Abstract
The mixed ligand complexes MLA of Cu(II) and Co(II) with Schiff base derived from 4-amino antipyrine and 5-NO2 salicylaldehyde (2,3 –dimethyl-1-phenyl-4-(2-hydroxy-5-nitro benzylideneamino)-pyrazol-5-one) as ONO donor (L) and A = 2,2 bipyridine (bpy),1,10 phenonthroline (1,10 phen) as N, N donor ligands have been prepared, owing to their biological and other applications. The structural features have arrived from their elemental analyses, magnetic susceptibility, molar conductance, Mass, IR, UV–VIS, powdered XRD and ESR spectral studies, that established MLA type of composition for the metal complexes. The electronic absorption spectral data of the complexes suggest an octahedral geometry around the central metal ion. The interaction of the complexes with Calf Thymus (CT) DNA has been studied using absorption spectra, viscosity measurements and fluorescence spectra. The binding constants (Kb) of the complexes were determined as 2.1 × 106 M−1 for complex 1, 2.5x106M−1 for complex 2, 1.16 × 106 M−1 for complex 3,1.25x106M−1 for complex 4, DNA cleavage experiments performed on pBR-322 plasmids using metal complexes in the presence of H2O2 showed that all the complexes afford a pronounced DNA cleavage. Molecular modelling studies were also performed to confirm the geometries of the complexes. The ligand and their metal complexes were screened for their antimicrobial activity against bacteria. The results showed that the metal complexes are biologically active.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrazolones are a class of organic compounds that have been studied extensively due to their pharmaceutical properties. Pyrazolone is a five membered lactam ring which contains two nitrogens and a ketone in the same molecule, and is an active moiety in pharmacological activity, such as anti inflammatory agents [1] for the treatment of arthritis [2] and as analgesics [3]. Anticancer activity has also been reported [4]. Pyrazolones have also applications outside the pharmaceutical field, such as in the solvent extraction of metal ions [5], for analytical purposes [6] and ligands in complexes with catalytic activity [7].
The interaction of transition metal complexes with DNA has been a subject of passionate research in the field of bioinorganic chemistry, ever since the discovery of cis- platin as an anticancer agent. As an important intention of anticancer drugs. DNA plays a central role in replication, transcription and regulation of genes. The presence of metal binding sites in DNA structure make different type of interactions possible such as intercalation between base pairs, minor groove binding and major groove binding [8].
The coordination chemistry of Schiff base as ligand having oxygen and nitrogen donor ligands be a focus for great attention from the biochemists due to their catalytic activity and their relevance to bioinorganic systems [9]. They also show a biological applications including antibacterial, antifungal, antoxidant and cytotoxic activity [10, 11].
The present investigation deals with the synthesis, characterization, antimicrobial and DNA binding, cleavage studies of Cu(II) and Co(II) complexes involving Schiff base derived from 4-amino antipyrine and 5-nitro salicylaldehyde (2,3 –dimethyl-1-phenyl-4-(2-hydroxy-5-nitro benzylideneamino)-pyrazol-5-one)as primary ligand, bipyridine and 1,10 phenanthroline as secondary ligands.
Experimental
Materials and Instruments
All the chemicals used for synthesis purposes were of AR grade, purchased from Sigma Aldrich chemicals, and for spectral analysis spectral grade solvents were used. Millipore water was used for preparing buffer solution.
Elemental analysis of the ligand and its metal complexes was carried out using a Perkin-Elmer 240C (USA) elemental analyser. Molar conductance of the metal complexes was measured in DMSO solution using Digisun digital conductivity meter. Magnetic susceptibilities of the complexes were measured on Guoy balance, model 7550 using Hg [Co(NCS)4] as standard. Thermo gravimetric analyses of the complexes were carried on Shimadzu DTG −60 H system . IR spectra were recorded Brucker FT-IR Spectrometer. Electronic spectra were recorded with Elico SL 159 UV -Visible Spectrophotometer from 200 to 1100 nm. The mass spectra were recorded by ESI technique on LCQ ion trap thermo Finningan Sanjose CA (USA) mass spectrometer. Molecular mechanics calculations were done with Argus lab software, an interactive graphics program that allows rapid structure building, geometry optimization and molecular display. Molecular modelling software Argus lab has ability to handle transition metal complexes. Energy minimization was repeated several times to find global minimum.
Synthesis of Ligands and Metal Complexes
Synthesis of Ligand
The ligand SAAP has been synthesized by the method that has been reported earlier by Gyanakumari et.al [12]. Methanolic solution of (40 ml) aminoantipyrine (4.06 g, 0.02 mol) was added to methanolic solution of 5-NO2 salicylaldehyde (3.34 g, 0.02 mol), the resultant mixture was refluxed for ca. 3 hr, the solid product formed was filtered and recrytastallized from methanol.

