Abstract
Local adaptations of host plants to climatic conditions along an elevation gradient can affect insect-plant interactions. Using local accessions sampled from different elevations within South America, plant defense responses and herbivore growth were evaluated on two host plants: a) cherry tomato, Solanum lycopersicum var. cerasiforme, and b) wild tomato, Solanum pimpinellifolium. The elevational origin of the accessions ranged from 100 to 3000 m above sea level. We hypothesized a higher level of defensive compounds in plants originating from lower elevations and, consequently, stronger resistance to insect herbivory. Interestingly, plant resistance to insect herbivory, as demonstrated by a reduction in Helicoverpa zea growth, was stronger for middle and high-elevation accessions. Total phenolic content increased with elevation in both herbivore-damaged and undamaged leaves, augmenting plant resistance. However, an elevational gradient was not evident for plant defensive proteins (polyphenol oxidase and trypsin protease inhibitors) or the density of leaf trichomes. Tradeoffs between constitutive and induced defenses were evident in both tomato genotypes. Future studies should test the role of plasticity in plant defense systems in restricting or facilitating range expansion of insect herbivores with climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elevational gradients can result in large variations in environmental conditions over relatively short distances (Körner 2007). Therefore, such gradients can be used in natural experiments to simulate climate change scenarios (Khairiyah et al. 2013; Buckley et al. 2019). Studies of elevational gradients and insect-plant interactions have primarily addressed woody vegetation and perennial herbaceous plants in natural environments (reviewed by Moreira et al. 2018). Inferences drawn from such studies, often conducted in natural but disparate environments, may be confounded by differences in herbivore populations, abiotic conditions, or plant phenotypic plasticity (Ohsawa and Ide 2008; Rokaya et al. 2016). Common garden experiments under controlled climatic conditions may provide a better understanding of plant defense plasticity and its effect on insect-plant interactions (Rokaya et al. 2016; Shah et al. 2020).
At higher elevations, a low-level of herbivore abundance and plant damage are predicted due to harsher environmental conditions (Salgado et al. 2016; Wu et al. 2021). Such reduction in herbivory pressure is expected to reduce the level of constitutive plant secondary metabolites (PSMs) leading to a lower level of plant resistance (Dostálek et al. 2016; Kergunteuil et al. 2018; Pellissier et al. 2014; Robert et al. 2019). However, widespread applicability of this prediction is problematic; upon closer inspection, there are cases that do not fit the pattern. In some cases, defense investments were enhanced with elevation (Buckley et al. 2019; Galmán et al. 2019), while others have found no or non-linear associations (Buckley et al. 2019; Pellissier et al. 2014). These inconsistencies, from studies conducted mostly on trees and herbs, are indicative of a complex interaction between insects and plants in relation to elevation. Unfortunately, there is limited knowledge of variation in plant defense responses along elevation gradients in agricultural systems highly sensitive to both biotic and abiotic stressors (Smilanich et al. 2016; Smith and Beaulieu 2009).
Elevation also affects the level of induced PSMs in response to herbivore damage. In pine species (Family: Pinaceae), the level of induced resins was reduced with elevation, whereas constitutive defenses were elevated (Moreira et al. 2014). A similar reduction in inducibility of glucosinolates (GLS) with elevation in Cardamine species was reported by Pellissier et al. (2016). In contrast, induction of GLS was stronger in mid- and high-elevation populations of Arabis alpina (Family: Brassicaceae) (Buckley et al. 2019). It is important to note that these studies analyzed different kinds of PSMs, which are affected by herbivory pressure and also by an adaptive response to different environmental stresses (e.g., ultraviolet, temperature, CO2) (Moreira et al. 2018). PSMs, such as total phenolics content (TPC), flavonoids, proline, tannins, and ascorbic acid were higher in leaves from higher elevation plants in several endemic plants from different families, including the Lamiaceae, Rosaceae, and Caryophyllaceae (Hashim et al. 2020). Induction of jasmonate-induced proteins (e.g., protease inhibitors) in response to adverse abiotic factors at higher elevations was also reported in the same study. TPC in leaves was enhanced at higher elevations in Plantago lanceolate (Family: Plantaginaceae) and Medicago minima (Family: Fabacae), presumably as a protective strategy against high ultraviolet radiation (Kabtni et al. 2020). Similarly, increased leaf trichome densities in plants from higher elevation were reported for Nepeta heliotropifolia (Family: Lamiaceae; Talebi et al. 2019), Artemisia tridentata sp. Wyomingensis (Family: Asteraceae; Dyslin 2012), accessions of Solanum berthaultii (Family: Solanaceae; Horgan et al. 2009), and several other flowering plants (Wu et al. 2021). High-density trichomes protect plants from UV-B radiation and heat loss at higher elevations (Karabourniotis et al. 1998; Kofidis and Bosabalidis 2008). Knowledge of plant defense responses in plants modulated by a combination of herbivore pressure and abiotic factors should help improve predictions of plant growth under climate warming.
