9, 10-diphenylanthralene (DPA) in DMSO as reference standard. The following relation has applied to calculate the fluorescence quantum yield [36]:
where F denotes the integral of the corrected fluorescence spectrum, A is the absorbance at the excitation wavelength, and n is the refractive index of the medium. The subscripts “s” and “r” refer to sample and reference, respectively.
The fluorescence quantum yield (φf) of AMQC depends strongly on the solvent properties (Table 1). The fluorescence quantum yield can be correlated with ET(30) of the solvent, where ET(30) is the solvent polarity parameter introduced by Reichardt [37] Fig. 5. The fluorescence quantum yield (Ф f ) of AMQC decreases with increasing solvent polarity. This could be attributed to efficient internal conversion and or intersystem crossing by extensive mixing between the close-lying 1(π–π*) and 1(n–π*) states.
Effect of Surfactant on Emission Spectrum of AMQC
A positively charged and cetyltrimethyl ammonium bromide (CTAB) and negatively charged sodium dodecyl sulphate (SDS) surfactants were selected for evaluating the emission behavior of the AMQC dye. The two specified surfactants were chosen because ionic charges possessed by AMQC dye can be influenced by the positively charged CTAB and negatively charged SDS. Thus, the charge attraction accounts for the AMQC emission behavior. Fluorescence emission spectra of AMQC in the absence and presence of CTAB and SDS were measured. Fluorescence intensities of AMQC increase when increasing the concentration of CTAB from 2 × 10−4 up to 1.6 × 10−3 M as shown Fig. 6. Such enhancement in the fluorescence intensity of 1 × 10−5 M AMQC at fixed concentrations with an increase in the CTAB concentration may likely be ascribed to the association mechanism of AMQC with CTAB. The fluorescence intensity of AMQC is quenched with an increase of the SDS concentration (2 × 10−3 up to 1.6 × 10−2 M Moreover, more significant reductions were noticed in fluorescence intensities of AMQC with SDS. The quenching of AMQC upon increasing SDS concentration can likely be ascribed to the association of AMQC with SDS. Figure 7 represents the influence of SDS on the relative emission intensity of 1 × 10−5 M AMQC. It can be observed that there was a subsequent decrease in the relative emission intensity of AMQC with an increase in the SDS concentration, strongly providing that there was an interaction between AMQC and SDS. It seems that the dye molecule located in the hydrocarbon core of CTAB aggregates, while in SDS, the dye located at micelle – water interface, with quenching role of water. As shown in Fig 6 & Fig 7, the emission intensity of AMQC increases with increasing the concentration of surfactant CTAB, and emission intensity of AMQC decreases with increasing the concentration of surfactant SDS an abrupt change in fluorescence intensity is observed at surfactant concentration of 7.96 × 10−4 and 6.10 × 10−3 mol dm−3 which are very close to the critical micelle concentration of CTAB and SDS [38] respectively; thus AMQC can be employed as a probe to determine the CMC of a surfactants Fig. 6 and Fig. 7. It was well known that aromatic molecules were generally solubilized in the palisade layer of micelle [39]. Thus the enhancement of emission intensity is attributed to the passage of dye molecule from the aqueous bulk solution to the palisade layer of micelle.
Nonlinear Measurements
Z-scan measurements were carried out to measure the sign and magnitude of nonlinear refractive index and nonlinear absorption. The intensity dependent nonlinear absorption and refraction are related by the following equations [15, 40]
where α 0 is the linear absorption coefficient, n 0 is the linear refractive index, β and n 2 are the nonlinear absorption coefficient and refractive index respectively. I is the intensity of the input laser beam.
Closed aperture Z- scan measurements were carried out to measure the nonlinear refractive index of AMQC. Figure 8 shows the variation of normalized transmittance versus Z-position for the different concentrations from 1 mM to 0.1 mM of the dye dissolved in chloroform CHCl 3. The plot shows a pre focal transmittance maximum (peak) followed by a post focal transmittance minimum (valley) which is the signature of negative nonlinear refractive index.