Synthesis of Metal Complexes
Ternary metal complexes [Cu(II) (L) (A)](1,2), Co(II)(L) (A)] (3, 4) were synthesized and isolated in aqueous methanol medium, where A = bipy/1,10 phen.To a methanolic solution of Schiff base (0.352 g, 0.001), an aqueous solution of copper acetate mono hydtrate (0.199 g, 0.001) / Cobalt acetate (0.249 g, 0.001) was added dropwise a green colour precipitate for Cu(II) / Orange colour precipitate for Co(II) were obtained after refluxing the reaction mixture for 2 hr. To this mixture, a methanolic solution of heterocyclic base (0.198 g phen / 0.156 g bipy) was added drop wise. The solution on refluxing for 3 hr, at room temperature gave a light green precipitate for Cu(II)/ orange precipitate for Co(II) . The precipitates were separated by filtration and finally air dried.
Analytical Data
[Cu(SAAP)(Bipy)(OAc)]
Pale green, colour,IR ѵmax : 3300 cm−1(νO-H/H2O), 1549 cm−1 (νC=N), 1642 cm−1 (νC=O), 1334 cm−1 (νC-O), 650 cm− νM-O, 416 cm−1 (νM-N) 1496 (νasym coo−) 1280(νsym coo−) Anal.Calc for C30 H30 N6 Cu O6 Cal: C 58.25, H 4.85, N 13.59, :found :C 58.20, H 4.82, N 13.44; μeff : 1.81 BM UV–vis(nm) 273,293680 :ESI-MS (m/z): 628 [Cu(L)(bipy)(oac)]+.
[Cu(SAAP)(Phen)(H2O)](oac)
Green colour,IR ѵmax : 3431 cm−1(νO-H/H2O), 1547 cm−1 (νC=N), 1654 cm−1 (νC=O), 1372 cm−1 (νC-O), 640 cm−1 νM-O, 486 cm−1 (νM-N) Anal.Calc for C30H31N6CuO5 Cal: C 57.05, H 4.91, N 13.31 :found :C 62.08, H 4.93, N 13.46;μeff : 1.78 BM UV–vis(nm) 260,298,342,416,693 :ESI-MS (m/z): 632, [Cu(L)(phen)(H2O]+ (M + 1).
[Co(SAAP)(Phen)(OAc)(H2O)]
Orange colour, IR ѵmax : 3400 cm−1(νO-H/H2O), 1536 cm−1 (νC=N), 1666 cm−1 (νC=O),1376 cm−1 (νC-O), 650 cm−1 νM-O, 495 cm−1 (νM-N) Anal.Calc for C32H32N6CoO7 Cal: C 56.97, H 5.04, N 12.46 :found :C 56.99, H 5.08, N 12.48;μeff : 4.92BM UV–vis(nm) 316,380,424,580,709 :ESI-MS (m/z): 708, [Co(L)(phen)(H2O)(Oac)]+.
[Co(SAAP)(H2O)]Bipy)(OAc)
Orange colour,IR ѵmax : 3383 cm−1(νO-H/H2O), 1521 cm−1 (νC=N), 1666 cm−1 (νC=O),1386 cm−1 (νC-O), 654 cm1 νM-O, 495 cm−1 (νM-N) Anal.Calc for C30H32N6CoO7 Cal: C 57.05, H 5.07, N 13.31 :found :C 57.09, H 5.08, N 13.39;μeff : 4.78BM UV–vis(nm) 312,380,424,,724 :ESI-MS (m/z): 589, [Co(L)(bipy)(H2O)(Oac)]+ (M + Na).
DNA Binding
Preparation of Stock Solution
All the experiments involving with the interaction of the ligand and complexes with CT DNA were carried out in doubly distilled water buffer containing 5 mM Tris [Tris (hydroxylmethyl)-aminomethane]. The solution of CT-DNA in the buffer gave ratios of UV absorbance of about 1.8–1.9 at 260 nm and 280 nm, indicating that the CT-DNA concentration per nucleotide was determined spectrometrically by employing an extinction coefficient of 6600 M−1 cm−1 at 260 nm [13]. The stock solution was stored at 40 °C.
Absorption Spectra
Absorption spectra were recorded on Elico SL 159 UV-Visible spectrophotometer using 1 cm quartz micro cuvettes. Absorption titrations were executed by maintaining the concentration of the complex constant (10 μM) and by altering the concentration of CT–DNA from 10 to 100 μM. The binding constants (Kb) of metal complexes have been determined from the spectroscopic titration data using the following Eq. 1.
The apparent extinction coefficient ɛa, was obtained by calculating Aobs / [complex], ɛf and ɛb correspond to the extinction coefficient for the free (unbound) and fully bound complex respectively. A plot of [DNA] / (ɛa -ɛf) vs [DNA] will have a slope equal to 1/(ɛb -ɛf) and an intercept equal to 1/ Kb(ɛb -ɛf), Kb is then given by the ratio of the slope and the intercept.