In the present study, we tested the hypothesis that the level of constitutive PSM and defensive proteins are higher in tomato accessions originating from a lower elevation and therefore strengthen plant resistance against herbivory (Buckley et al. 2019; Dostálek et al. 2016). Conversely, the strong inducibility of these defensive compounds in tomato accessions from higher elevations has been hypothesized (Rasmann et al. 2014). Plant responses to changes in biotic (insect pests) and abiotic effects (environmental variations) were assessed by measuring defensive proteins [polyphenol oxidase (PPO), trypsin protease inhibitors (TPI)], TPC, leaf trichome density, and herbivore bioassays. PPO and TPI play important roles in enhancing plant defenses in tomato by interfering with the digestive physiology of insect herbivores (Felton et al. 1989). The activity of TPI, PPO, and phenolics are affected by biotic and abiotic stresses in tomatoes (Lin et al. 2021; Paudel et al. 2020). Two wild relatives of tomato, Solanum lycopersicum var. cerasiforme (SLC) and S pimpinellifolium (SP), both native to the Andes in South America (Blanca et al. 2015), were used in this study. Experiments were conducted under common-garden conditions to control the abiotic habitat (Garrido et al. 2012).
Materials and Methods
Plant Materials
Tomato seeds were collected from the Andes Mountains in South America and obtained from the C.M. Rick Tomato Genetics Resource Center, UC Davis, Davis, CA, USA (https://tgrc.ucdavis.edu). Three accessions each of SLC and SP originated from a broad elevation range (100 m to 3000 m; Table 1) and were used for the experiments. One accession each from SP (LA 0400) and SLC (LA 2213) were sourced from a single plant; the rest of the accessions were from multiple individuals/populations. As both SP and SLC are of South American origin, we assumed that the plants used in the experiment are the product of generations of exposure to their respective elevational gradient (R. Chetelat, TGRC, personal communication, Sept 10, 2019). Plants were grown in a greenhouse environment (27 °C ± 2 °C, 60–70% RH, 16:8 L:D) at Penn State University campus in central Pennsylvania, USA. Seedlings were grown in Metromix 400 potting mix (Premier Horticulture, Quakertown, PA, USA) and watered every 1–2 d until the end of the experiment.
Insect Herbivore
Eggs of Helicoverpa zea were purchased from Benzon Research (Carlisle, PA, USA). Neonates were reared individually inside a plastic cup (30 ml) on a wheat germ and casein-based diet (Bioserv, Frenchtown, USA) until used for the experiment. Fifth instars were used to damage leaflets, whereas 1st instars were used for bioassay experiments (details below).
Defensive Proteins
Two groups of plants for the two treatments (damaged and undamaged) were randomly assembled from plants growing in the greenhouse. Constitutive and induced levels of two jasmonic acid (JA)-related defensive proteins, polyphenol oxidase (PPO) and trypsin protease inhibitor (TPI), were measured in undamaged and caterpillar-damaged leaves. At the four-leaf stage, fully expanded terminal leaflets were used as the focal leaves for defensive protein bioassays (Tan et al. 2018). Leaves were damaged, allowing a 5th instar H. zea to feed inside a clip cage (3.15 cm2) attached to a leaflet. Average time to consume the entire section of leaf tissue inside the clip cage was between 2 and 3 h. An empty clip cage from a separate plant was used as a ‘control’. Following the completion of feeding, the insect and the clip cage were removed from the plant. Leaf tissues (50 mg) from both damaged and control leaflets were collected 48 h later using liquid nitrogen and stored at −80 °C until analysis. Tissues from damaged leaves were collected around the edges bordering the herbivore-damaged area. Similar portions of leaf tissues were also collected from the control. PPO and TPI activities were measured using a spectrophotometric method described by Acevedo et al. (2017). PPO activity was determined based on reaction with caffeic acid and measured as the change in absorbance at 450 nm over 5 min. The values were then normalized by the amount of protein in the sample and expressed as mean optical density (mOD)/min/mg protein. Bradford assay, using Coomassie brilliant blue, was used to quantify protein content in leaf samples (Bradford 1976). TPI activity was estimated using an inhibition rate for the reaction between trypsin and p-toluene-sulfonyl-L-arginine methyl ester and calculated as % inhibition/mg protein. The protocol to measure TPI activity is described in detail by Zhao et al. (2019). The inducibility of defensive proteins was estimated by dividing the level of induced activity by the constitutive levels and expressed as fold change.