We have evaluated the nonlinear refractive index, n2 and the nonlinear absorption coefficient β by fitting the normalized transmittance of close aperture scan data with the following equation [41]
where, \( \rho =\frac{\varDelta \varPsi }{\varDelta {\varPhi}_0} \); ΔΦ 0 = kn 2 L eff I 0 and \( \varDelta \psi =\beta {I}_0\frac{L_{eff}}{2} \) are the phase shifts due to nonlinear refraction and nonlinear absorption. \( x=\frac{z}{z_0} \)is related to diffraction length of the beam, z 0, \( {z}_0=\frac{k{w}_0^2}{2} \); \( {L}_{eff}=\frac{\left[1- \exp \left(-{\alpha}_0L\right)\right]}{\alpha_0} \), which is effective thickness of the sample, α 0 is linear absorption coefficient, L is the actual thickness of the sample, I0 is the on-axis irradiance at the focus \( {I}_0=\raisebox{1ex}{$2P$}\!\left/ \!\raisebox{-1ex}{$\pi {w}_0^2$}\right. \). w 0 is the beam waist at focus. The values for the nonlinear refractive index, n2 at different concentration are summarized in Table 2. However the values of β obtained by the least square fit of above equation are very low and inconsistent. In order to evaluate nonlinear absorption, we also recorded the open aperture Z-scan data. The scan reveals a flat horizontal line for the normalized transmittance. This indicates the value of nonlinear absorption is very low for this dye. So it is not possible for us to measure β from this data. Hence it confirms the results obtained from closed aperture scan data. We also calculated the values of χ(3) based on the standard equations [14, 17], taking β = 0, and these are also presented in Table 2.
As it is an established fact that the nonlinear refraction arises from thermal, electrostrictive, molecular and electronic effects. But in our case origin of the nonlinearity is mainly due to thermal effects since we have used a cw laser [42]; it is also evident from peak-valley separation being more than 1.7 times the Rayleigh range z0. Figure 9 shows the variation of nonlinear refractive index versus concentrations. It is observed that as the concentration increases the (negative) nonlinearity also increases.
Conclusion
Blue emitting dye 2-amino-4-(3, 4, 5-tri methoxyphenyl)-9-methoxy-5,6 dihydrobenzo [f] isoquinoline-1-carbonitrile (AMQC) was synthesized by one-pot multicomponent reactions (MCRs). AMQC dye displays red shift in fluorescence spectrum as solvent polarity increases. AMQC dye undergoes solubilization in different micelles and may be used in the determination of CMC of surfactants (e.g. SDS and CTAB) and it can be used as quencher or probe for dyes. Nonlinear optical parameters of AMQC dye shows relatively lower nonlinear refractive index and nonlinear absorption coefficient at the power levels. Variation of n2 with concentration is linear in the concentration range used in the present study. Although the dye has excellent fluorescence yield in the blue spectral region, however it has relatively poor nonlinear response at the operating parameters.
References
Boyd RW (2008) Nonlinear Optics, 3rd edn. Academic Press, Boston Rochester, New York
Shettigar S, Umesh G, Purnesh P, Manjunatha KB, Asiri AM (2009) The third-order nonlinear optical properties of novel styryl dyes. Dyes Pigments 83:207–210
Sun R, Yan B, Ge J, Xu Q, Li N, Wu X, Song Y, Lu J (2013) Third-order nonlinear optical properties of unsymmetric pentamethine cyanine dyes possessing benzoxazolyl and benzothiazolyl groups. Dyes Pigments 96:189–195
Al-Ahmad AY, Hassan QMA, Badran HA, Hussain KA (2012) Investigating some linear and nonlinear optical properties of the azo dye (1-amino-2-hydroxy naphthalin sulfonic acid-[3-(4-azo)]-4-amino diphenyl sulfone). Opt Laser Technol 44:1450–1455
He T, Wang C, Zhang C, Lu G (2011) Nonlinear optical properties of an azo-based dye irradiated by picosecond and nanosecond laser pulses. Phys B Condens Matter 406:488–493
Li D, Yu D, Zhang Q, Li S, Zhou H, Wu J, Tian Y (2013) Synthesis, crystal structure and third-order nonlinear optical properties in the near-IR range of a novel stilbazolium dye substituted with flexible polyether chains. Dyes Pigments 97:278–285
Castanon SL, Beristain MF, Ortega A, Gomez-Sosa G, Munoz E, Perez-Martinez AL, Ogawa T, Halim MF, Smith F, Walser A, Dorsinville R (2011) The synthesis, characterization and third-order nonlinear optical character of poly(2,5-dipropargyloxybenzoate) containing a polar aromatic diacetylene. Dyes Pigments 88:129–134