Viscosity Measurements
Viscosity measurements were carried out using an Ostwald viscometer maintained at a constant temperature at 28.0 ± 0.1 °C in a thermostatic bath. Flow time was measured with a digital stopwatch and each sample was sample was measured five times for which an average flow time was calculated. The graph from (η / ηo)1/3 versus the ratio of the concentration of the complex and CT-DNA, where η is the viscosity of metal complex with DNA and ηo is the viscosity of DNA without metal complex. Viscosity values were calculated from the equation η = t − to,t is flow time of DNA containing solution t0 flow time of buffer alone .
Fluorescence Titration
Fluorescence titration experiment was carried out in 5 mM Tris–HCl, 50 mM NaCl buffer at pH 7.4 using ethidium bromide (EB) displacement method [14].The emission spectra of metal complexes have been measured absence and presence of CT DNA. It was reported that the enhanced fluorescence intensity quenched by the addition of a metal complex solution. In each experiments, 40 μL of CT DNA solution (A = 1.0) was added to 2.25 ml of EB in buffer solution and the fluorescence intensity was measured at emission wavelength 600,610 nm and excitation wavelength 490,480 nm. The concentrations of the metal complexes were increased from 0 to 100 μM. The Stern–Volmer quenching constant was calculated according to the classical Stern–Volmer equation [15]
where I0 and I are the fluorescence intensities at600,610 nm in the absence and presence of the quencher, respectively, Ksv is the linear Stern–Volmer quenching constant, r is the concentration of the quencher. Ksv is the slope, obtained from the plot of I0/I versus r.
DNA Cleavage Studies
TE buffer (10 mM Tris–HCl and 1 mM Na2EDTA) Used for dilution of pBR322 DNA, TAE buffer (pH 8.0; 40 mM tris base, 20 mM acetic acid, 1 mM EDTA) was used for gel-electrophoresis. Super coiled pBR322 DNA (0.2 μg/μL) was treated with Cu(II) complexes (16 μM) in a clean Eppendorf tube. The contents were incubated for 1 h at 37 °C and loaded onto a 1 % agarose gel after mixing 5 μl of loading buffer (0.25 % bromophenol blue). The electrophoresis was performed at a constant voltage 70 V for 1 hrs until the bromophenol blue had travelled through 70 % of the gel. Afterwards, the gel was stained for 15 min in ethidium bromide solution. The plasmid bands were photographed in a Gel doc system (Alpha InfoTech Corporation).
Antibacterial Study
The free ligand SAAP and its ternary metal complexes were tested against the bacterial species, Klebsiella pneumonia, Bacillus subtilis, Escherichia coli, Staphylococcus aureus by ampicillin as a standard antibacterial agent. The metal complexes and ligands were prepared in DMSO at a concentration of. 10 μl. Paper discs saturated with solution of metal complexes in DMSO or DMSO as a negative control and were placed aseptically in the petridishes containing nutrient agar media inoculated with the above mentioned two bacteria separately. The petridishes were incubated at 37 °C and the inhibition zones were recorded after 24 h of incubation period.
Results and Discussion
Synthesis and Characterization
The synthesis of 1, 2, 3 and 4 was schematically presented in Scheme I. The complexes were highly soluble in DMSO, DMF and MeOH. They were non hygroscopic and stable in solid and solution phases. The analytical data for Cu(II) and Co(II) complexes are in good agreement with the molecular formulae of the complexes. The molar conductance value for complexes 1, 3 & 4 are too low, indicating that complexes are non electrolytic. For 2 molar conductance values are high indicating these complexes are electrolytic in nature.
Infrared Spectra
The important IR spectral data of the ligand and it complexes are summarized in table 1. The ѵC=O and ѵC=N (azomethine) observed at 1666 cm−1 and 1595 cm−1 respectively in the spectra (except Co(II)-L) of the ligand show downward shift in all the complexes. These are suggestive of the participation of the pyrazol-carbonyl and azomethine groups in coordination [16]. In the spectra of Co(II) complexes no shift is observed in pyrazol –carbonyl(ѵC=O) band, indicating the non- participation of C = O group in coordination with the metal ion. The IR broad bands of metal complexes in the range 3300–3431 cm−1 indicate the presence of coordinated / lattice water molecules [17]. Appearance of a band around 896-831 cm−1 at lower frequency region indicates presence of coordinated water molecules in the metal complexes also supported by thermal analysis [18]. The ѵC-O(phenolic) modes of the ligand appear at 1381 cm−1, shifted in the complexes indicate the complex formation via deprotonation of phenolic OH group [19]. The non ligand bands 650–651 cm−1 and 416–495 cm−1 are assigned to ѵM-O and ѵM-N respectively [20]. The peaks corresponding to the ring stretching frequencies ѵC=C and ѵC=N at 1503, 1421 cm−1 of free phenanthroline and at 1527, 1443 cm−1 of free bipy were shifted to higher frequencies upon complexation, indicating the coordination of the heterocyclic nitrogen atoms to the metal ion. The characteristic out of plane hydrogen bending modes of free phenanthroline observed at 853 and 738 cm−1 (for bipyridine at 851, 691 cm−1) were shifted upon metal complexation [21]. The complexes (2, 3, 4) show 1544, 1550, 1580 cm−1 and 1397, 1290, 1390 cm−1 which can be assigned respectively to ѵasym (COO) and ѵsym(COO) of carboxylato group [22].