Total Phenolics Content
TPC was analyzed in both damaged and undamaged leaves using a colorimetric assay as described by Ainsworth and Gillespie 2007. Briefly, leaf tissues (20 mg) were harvested and stored as described above for defensive proteins. Leaf tissues were homogenized, and 1.5 ml ice-cold methanol (95%) was added and allowed to extract for 48 h in the dark. Samples were centrifuged and F-C reagent and Na2CO3 added to 100 ml of the supernatant. Absorbance was recorded at 765 nm using a spectrophotometer and the amount of total phenolics was calculated (μg/mg tissue) using gallic acid equivalents. Inducibility was estimated by dividing the level of induced activity by the constitutive levels and expressed as fold change.
Density of Leaf Trichomes
The youngest terminal leaflets from randomly selected three-week old tomato seedlings were used to compare the density of trichomes on the adaxial leaf surface (Paudel et al. 2020). Two leaf discs (0.6 cm diam.) were punched out from each side of the mid-vein of a leaflet. The density (number/cm2) of all glandular and non-glandular trichomes was determined by counting all trichomes under a light microscope.
Herbivore Bioassays
Herbivore feeding bioassays with excised leaves (damaged or undamaged) in a laboratory were used as a proxy for measuring plant resistance. Leaves were damaged using 5th instar H. zea as described above for defensive proteins. At 48 h post-damage, randomly selected 1st instars (n = 11–14/treatment) from a stock colony were weighed (‘day 0’) and placed into plastic cups (30 ml) with damaged and undamaged leaves under ambient temperature. Individual larvae were weighed 48 h later (‘day 2’) and the relative growth rate (RGR) was calculated as:
Wo and W2 are larval weight at days d0 and d2 (Waldbauer 1968).
Statistical Analysis
Activities of plant defensive proteins (PPO and TPI), TPC, and RGR of insect larvae were analyzed using a two-way ANOVA with the main effects being ‘tomato accessions’ and ‘insect treatment, damaged or undamaged, plus all interaction terms (Minitab Inc. 2018). Trichome density data were analyzed using one-way ANOVA with ‘tomato accessions’ as the main effect. PPO activity, TPI activity, and trichome density data were LOG10 transformed to meet the normality assumption; data were back transformed for presentation.
The relationships between RGR and PPO, TPI, or TPC were examined using linear regression (Minitab Inc. 2018). The average values for RGR, PPO, TPI and TPC were calculated for each tomato accession originating from different elevations and two treatments (damaged vs. undamaged), resulting in 12 data points (6 accessions × 2 treatments).
Results
Defensive Proteins
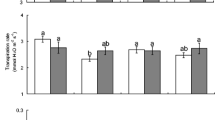
Activity of TPI in leaves from both tomato genotypes (SLC and SP) did not vary with elevation origin (SLC: F2, 35 = 0.30, p = 0.75, SP: F2, 35 = 0.07, p = 0.93), but was affected by insect damage (SLC: F1, 35 = 153.80, p < 0 .001, SP: F1, 35 = 33.90, p < 0.001) (Fig. 1a, b). Interactive effects of elevation origin and insect damage were significant for SLC accessions (F2,35 = 5.80, p < 0.001), but not for SP accessions (F2,35 = 0.4, p = 0.68).
Activity of trypsin protease inhibitor (TPI) in undamaged and damaged leaves from four-leaf stage tomato plants: a Solanum lycopersicum var. cerasiforme (SLC) accessions collected from three different elevations, LA 1286 (150 m), LA 2213 (800 m) and LA 4708 (2800 m), and b Solanum pimpinellifolium (SP) accessions collected from three different elevations, LA 0375 (100 m), LA 0400 (800 m), and LA 1547 (3000 m). Fifth instar Helicoverpa zea were used to damage leaves. Bars are mean ± SEM. Means with different letters are different (as determined by Tukey HSD, P < 0.05) among tomato accessions, whereas * denotes a difference between the damaged and undamaged plants within individual tomato accessions
Among SLC accessions, LA 2213 (800 m) had the highest TPI activity in undamaged leaves, whereas LA 1286 (150 m) had the highest post-herbivore damage. Inducibility of TPI was highest for LA 1286 (150 m) with a 3.3-fold increase, followed by LA 2213 (800 m; 2.0-fold increase) and LA 4708 (2800 m; 1.9-fold increase). Among SP accessions, LA 0375 (100 m) had the highest TPI activity in undamaged leaves, whereas LA 0400 (800 m) had the highest post-herbivore damage. Inducibility of TPI was highest for LA 1547 (3000 m) with a 2.6-fold increase, followed by LA 0400 (800 m; 2.1-fold increase) and LA 0375 (100 m; 1.8-fold increase).