Marvin J, Weber editor-in-chief (2002) Handbook of Optical Materials. CRC Press, Boca Raton
Weber MJ, Milam D, Smith WL (1978) Nonlinear refractive index of galsses and crystals. Opt Eng 17:463–465
Moran MJ, She CY, Carman RL (1975) Interferometeric Measurement of the Nonlinear Refractive -Index coefficient Relative to CS2 in Laser system-Related materials. IEEE J Quantum Electron QE-11:259–263
Friberg SR, Smith PW (1987) Nonlinear refractive index of glasses for ultrafast optical switches. IEEE J Quantum Electron QE-23:2089–2094
Owyoung A (1973) Ellipse Rotation studies in Host Materials. IEEE J Quantum Electron QE-9:1064–1069
Williams WE, Soileau MJ, Van Stryland EW (1984) Optical switching and n2 leasurements in CS2. Opt Commun 50:256–263
Sheik-Bahae M, Said AA, Van Stryland EW (1989) High-sensitivity, single-beam n2 measurements. Opt Lett 14:955–957
Sheik-Bahae M, Said AA, Wei T, Hagan DJ, Van Stryland EW (1990) IEEE sensitive measurements of optical nonlinearities using a single beam. J Quantum Electron QE-26:760–769
Wang J, Sheik-Bahae M, Said AA, Hagen DJ, Van Stryland EW (1994) Time-resolved Z-scan measurements of optical nonlinearities. J Opt Soc Am B 11:1009–1017
Said AA, Sheik-Bahae M, Hagan DJ, Wei TH, Wang J, Young J, Ven Stryland EW (1992) Determination of bound and free carrier nonlinearities in ZnSe, GaAs, CdTe and ZnTe. J Opt Soc Am B 9:405–414
Asiri AMSA, Khan SA (2012) Synthesis, characterization, and in vitro antibacterial activities of macromolecules derived from bis-chalcon Abdullah M. Asiri, Salman a. Khan. J Heterocycl Chem 49:1434–1438
Mamtimin X, Matsidik R, Nurulla I (2010) New soluble rigid rod copolymers comprising alternating 2-amino-pyrimidine and phenylene repeat units: syntheses, characterization, optical and electrochemical properties. Polymer 51:437–446
Koytepe S, Paşahan A, Ekinci E, Seçkin T (2005) Synthesis, characterization and H2O2-sensing properties of pyrimidine-based hyperbranched polyimides. Eur Polym J 41:121–127
Zhang Q, Luo J, Ye L, Wang H, Huang B, Zhang J, Wu J, Zhang S, Tian Y (2014) Design, synthesis, linear and nonlinear photophysical properties and biological imaging application of a novel Λ-type pyrimidine-based thiophene derivative. J Mol Struct 1074:33–42
Achelle S, Ramondenc Y, Dupas G, Ple N (2008) Bis- and tris(arylethynyl)pyrimidine oligomers: synthesis and light-emitting properties. Tetrahedron 12:2783–2791
Baranov VI, Gribov LA, Djenjer VO, Zelentsov DY (1997) Adiabatic semi-empirical parametric method for computing electronic-vibrational spectra of complex molecules part 3. Azines. J Mol Struct 407:209–216
Jacobs JF, Koper GJM, Ursem WNJ (2007) UV protective coatings: a botanical approach. Prog Org Coat 58:166–171
Asiri AM, Khan SA (2011) Synthesis, characterization and optical properties of mono- and bis-chalcone. Mater Lett 65:1749–1752
Razvi MAN, Bakry AH, Afzal SM, Khan SA, Abdullah M, Asiri AM (2015) Synthesis, characterization and determination of third-order optical nonlinearity by cw z-scan technique of novel thiobarbituric acid derivative dyes. Mater Lett 144:13
Khan SA, Razvi MAN, Bakry AH, Afzal SM, Asiri AM, El-Daly SA (2015) Microwave assisted synthesis, spectroscopic studies and non Linear optical properties of bis-chromophores. Spectrochim Acta A 137:1100
Asiri AM, Khan SA, Al-Thaqafy SH, Sharma K (2015) One pot synthesis, photophysical and X-ray studies of novel highly fluorescent isoquinoline derivatives with higher antibacterial efficacy based on the in-vitro and density functional theory. J Fluoresc 2015:503–518
Chakraborty A, Kar S, Nath DN, Guchhait N (2007) Photoinduced intramolecular charge-transfer reactions in 4-amino-3- methyl benzoic acid methyl ester: A fluorescence study in condensedphase and jet-cooled molecular beams. J Chem Sci (Indan Academy of Science) 119:195–203
Asiri AM, Khan SA, Marwani HM, Sharma K (2013) Synthesis, spectroscopic and physicochemical investigation of environmentally benign heterocyclic Schiff base derivatives as antibacterial agents on the bases of in vitro and density functional theory. J Phtochem Phtobio B: 120:82–89
Suppan S. (1990) Invited review solvatochromic shifts: the influence of the medium on the energy of electronic states. J. Photochem. Photobiol., A: 50: 293–330.