Electronic Absorption Spectra and Magnetic Moment Studies
UV–VIS Spectra of Cu(II) complexes (1 & 2) in DMSO show a broad band (Fig. 1b, c) in 600,680,620,693 nm region corresponding to 2T2g → 2Eg transition which is in consistent with that of distorted octahedral geometry [23, 24]. It is also supported by magnetic moments of the complexes. The broadness and position of the band favor distorted octahedral geometry. In the UV–VIS band at 336 nm for complex 1 and 342 nm for complex 2 are due to the n-π* transitions of azo methine (C = N) function of Schiff base (Fig. 1a) and remaining bands in the UV region at 273 nm, 297 nm for complex 1 and 260 nm, for complex 2 are due to the π-π* transitions of coordinated phen and bipy ligands respectively [25].
The two Co(II) complexes(3&4) exhibit four absorption bands (Fig. 1d,) in the region 257–312 nm, 380–396 nm, 400–450 nm, 607–724 nm and 580–709 nm. The first two intense maxima are due to intraligand transitions of the organic moiety. The other two transitions may be assigned to 4T1g → 4T2g (P) and 4T1g(F) → 4T2g (F) respectively, suggesting an octahedral geometry for these complexes. The magnetic moment of the same complexes also indicate octahedral geometry [26, 27]. The important uv spectral data of the ligand and it complexes are summarized in (Table 2.)
Mass Spectra
The ESI-Mass spectra of four complexes have been recorded. The molecular ion [M]+ peaks obtained from 1, 2, 3 and 4 complexes are as follows: m/z = 596 (M + Na) [Cu(SAAP)(bipy)(OAc)] (1), m/z = 632 (M + H) [Cu(SAAP)(phen)(H2O] (2), m/z = 586 (M+) [Co(SAAP)(bipy)(OAc)(H2O)] (3) and m/z = 676 (M) [Co(SAAP)(phen)(OAc)(H2O)] (4). The data obtained are in good agreement with the proposed molecular formula for Cu(II) and Co(II) complexes.
TGA-DTA
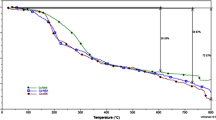
Generally, two types of water molecules are associated with the complexes viz lattice water and coordinated water. The lattice water lost at low temperature (60–120 °C) where as the coordinated water molecules lost at high temperatures (150–200 °C) [28]. Thermogarms of [Cu(SAAP)(phen)H2O](oac) Fig. 2b indicated a total weight loss of 71% upto 1000 °C. The thermogram of the complex is stable upto 120 °C indicates the absence of lattice water. The first decomposition step in the temperature range of 180–200 °C corresponds to loss of coordinated water and the second decomposition step in the temperature range of 330 °C [29] corresponds to loss of acetate moiety. In the third step decomposition of organic moiety up to 620 °C. The residue at 1000 °C indicates the non–volatile metal component present in the complex. The DTA of the complex showed endothermic peaks at 150 °C, 300 °C, 450 °C and 925 °C which are due to the loss of water and subsequent decomposition of the metal complexes [30]. Thermogarms of [Co(SAAP)(phen)(oac)(H2O)] indicated Fig. 2a, a total weight loss of 81% upto 1000 °C. The thermogram of the complex is stable up to 120 °C indicates the absence of lattice water The first decomposition step in the temperature range of 120 °C-180 °C corresponds to loss of coordinated water and the second decomposition step corresponds to 350 °C loss of acetate moiety. The third step decomposition of organic moiety up to 620 °C. The residue at 1000 °C indicates the non—volatile metal component present in the complex. The DTA of the complex showed endothermic peaks at 100 °C, 200 °C, 350 °C and 780 °C which are due to the loss of water and subsequent decomposition of the metal complexes [31].