Activity of PPO varied between SLC and SP accessions originating from different elevations. There was an effect of elevation origin within SLC accessions (SLC: F2,35 = 5.20, p = 0.01) but not for SP accessions (SLC: F2,35 = 1.70, p = 0.20) (Fig. 2a, b). Insect damage affected PPO activity in leaves (SLC: F1,35 = 133.40, p < 0.001, SP: F1,35 = 80.00, p < 0.001). Interactive effects of elevation origin of genotypes and insect damage were not significant for both SLC and SP accessions (SLC: F2,35 = 1.90, p = 0.20, SP: F2,35 = 1.40, p = 0.30).
Activity of polyphenol oxidase (PPO) in undamaged and damaged leaves from four-leaf stage tomato plants: a Solanum lycopersicum var. cerasiforme (SLC) accessions collected from three different elevations, LA 1286 (150 m), LA 2213 (800 m) and LA 4708 (2800 m), and b Solanum pimpinellifolium (SP) accessions collected from three different elevations, LA 0375 (100 m), LA 0400 (800 m), and LA 1547 (3000 m). Fifth instar Helicoverpa zea were used to damage leaves. Bars are mean ± SEM. Means with different letters are different (as determined by Tukey HSD, P < 0.05) among tomato accessions, whereas * denotes a difference between damaged and undamaged plants within individual tomato accessions
Among SLC accessions, LA 4708 (2800 m) had the highest PPO activity in undamaged leaves, whereas LA 1286 (150 m) had the highest post-herbivore damage. Inducibility of PPO was highest for LA 2213 (800 m) with a 11.5-fold increase, followed by LA 1286 (150 m; 9.3-fold increase) and LA 4708 (2800 m; 5.2-fold increase). Among SP accessions, LA 0375 (100 m) had the highest PPO activity in both damaged and undamaged leaves. Inducibility of PPO was highest for LA 1547 (3000 m) with a 20.5-fold increase, followed by LA 0400 (800 m; 10.8-fold increase) and LA 0375 (100 m; 8.3-fold increase),
Total Phenolic Content
TPC varied with elevational origin in both SLC and SP accessions (SLC: F2,29 = 9.12, p = 0.001, SP: F2,30 = 0.51, p = 0.001) (Fig. 3a, b). Insect damage, however, did not affect TPC activity in leaves (SLC: F1,29 = 3.19, p = 0.085, SP: F1,30 = 2.96, p = 0.096). Interaction effects of elevation origin of genotypes and insect damage were not significant for both SLC and SP accessions (SLC: F2,29 = 0.29, p = 0.75, SP: F2,30 = 2.02, p = 0.15).
Total phenolics content (μg/mg tissue) in undamaged and damaged leaves from four-leaf stage plants: a Solanum lycopersicum var. cerasiforme (SLC) accessions collected from three different elevations, LA 1286 (150 m), LA 2213 (800 m) and LA 4708 (2800 m), and b Solanum pimpinellifolium (SP) accessions collected from three different elevations, LA 0375 (100 m), LA 0400 (800 m), and LA 1547 (3000 m). Bars are mean ± SEM and means with different letters are different (as determined by Tukey HSD, P < 0.05) among tomato accessions
Among SLC accessions, LA 4708 (2800 m) had the highest activity of TPC in both damaged and undamaged leaves. Inducibility was highest for LA 2213 (800 m) with a 1.12-fold increase, followed by LA 1286 (150 m; 1.06-fold increase) and LA 4708 (2800 m; 1.03-fold increase). Among SP accessions, LA 1547 (3000 m) had the highest level of TPC in both damaged and undamaged leaves. Inducibility of TPC was highest for LA 0375 (100 m) with a 1.22-fold increase, followed by LA 0400 (800 m; 1.04-fold increase) and LA 0375 (100 m; 1.0-fold increase),
Density of Leaf Trichomes
On both SLC and SP accessions, elevation origin affected leaf trichome density on undamaged leaves (SLC: F2,60 = 3.90, p < 0.01, SP: F2,60 = 12.70, p < 0.001) (Fig. 4a, b). Trichome density in SP accessions was highest for LA 1286 (150 m) followed by LA 2213 (800 m) and LA 4708 (2800 m). Among SP accessions, the highest trichome density was observed in LA 2213 (800 m) followed by LA 1286 (150 m) and LA 4708 (2800 m).