Bordeau G, Lartia R, Teulade-Fichou M (2010) Meta-substituted triphenylamines as new dyes displaying exceptionally large stokes shifts. Tetrahedron Lett 51:4429
Aktan E, Babür B, Seferoğlu Z, Hokelek T, Şahin E (2011) Synthesis and structure of a novel hetarylazoindole dye studied by X-ray diffraction, FT-IR, FT-Raman, UV–vis, NMR spectra and DFT calculations. J Mol Struct 1002:113–120
Kawashita Y, Yabana K, Noda M, Nobusada K, Nakatsukasa T (2009) Oscillator strength distribution of C60 in the time-dependent density functional theory. J Mol Struct 914:130–135
El-Daly S, Asiri AM, Khan SA, Alamry KA (2013) Ravi Spectral Properties and Micellization of 1-(2, 5-Dimethyl-thiophen-3-yl)-3-(2, 4, 5-trimethoxy-phenyl)-propenone (DTTP) in Different Media. J Lumin 134:819–824
Kotaka H, Konishi G, Mizuno K (2010) Synthesis and photoluminescence properties of π-extended fluorene derivatives: the first example of a fluorescent solvatochromic nitro-group-containing dye with a high fluorescence quantum yield. Tetrahedron Lett 51:181–184
Royzen M, Canary JW (2013) Structural parameters of Zn(II) complexes of 8-hydroxyquinoline-based tripodal ligands affect fluorescence quantumyield. Polyhedron 58:85–91
Mukherjee K, Moulik SP, Mukherjee DC (1993) Thermodynamics of micellization of aerosol OT in polar and nonpolar solvents. A Calorimetric Study Langmuir 9:1727–1730
Mukerjee P, Cardinal JR (1978) Benzene derivatives and naphthalene solubilized in micelles. Polarity of microenvironments, location and distribution in micelles, and correlation with surface activity in hydrocarbon-water systems. J Phys Chem 82:1620–1627
Sutherland RL (1966) Handbook of Nonlinear Optics. Marcel Dekker, Inc, New York
Liu X, Guo S, Wang H, Hou L (2001) Theoretical study on the closed aperture z-scan curves in the materials with nonlinear refraction and strong nonlinear absorption. Opt Commun 197:431–438
Christodoulides DN, Khoo IC, Salamo GJ, Stegeman GI, Van Stryland EW (2010) Nonlinear refraction and absorption: mechanisms and magnitudes. Adv Opt Photon 2:60
Acknowledgments
This Project was funded by Saudi Basic Industries Corporation (SABIC) and the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. 25-130-1436-S. The authors, therefore, acknowledge with thanks SABIC and DSR technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afzal, S.M., Asiri, A.M., Razvi, M.A.N. et al. Synthesis, Spectrofluorometric Studies, Micellization and non Linear Optical Properties of Blue Emitting Quinoline (AMQC) Dye. J Fluoresc 26, 559–566 (2016). https://doi.org/10.1007/s10895-015-1740-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1740-9