ESR Spectra
ESR spectra of Cu(II) complexes were recorded DMSO at liquid nitrogen temperature(LNT). ESR spectral studies gives information about the unpaired electrons and bonding nature of metal ion and its ligands. There is many results regarding the applications of ESR of Cu(II) complexes, covalency of the metal ligand bonding. The ESR spectra of the Cu(II) complexes were recorded on Jeol, JES-FA 200 ESR spectrometer at room temperature. The present Cu(II) complexes exhibited high resolved signals (Fig. 3). The observed data showed that gΠ = 2.0–2.49 and g┴ = 2.09–2.24. The gΠ values are greater than g┴ suggesting major distortion from octahedral symmetry in the Cu(II) complexes [32]. The gII is a moderately sensitive function for indicating covalency. The gΠ > 2.3 is characteristic of anionic environment and gΠ <2.3 of covalent environment in M –L bonding [33]. The observed gΠ values for all the complexes (except Cu-L2 and Cu-L5) are less than 2.3, in agreement with the covalent character of the M-L bond. These two (Cu-L2 and Cu-L5) complexes showed gΠ > 2.3 which is characteristic of anion environment. The trend gΠ > g┴ > 2.0023 for the complexes specifies that unpaired electron is localize in dx 2 -y 2 orbital. Thus we expect tetragonal geometry for the complexes. Axial symmetry parameter G = (gΠ-2) /(g┴-2), which measure the exchange interaction between the metal centres in a polycrystalline solid has been calculated. If G > 4 the exchange interaction is negligible, if G < 4 indicates considerable exchange interaction in the solid complexes [34]. The above reported complexes showed G values < 4 indicating the exchange interaction in complexes. Earlier works [35] reported that gΠ is 2.4 for copper-oxygen bonds and 2.3 for copper - nitrogen bonds. For the complexes reported here, gΠ values between 2.3 and 2.4 further confirms the presence of mixed copper-nitrogen and copper–oxygen bonds in these chelate complexes.
Powder XRD Studies
The X-ray diffraction pattern of [Cu(SAAP)(bipy)(oac)], [Co(SAAP)(phen)(oac)(H2O)] Complexes are given (Fig. 4). The single crystals of complexes could not be isolated because of Their insolubility in most of organic solvents . The powder XRD of Cu(II), Co(II) Complexes show a sharp crystalline peaks indicating its crystalline phase. XRD patterns of complexes recorded in the range (2ϴ = 10–600). The average crystallite size (dXRD) of complexes was calculated using scherer’s formula [36]. The Co(II), Cu(II) complexes having average crystallite size 44, 32 and 12 nm respectively.
Where ⋌ is the wave length of X-ray radiation, β is the full width at half maximum of diffraction line and ϴ is the diffraction angle.
SEM
Purity and morphology of the complexes obtained were studied by SEM, the obtained SEM micrographs shown in (Fig. 5) the sem picture of metal complexes shown that the particles are agglomerated with the controlled morphological structure and the presence of irregular brick like structure in Co (II) complexes. The sem images of Cu (II) complexes exhibit cauliflower-like structure. However, particles size less than 100 nm, were also observed which groups to form a agglomerates of larger size. The average crystalline size obtained from XRD also shows that these complexes have poly crystalline with nano size grains.
DNA Binding Studies
Electronic Absorption Spectral Studies
Electronic absorption spectroscopy is one of the most common ways to investigate the interactions of complexes with DNA. The absorption spectra of four complexes Fig. 6a-d shows both hypochromic and bathochromic shifts of the addition of increasing amounts of CT –DNA. Hypochromism is suggested to arise due to intercalative mode of binding involving a strong stacking interaction between an aromatic chromophore and the base pairs of DNA [37]. After intercalating the base pairs of DNA, the π* orbital of the intercalated ligand can couple with the π orbital of base pairs thus decreasing the π-π* transition energy and resulting bathochromism [38]. The absorption spectra of the complexes, Cu(II)-SAAP-bipy (1), Cu(II)-SAAP-phen (2), Co(II)-SAAP-bipy (3) and Co(II)- SAAP-phen (4) in the absence and presence of calf thymus DNA are illustrated in Fig. 7. The plot of the absorption titration data according to equation 1 gave a linear plot and resulted in an intrinsic binding constant (Kb) of 2.1 × 106 M−1 for complex 1, 2.5x106M−1 for complex 2, 1.16 × 106 M−1 for complex 3,1.25x106M−1 for complex 4 [39, 40].
Absorption spectral changes of complexes in DMSO solution upon the addition of CT-DNA at 250c (↓) indicates absence and presence of by adding increasing the DNA concentration. Intercept plot of [DNA]/ε a-εbVs [DNA], a) [Cu(SAAP)(bipy)(OAc)], (b) [Cu(SAAP)(phen)(H2O].(c) [Co(SAAP)(bipy)(OAc)(H2O)] and (d) [Co(SAAP)(phen)(OAc)(H2O)]
Viscosity Studies
To investigate further clarification for the binding nature of complexes to DNA, viscosity of the complex solutions with DNA was measured (Fig. 7). The viscosity of DNA solution is increased with the addition of metal complexes which can bind to DNA due to the DNA helix length increases as base pairs are lengthened to accommodate the complex aromatic planar moiety. The viscosity of DNA is slightly increased with the increase of the concentration of complexes, in contrast to that of proven DNA intercalator EtBr (=ethidium bromide) as shown in (Fig. 7). Based on the viscosity values, it was indicates a strong intercalating mode of interaction of these complexes binding [41].