Density of glandular and non-glandular trichomes (number of trichomes/cm2) on undamaged leaf surfaces (adaxial): a Solanum lycopersicum var. cerasiforme (SLC) accessions collected from three different elevations, LA 1286 (150 m), LA 2213 (800 m) and LA 4708 (2800 m), and b Solanum pimpinellifolium (SP) accessions collected from three different elevations, LA 0375 (100 m), LA 0400 (800 m), and LA 1547 (3000 m). Bars are mean ± SEM and means with different letters are different as determined by Tukey HSD
Herbivore Performance
On both SLC and SP accessions, RGR varied with elevation origin on undamaged leaves (SLC: F2,78 = 8.10, p < 0.01, SP: F2,78 = 17.70, p < 0.001) (Fig. 5a, b). Previous insect damage affected RGR in SLC accessions (F1,78 = 5.30, p = 0.02) but not for SP accessions (F1,78 = 2.20, p = 0.13). Among SLC accessions, RGR of H. zea was lowest on undamaged leaves from LA 4708 (2800 m), followed by LA 1286 (150 m) and LA 2213 (800 m). When fed on previously damaged leaves, RGR was lowest on LA 4708 followed by LA 2213 and LA 1286. The percent reduction in RGR was comparatively higher on damaged leaves from LA 2213. On average, RGR was reduced by 1.34-fold, 1.14-fold, and 1.04-fold on damaged leaves compared to undamaged leaves from LA 2213, LA 4708, and LA 1286, respectively. Interactive effects of elevation origin and insect damage were not significant for both genotypes (elevation × insect damage; SLC: F2,78 = 1.50, p = 0.23, SP: F2,78 = 0.06, p = 0.94).
Relative growth rate (RGR) (mass gained/g/day) of first instar Helicoverpa zea fed on detached leaves (damaged or undamaged): a Solanum lycopersicum var. cerasiforme (SLC) accessions collected from three different elevations, LA 1286 (150 m), LA 2213 (800 m) and LA 4708 (2800 m), and b Solanum pimpinellifolium (SP) accessions collected from three different elevations, LA 0375 (100 m), LA 0400 (800 m), and LA 1547 (3000 m). Fifth instar H. zea were used to damage leaves and the bioassay was conducted 48 h post-damage. Bars are mean ± SEM. Means with different letters are different (as determined by Tukey HSD, P < 0.05) among tomato accessions, whereas * denotes difference between damaged and undamaged plants within individual tomato accessions
Among SP accessions, RGR of H. zea was lowest on both undamaged and damaged leaves from LA 1547 (3000 m), followed by LA 0400 (800 m) and LA 0375 (100 m). The reduction in growth was also comparatively higher on damaged leaves from LA 1547. On average, RGR was reduced by 1.11-fold, 1.08-fold, and 1.05-fold on damaged leaves compared to undamaged leaves from LA 1547, LA 0375, and LA 0400, respectively. In both SLC and SP accessions, interactive effects of elevation origin and insect damage were not significant.
Statistical differences were not observed between the two wild tomato species (SLC and SP) based on linear regression analysis of RGR with TPI, PPO, and TPC. Therefore, the relationship between RGR and TPI, PPO, and TPC are presented using a single regression line regardless of the two wild tomato species (Fig. 6a–c). RGR of H. zea was negatively correlated with all three defensive chemicals (TPI, PPO, TPC) and was significant for TPC (F1,10 = 1.50, p = 0.002).
Relationship between relative growth rate (RGR) (mass gained/g/day) and defensive chemicals based on linear regression analysis on Solanum lycopersicum var. cerasiforme (SLC) and Solanum pimpinellifolium (SP) accessions: a Trypsin protease inhibitor (TPI) b Polyphenol oxidase (PPO), and c Total phenolics content (TPC). S. lycopersicum var. cerasiforme (SLC) accessions collected from three different elevations, LA 1286 (150 m), LA 2213 (800 m) and LA 4708 (2800 m), and b S. pimpinellifolium (SP). The two wild tomato species were not different; therefore, relationships are presented using a single regression line between RGR and defensive chemicals. Arithmetic value of regression line obtained from the analysis along with the p value are presented. Each dot represents average values of RGR and defensive chemicals for individual tomato accessions; SLC accessions are presented in black round circles, whereas red squares denotes SP accessions
Discussion
The elevational origin of tomato plants affected both host plant defenses and herbivory. Tomato accessions from mid and high elevations were most resistant against H. zea, whereas those originating from low elevation were most susceptible. There was a substantial variation in level of constitutive and induced PSMs between two tomato genotypes and among accessions within each genotype. This suggests a complex plant response to varying levels of herbivore pressure and environmental factors along elevational gradients.