Fluorescence Titration
EB was used as fluorescence tag for these Cu(II),Co(II) complexes, and competitive binding studies of the metal complexes were carried out with the help of EB bound to DNA. Figure 8 shows the changes in the emission intensity of EB bound to DNA in absence and presence of the complexes 2,3 The fluorescence intensity decreases with increase in concentration of the metal complexes. signifying that the complex compete with EB in binding to DNA. The Kq values for the complexes 2,3 and are 3.046 × 105 M−1 and 1.211 × 106 M -1,respectively. The quenching of EB bound to CT-DNA by the Cu(II),Co(II) complexes are in good agreement with the linear Stern–Volmer equation. These results shows that all the complexes interact with DNA through a minor groove binding. which provides further evidence that the metal complexes bind to DNA. Based on the K values the order of binding strength of metal complexes is Cu(II)˃Co(II).these results should be believed as capable lead compounds for the development of targeted drug for cancer therapy.
DNA Cleavage Studies
DNA cleavage experiments using pBR322 DNA were executed with complexes in the presence of H2O2 as an oxidant. The nuclease activity was greatly enhanced by the incorporation of metal ion in the presence of an oxidant.; it is evident from (Fig. 9), which shows that the complexes 3, 4 and 5 cleave DNA more efficiently in the presence of oxidant, which may be attributed to the formation of hydroxyl free radicals. The production of hydroxyl free radical is due to the reaction between the metal complex and oxidant. These hydroxyl radicals participate in the oxidation of the de-oxy ribose moiety, followed by hydrolytic cleavage of the sugar phosphate backbone. The more nuclease activity in the metal complexes in the presence of oxidant may be due to the enhanced production of hydroxyl radicals. The cleavage efficiency was measured by determining the ability of the complex to convert the super coiled DNA to nicked form. As it is evident from Fig. 9 (lane 3, 4, 5) there is a considerable increase in the intensity of bands for open circular form in case of [Co(SAAP)(bipy)(OAc)(H2O)], [Cu(SAAP)(bipy)(OAc)], [Cu(SAAP)(phen)(H2O] complexes. This suggests that samples have complexes nicking activity.
Antimicrobial Studies
The free ligand SAAP and its ternary metal complexes were tested against the bacterial species (Fig. 10), Klebsiella pneumonia, Bacillus subtilis, Escherichia coli and Staphylococcus aureus by ampicillin as a standard antibacterial agent. The results were tabulated in (Table 3). From the results it has been observed that the highest zones of inhibition i.e. 11.3, 9 and 9 mm were measured in B. Subtitles, K. Pneumonia, S. Aureus in case of Cu(II) complexes. Co(II) complexes show highest inhibition zone (10 mm) against B.Subtitles and E.coli. The complexes show better antibacterial activity than their parental ligand and Cu(II)-L-bpy complex has maximum antibacterial effect. This is due to chelation theory, chelation reduces the polarity of the metal atom mainly because of partial sharing of it positive charge with the donor groups and possible π electron delocalization within the whole chelate ring, also, the chelation increases the lipophilic nature of the central atom which subsequently favors its permeation through the lipid layer of the cell membrane [42, 43]. All the test compounds show lesser activity than the standard antibiotic ampicillin. All these were shown in Table 3.
Molecular Modelling
The possible geometries of metal complexes were assessed using the molecular calculation with Arguslab software [44–46]. The geometrical optimization of the structures obtained are presented in (Fig. 11).
-
(a) [Cu(SAAP)(bipy)(OAc)],(b) [Cu(SAAP)(phen)(H2O]
-
(c) [Co(SAAP)(bipy)(OAc)(H2O)], (d) [Co(SAAP)(phen)(OAc)(H2O)
Conclusion
Mononuclear Cu(II) and Co(II) complexes of Schiff base (SAAP) with neutral coligands(bpy,phen) were synthesized, characterized using the micro analytical, thermal, molar conductance,electronic and vibrational spectra suggested Cu(II),Co(II) complexes are Octahedral arrangement(Fig. 12). TGA showed that the complexes are thermally more stable. XRD and SEM analysis suggest crystalline and morphological structural studies of complexes. DNA binding properties have been investigated by using UV absorption spectra, viscosity and Fluorescence measurements. All these results indicate that the Cu(II) and Co(II) complexes bind to CT-DNA moderately in an intercalative mode,Cu(II) complexes are effective binding then Co(II) complexes. The complexes have been found to promote cleavage ability of pBR 322 plasmid DNA by oxidative cleavage mechanism. The anti microbial activity data shown that Cu(II)L-bipy, Co(II)L-bipy complexes displayed higher activity among other complexes.
References
Sondi SM, Sharma VK, Verma RP, Singhal N, Shukla R, Raghubir R, Dubey MP (1999) Synthesis, anti inflammatory and analegesic activity evalution of some mercapto pyrimidine and pyrimidobenzimidazole derivatives. Synthesis 5(131):878–884
Hardcastle SA, Dieppe P, Gregson CL, Hunter D, Thomas GER, Arden NK, Spector TD, Hart DJ, Laugharne MJGA, Clague GA (2014) Prevalence of radiographic hip osteoarthritis is increased in high bone mass. Osteoarthr Cartil 22(8):1120–1128
Koo HJ, Yoon WJ, Sohn EH, Ham YM, Jang SA, Kwon JE, Jeong YJ (2014) The analgesic and anti-inflammatory effects of Litsea japonica fruit are mediated via suppression of NF-κB and JNK/p38 MAPK activation. Int Immunopharmacol 22:84–97
Sampath K, Raja G, Butcher RJ, Jayabalakrishnan C (2013) DNA binding/cleavage, antioxidant and cytotoxic activities of water soluble cobalt(II) and copper(II) antipyrine complexes. Inorganica Chimica Act 406:44–52
Ito T, Goto C, Noguchi K (2001) Lanthanoid ion-selective solvent polymeric membrane electrode based on 1-phenyl-3-methyl-4-octadecanoyl-5-pyrazolone. Anal Chim Acta 443:41–51
Sondhi SM, Singhal N, Verma RP, Arora SK, Dastdar SG (2001) Synthsis hemin and porphyrin derivatives and their evalution for anti cancer activity. Indian J Chem b 40:113–119
Bao F, Lu X, Kang B, Wu Q (2006) Vinyl polymerization of norbornene catalyzed by a series of bis(b-ketoiminato)nickel (II) complexes in the presence of methylaluminoxane. Eur Polym J 42:928–934
Hurley LH (2002) Nat.Rev.2 188
Shanker K, Rohini R, Shravan kumar K, Muralidhar Reddy P, Ho YP, Ravinder (2009) Ru(II) complexes of N4and N2O2 macrocyclic Schiff base ligands, their antibacterial and antifungal studies. Spectrochim Acta A Mol Biomol Spectrosc 73(1):205–211
Raman N, Sobha S, Mitu L (2013) Design, synthesis, DNA binding ability, chemical nuclease activity and antimicrobial evaluation of Cu(II), Co(II), Ni(II) and Zn(II) metal complexes containing tridentate Schiff base. J Saudi Chem Soc 17:151–159
Sathiyaraj S, Sampath K, Raja G, Butcher RJ, Gupta SK, Jayabalakrishnan C (2013) DNA binding/cleavage, antioxidant and cytotoxic activities of water a soluble cobalt(II) and copper(II) antipyrine complexes. Inorg Chim Acta 406:44–52
Anupama B, Sunitha M, Kumari C, Gyana (2011) Synthesis, characterization, DNA binding and antimicrobial activity of copper (II) complexes with 4-aminoantipyrine schiff bases. Asian J Res Chem 4(10):1529–1535
Reichmann MF, Rice SA, Thomas CA, Doty P (1954) A further examination of the molecular weight an size of desoxypentose nucleic acid. J Am Chem Soc 76:3047–3053
Dixit N, Koiri RK, Maurya BK, Trigun SK, Hobartner C, Mishra L (2011) One pot synthesis of Cu(II) 2,2-bipyridyl complexes of 5-hydroxy-hydurilic acid and alloxanic acid: synthesis, crystal structure, chemical nuclease activity and cytotoxicity. J Inorg Biochem 105:256–267
Lakowicz JR, Webber G (1973) Quenching of fluorescence by oxygen probe for structural fluctuations in macromolecules. Biochemistry 12:4161–4170
Waheb ZH, Mashalay MM, Fahem AA (2005) Chem Pap 59:25
Alemi AA, Shaabani B (2000) Synthesis and characterizationof a p-tert-Butylcalix [4] arene and its complex with copper (II). Acta Chim Solv 47:363–369
Makode JT, Aswar AS (2004) Synthesis, characterization, biological and thermal properties of some new Schiff base complexes derived from 2-hydroxy-5-chloroacetophenone and S-methyldithiocarbazate. Indian J Chem A43(10):2120–2125
Naik VM, Mallur NB (2002) Synthesis and characterization of niobium(V) complexes with terdentate ONO donor hydrazones. Indian J Chem Sect A 41:780–784
Nakamoto K (1997) Infrared and raman spectra of inorganic and coordination compounds, 3rd edn. Wiley, New York
Rabindra Reddy P, Shilpa A, Raju N, Raghavaiah P (2011) Synthesis,structure, DNA binding and cleavage properties of ternary amino acidSchiff base-phen/bipy Cu(II) complexes. J Inorg Bio Chem 105:1603–1612
Yang L, La CA, Anderson OP, Crans DC (2002) 4-Hydroxypyridine-2,6-dicarboxylatodioxovanadate(V) complexes. Solid State Aqueous Chem Inorg Chem 41:6322–6331