Growth of H. zea was reduced on both damaged and undamaged leaves from higher elevation accessions for both tomato genotypes (SLC and SP). The level of TPC in leaves was higher on plants from higher elevations, which negatively affected herbivore growth rate, as documented in other systems (e.g., Oak, Quercus robur, Abdala-Roberts et al. 2016; Himalayan Yellow Sage, Salvia nubicola, Dostálek et al. 2016). The results, however, contrast with the general prediction of relatively less defensive investment at higher elevations due to decreasing herbivory rates (Moreira et al. 2018; Rasmann et al. 2014). This indicates that herbivore pressure alone is inadequate for explaining variations in plant defense investment strategies along elevational gradients. Therefore, the effect of changing abiotic conditions on plant defenses is likely an important consideration (Robert et al. 2019). For example, phenolic compounds were higher at high elevations (as found in the present study), presumably to protect plants from an increased level of UV radiation (Close and McArthur 2002). Increases in the levels of other PSMs, such as cyanogenic glycosides (CNglcs) and glucosinolates, at higher elevation have also been reported in Bird’s-foot trefoil, Lotus corniculatus (Salgado et al. 2016), and Cardamine species (Pellissier et al. 2016), respectively.
Elevational trends in constitutive and induced levels of defensive proteins were not apparent. However, tradeoffs between two defense strategies were evident for both tomato genotypes in accordance with previous studies (Kempel et al. 2011; Rasmann et al. 2014). Mid and high elevation SLC accessions exhibited the highest levels of constitutive TPI, PPO, and TPC. In contrast, the inducibility of these defenses was highest for low and mid-elevation accessions. Among SP accessions, higher levels of constitutive TPI and PPO were recorded in low elevation plants, whereas TPC was highest in plants originating from high elevations. Conversely, strong inducibility of TPI and PPO were recorded in high elevation plants, whereas TPC was highest in plants originating from the lowest elevation. Similar asymmetric patterns were also observed with leaf trichomes.
Concluding remarks and future directions
Variations in host plant defense responses to elevation, as observed in this study, may have important ecological consequences in the context of climate change. Such variations indicate plasticity in host plant responses adapted to different elevations or locations. As insect species expand their range further from the equator, the strength of genetic-based host plant defense mechanisms may play an important role in either restricting or facilitating range expansion (Marta et al. 2019; Shah et al. 2020). Further, crop losses are predicted to rise in the face of global climate change (Deutsch et al. 2018); therefore, variation in plant-defensive traits from plant genotypes/accessions adapted to different climates may be utilized to produce resilient agricultural crops (Paudel et al. 2019). Future studies should consider involving reciprocal transplants along a continuous elevation gradient at one or more sites to support the results and conclusions of this study. Similarly, multi-trophic interactions with a complete set of insect pests associated with a crop are necessary to further our understanding of how biotic and abiotic ecological factors, in combination with evolutionary processes, affect ecosystem dynamics.
References
Abdala-Roberts L, Rasmann S, Berny-Mier y Terán JC, Covelo F, Glauser G, Moreira X (2016) Biotic and abiotic factors associated with altitudinal variation in plant traits and herbivory in a dominant oak species. Am J Bot 103(12):2070–2078
Acevedo FE, Peiffer M, Tan CW, Stanley BA, Stanley A, Wang J, Felton G (2017) Fall armyworm-associated gut bacteria modulate plant defense responses. Mol Plant-Microbe Interact 30(2):127–137
Agrawal AA (1999) Induced plant defense: evolution of induction and adaptive phenotypic plasticity. In: Inducible plant defenses against pathogens and herbivores: biochemistry, ecology, and agriculture. American Phytopathological Society Press, St. Paul, pp 251–268
Agrawal AA, Conner JK, Johnson MT, Wallsgrove R (2002) Ecological genetics of an induced plant defense against herbivores: additive genetic variance and costs of phenotypic plasticity. Evolution 56(11):2206–2213
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2(4):875–877
Blanca J, Montero-Pau J, Sauvage C, Bauchet G, Illa E, Díez MJ et al (2015) Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genomics 16(1):1–19