Lever ABP (1971) Inorganic electronic spectroscopy, New York
Dunn TM (1960) The visible and ultraviolet spectra of complex compounds in moderm coordination chemistry interscience, New York
Rabindra Reddy P, Shilpa A (2011) Oxidative and hydrolytic DNA cleavage by Cu(II) complexes of salicylidene tyrosine schiff base and 1,10 phenanthroline/bipyridine. Polyhedron 30:565
Raman N, Kulandaisamy A, Jeyasubramanian K (2002) Synthesis,spectral,redox,and anti microbial activity of Schiff base transition metal(II)complexes derived from 4-aminoantipyrine and benzyl. Syn React Inorg Metal Org Nano Metal Chem 32:1583–1610
Raman N, Kulandaisamy A, Shanmugasundaram A (2001) Synthesis, spectral, redox and antimicrobial activities of Schiff base complexes derived from 1-phenyl-2,3-dimethyl-4-aminopyrazol-5-one and acetoacetanilide. Trans Met Chem 26:131–135
Nikolaev AV, Myachina LI, Logvinenko VA (1969) Therm Anal 2:779
Daniels T (1973) Thermal analysis. Wiley, New York
Freeman ES, Carroll BJ (1958) Phys Chem 62:39
Mishra AP, Khare M (2000) Non-isothermal degradation and kinetic parameters of some Schiff base complexes. J Indian Chem Soc 77:367–370
Choi SN, Menzel ER, Wasson JR (1977) Electronic spectra of copper(II) dithiocarbamates. J Inorg Nucl Chem 39:417
Kumar DN, Singh BK, Garg BS, Singh PK (2003) Spectral studies on copper(II) complexes of biologically active glutathione. Spec Chim Acta 59:1487–1496
Hathway BJ, Bardley GN, Gillard RD (1971) Eds, Essays in Chemistry Academic press Newyork, USA
Suresh Babu V, Ramesh A, Raghuram P and Raghava Naidu R(1997) Electron spin resonance studies on complexes of Copper(II) with o-phenolic oximes. Polyhedron1(7–8):607–610
Dhanaraj CJ, Nair MS (2009) Synthesis, characterization, and antimicrobial studies of some Schiff-base metal(II) complexes. J Coord Chem 62:4018–4028
Peng B, Chao H, Sun B, Li H, Gao F, Ji LN (2007) Synthesis, DNA-binding and photocleavage studies of cobalt(III) mixed-polypyridyl complexes: [Co(phen)2(dpta)]3+ and [Co(phen)2(amtp)]3+. J Inorg Biochem 101:404–411
Uma V, Kanthimathi M, Weyhermuller T, Nair BU (2005) Oxidative DNA cleavage mediated by a new copper(II) terpyridine complex: Crystal structure and DNA binding studies. J Inorg Biochem 99:2299–2307
Kumar CV, Asuncion EH (1993) DNA binding studies and site selective fluorescence sensitization of an anthryl probe. J Am Chem Soc 115:8547–8553
Kumar LS, Revanaslddappa HD (2011) Synthesis, characterization, antimicrobial, DNA binding, and oxidative cleavage activities of Cu(II) and Co(II) complexes with 2-(2-hydroxybenzylideneamino)isoindoline-1,3-dione. J Coord Chem 64:699–714
Wolfe A, Shimer GH, Meehan T (1987) Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. BioChem 26:6392
Fahmi N, Gupta IJ, Singh RV (1998) Sulfur bonded palladium(II)and platinum(II) complexes of biologically potent thioamides. Phos Sulfur Silicon 132:1–8
Chaudhary A, Singh RV (2003) Studies on biologically potent tetraazamacrocylic complexes of bivalent tin.Phos Sulfur. Silicon 178:615–626
Arguslab 4.0 Marky Thomson, Planaria software IC, Seattle, WA; www.arguslab.com/
Thompson MA, Zerner MC (1991) A theoretical examination of the electronic structure and spectroscopy of the photosynthetic reaction center from Rhodopseudomonas viridis. J Am Chem Soc 113:8210–8215
Thompson MA, Eric DG, Feller D (1996) J Phys Chem 98:10465
Acknowledgments
We thank to University grants commission (UGC, NEW Delhi, India) for financial support in the form of junior research fellow to me. We thank to HCU for extending the ESR facility and Department of Bio chemistry (Osmania University, Hyderabad) for microbial activity.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
leela, D.S., Ushaiah, B., Anupama, G. et al. Synthesis, Characterization, Antimicrobial, DNA Binding and Cleavage Studies of Mixed Ligand Cu(II), Co(II) Complexes. J Fluoresc 25, 185–197 (2015). https://doi.org/10.1007/s10895-014-1496-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1496-7