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buckley J, Widmer A, Mescher MC, De Moraes CM (2019) Variation in growth and defence traits among plant populations at different elevations: implications for adaptation to climate change. J Ecol 107(5):2478–2492
Close DC, McArthur C (2002) Rethinking the role of many plant phenolics–protection from photodamage not herbivores? Oikos 99(1):166–172
Deutsch CA, Tewksbury JJ, Tigchelaar M, Battisti DS, Merrill SC, Huey RB, Naylor RL (2018) Increase in crop losses to insect pests in a warming climate. Science 361(6405):916–919
Dostálek T, Rokaya MB, Maršík P, Rezek J, Skuhrovec J, Pavela R, Münzbergová Z (2016) Trade-off among different anti-herbivore defence strategies along an altitudinal gradient. AoB Plants 8:plw026. https://doi.org/10.1093/aobpla/plw026
Dyslin M (2012) Big Wyoming sagebrush screens UV radiation more effectively at higher altitudes. Undergraduate Research Symposium, Minnesota State University, Mankato, MN, USA, April 9, 2012. https://cornerstone.lib.mnsu.edu/urs/2012/poster-session-A/8/. Accessed 22 June 2018
Felton GW, Donato K, Del Vecchio RJ, Duffey SS (1989) Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J Chem Ecol 15(12):2667–2694
Galmán A, Abdala-Roberts L, Covelo F, Rasmann S, Moreira X (2019) Parallel increases in insect herbivory and defenses with increasing elevation for both saplings and adult trees of oak (Quercus) species. Am J Bot 106(12):1558–1565
Garrido E, Andraca-Gómez G, Fornoni J (2012) Local adaptation: simultaneously considering herbivores and their host plants. New Phytol 193(2):445–453
Hashim AM, Alharbi BM, Abdulmajeed AM, Elkelish A, Hozzein WN, Hassan HM (2020) Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 9(7):1–23
Horgan FG, Quiring DT, Lagnaoui A, Pelletier Y (2009) Effects of altitude of origin on trichome-mediated anti-herbivore resistance in wild Andean potatoes. Flora-Morphology, Distribution, Functional Ecology of Plants 204(1):49–62
Kabtni S, Sdouga D, Rebey IB, Save M, Trifi-Farah N, Fauconnier ML, Marghali S (2020) Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci Rep 10(1):1–15
Karabourniotis G, Kofidis G, Fasseas C, Liakoura V, Drossopoulos I (1998) Polyphenol deposition in leaf hairs of Olea europaea (Oleaceae) and Quercus ilex (Fagaceae). Am J Bot 85(7):1007–1012
Kempel A, Schädler M, Chrobock T, Fischer M, van Kleunen M (2011) Tradeoffs associated with constitutive and induced plant resistance against herbivory. Proc Natl Acad Sci 108(14):5685–5689
Kergunteuil A, Descombes P, Glauser G, Pellissier L, Rasmann S (2018) Plant physical and chemical defence variation along elevation gradients: a functional trait-based approach. Oecologia 187(2):561–571
Khairiyah MS, Usman S, Suzita Y, Florinsiah L, Shahirah NN (2013) The effect of elevations on diversity and abundance of class Insecta at Taman Negara Gunung Ledang, Johor. In: 2013 IEEE business engineering and industrial applications colloquium (BEIAC), pp 246–250
Knapp S, Peralta IE (2016) The tomato (Solanum lycopersicum L., Solanaceae) and its botanical relatives. In: The tomato genome. Springer, Berlin, Heidelberg, pp 7–21
Kofidis G, Bosabalidis AM (2008) Effects of altitude and season on glandular hairs and leaf structural traits of Nepeta nuda L. Bot Stud 49(4):363–372
Körner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22(11):569–574
Lin PA, Paudel S, Afzal A, Shedd NL, Felton GW (2021) Changes in tolerance and resistance of a plant to insect herbivores under variable water availability. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2020.104334
Marta S, Lacasella F, Cesaroni D, Sbordoni V (2019) Effects of Holocene climate changes on alpine ecosystems: nonequilibrium dynamics drive insect species richness on alpine islands. J Biogeogr 46(10):2248–2259
Minitab (Version 18) (2018) Available online: http://www.minitab.com/en-US/products/minitab/default.aspx (accessed on 22 June 2020)
Moreira X, Mooney KA, Rasmann S, Petry WK, Carrillo-Gavilán A, Zas R, Sampedro L (2014) Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecol Lett 17(5):537–546
Moreira X, Petry WK, Mooney KA, Rasmann S, Abdala-Roberts L (2018) Elevational gradients in plant defences and insect herbivory: recent advances in the field and prospects for future research. Ecography 41(9):1485–1496
Ohsawa T, Ide Y (2008) Global patterns of genetic variation in plant species along vertical and horizontal gradients on mountains. Glob Ecol Biogeogr 17(2):152–163
Paudel S, Lin PA, Foolad MR, Ali JG, Rajotte EG, Felton GW (2019) Induced plant defenses against herbivory in cultivated and wild tomato. J Chem Ecol 45(8):693–707
Paudel S, Lin PA, Hoover K, Felton GW, Rajotte EG (2020) Asymmetric responses to climate change: temperature differentially alters herbivore salivary elicitor and host plant responses to herbivory. J Chem Ecol 46(9):891–905
Pellissier L, Roger A, Bilat J, Rasmann S (2014) High elevation Plantago lanceolata plants are less resistant to herbivory than their low elevation conspecifics: is it just temperature? Ecography 37(10):950–959
Pellissier L, Moreira X, Danner H, Serrano M, Salamin N, van Dam NM, Rasmann S (2016) The simultaneous inducibility of phytochemicals related to plant direct and indirect defences against herbivores is stronger at low elevation. J Ecol 104(4):1116–1125
Peralta IE, Spooner DM, Knapp S (2008) Taxonomy of wild tomatoes and their relatives (Solanum sect. Lycopersicoides, sect. Juglandifolia, sect. Lycopersicon; Solanaceae). Syst Bot Monogr 84:1–186
Ranc N, Muños S, Santoni S, Causse M (2008) A clarified position for Solanum lycopersicum var. cerasiforme in the evolutionary history of tomatoes (solanaceae). BMC Plant Biol 8(1):1–16
Rasmann S, Pellissier L, Defossez E, Jactel H, Kunstler G (2014) Climate-driven change in plant–insect interactions along elevation gradients. Funct Ecol 28(1):46–54
Rivero RM, Ruiz JM, Garcıa PC, Lopez-Lefebre LR, Sánchez E, Romero L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci 160(2):315–321
Robert CA, Pellissier L, Moreira X, Defossez E, Pfander M, Guyer A et al (2019) Correlated induction of Phytohormones and Glucosinolates shapes insect herbivore resistance of Cardamine species along elevational gradients. J Chem Ecol 45(7):638–648
Rokaya MB, Dostálek T, Münzbergová Z (2016) Plant-herbivore interactions along elevational gradient: comparison of field and common garden data. Acta Oecol 77:168–175
Salgado AL, Suchan T, Pellissier L, Rasmann S, Ducrest AL, Alvarez N (2016) Differential phenotypic and genetic expression of defence compounds in a plant–herbivore interaction along elevation. R Soc Open Sci 3(9):1–10
Shah AA, Dillon ME, Hotaling S, Woods HA (2020) High elevation insect communities face shifting ecological and evolutionary landscapes. Current Opinion in Insect Science. https://doi.org/10.1016/j.cois.2020.04.002
Smilanich AM, Fincher RM, Dyer LA (2016) Does plant apparency matter? Thirty years of data provide limited support but reveal clear patterns of the effects of plant chemistry on herbivores. New Phytol 210(3):1044–1057
Smith SA, Beaulieu JM (2009) Life history influences rates of climatic niche evolution in flowering plants. Proc R Soc B Biol Sci 276(1677):4345–4352
Talebi SM, Nohooji MG, Yarmohammadi M, Khani M, Matsyura A (2019) Effect of altitude on essential oil composition and on glandular trichome density in three Nepeta species (N. sessilifolia, N. heliotropifolia and N. fissa). Mediterranean Botany 40(1):81–93
Tan CW, Peiffer M, Hoover K, Rosa C, Acevedo FE, Felton GW (2018) Symbiotic polydnavirus of a parasite manipulates caterpillar and plant immunity. Proc Natl Acad Sci 115(20):5199–5204
Waldbauer GP (1968) The consumption and utilization of food by insects. Advances in insect physiology, 5, 229–288
Wu R, Lev-Yadun S, Sun L, Sun H, Song B (2021) Higher elevations tend to have higher proportion of plant species with glandular Trichomes. Front Plant Sci. https://doi.org/10.3389/fpls.2021.632464
Zhao A, Li Y, Leng C, Wang P, Li Y (2019) Inhibitory effect of protease inhibitors on larval midgut protease activities and the performance of Plutella xylostella (Lepidoptera: Plutellidae). Front Physiol 9:1963
Zuriaga E, Blanca JM, Cordero L, Sifres A, Blas-Cerdán WG, Morales R, Nuez F (2009) Genetic and bioclimatic variation in Solanum pimpinellifolium. Genet Resour Crop Evol 56(1):39–51
Acknowledgments
We thank Michelle Peiffer for her continuous assistance in carrying out the study. SP was funded through the Integrated Pest Management Innovation Lab (IPM IL), United States Agency for International Development (USAID) Agreement No. AID-OAA-L-15-00001. This research was also partially supported by US Department of Agriculture Grant AFRI 2017- 67013-26596 and National Science Foundation Grant IOS- 1645548.
Author information
Authors and Affiliations
Contributions
Conception and design: SP, GWF, and EGR; Data recording, analysis, and interpretation: SP, GWF, and EGR; Preparation of manuscript and review: SP, GWF, and EGR; Obtaining of funding: EGR and GWF.
Corresponding author
Ethics declarations
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Paudel, S., Felton, G.W. & Rajotte, E.G. Anti-Herbivore Resistance Changes in Tomato with Elevation. J Chem Ecol 48, 196–206 (2022). https://doi.org/10.1007/s10886-021-01341-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01341-3