Abstract
Animals, including herbivores and predators, use diet-mixing to balance their macro- and micronutrient intake. Recent work demonstrated that lady beetles fed only pea aphids from fava beans had reduced fitness caused by a deficiency of dietary sterols. However, beetles redressed this deficit by eating fava bean leaves. In the current study we used Coccinella septempunctata as a model to test the hypotheses that pea aphids are a poor sterol resource independent of their host plant, and that fava beans produce low quality prey regardless of aphid species. Additionally, we tested the reproductive rescue capacity of alfalfa and barley foliage compared to fava, and profiled the sterols of phloem exudates, foliage, and aphids reared on these different hosts. Beetle fecundity and egg viability was significantly better when provided pea aphids reared on alfalfa (compared to fava beans) and green peach aphids reared on fava plants. Alfalfa and barley leaves were not consumed by beetles and did not support beetle reproduction. The sterol profile of aphids largely reflected their host plant phloem. However, green peach aphids from fava acquired 125-times more sterol than pea aphids from fava. Our findings show how the sterol content of different host-plants can affect the third trophic level. Our results suggest that 1) prey quality varies depending on prey species, even when they occur on the same plant, 2) plant species can mediate prey quality, 3) host plant-mediated effects on prey quality partially drive omnivory, and 4) diet-mixing benefits growth and reproduction by redressing micronutrient deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diet mixing is a widespread behavioral trait that is employed by taxa across the animal kingdom. It is known to serve a number of specific functions (Hailey et al. 1998; Singer and Bernays 2003) including toxin dilution (Behmer et al. 2002; Singer et al. 2002), maximizing the intake of nutrients when they are available in space and time (Behmer et al. 2001; Coogan et al. 2014), providing complementary sets of nutrients (Joern et al. 2012; Unsicker et al. 2008), and supplementing a diet deficient in micronutrients (Ugine et al. 2019; Simpson and Raubenheimer 2015). Determining the evolutionary forces that drive food selection is an especially complicated issue when it comes to the diets of omnivores, which can choose to feed at multiple trophic levels. Uncovering the nutritional ecology of functional groups of consumers like omnivores can provide valuable insights into their behavior as they seek to optimize their diet for development, defense and reproduction.
Omnivores exist on a diet-mixing spectrum ranging from herbivores that occasionally consume animal tissues (phytozoophagy), to predators, like lady beetles, that sometimes consume plant and fungal tissues (zoophytophagy; Coll and Guershon 2002). Omnivores are able to switch between feeding on prey and plants when one resource becomes depleted (Sanchez 2008), or when host plant defenses make prey a more favorable option (Agrawal et al., 1999). Zoophytophagy is a particularly interesting phenomenon as these primarily-predacious animals feed down the food chain at least two trophic levels, which is a curious behavior because prey are generally thought of as being a nutritionally complete resource and a more nutrient-dense resource than foliage (Mayntz et al. 2005). However, examples of predators consuming foliage, pollen and other non-prey foods, and examples of reproductive failures of predators consuming prey-only diets are fairly common in the literature (Eubanks and Denno 1999; Leigh et al. 2018; Toft 2005 and references therein; Ugine et al. 2019, 2020; White et al. 2017). This suggests that not all prey provide an adequate balance of macro and micronutrients to support reproduction, or that prey contain toxic compounds that affect predator performance (White et al. 2017).
Zoophytophagy has been reported from a diverse array of taxa, ranging from sharks, cats and spiders, to true bugs, lady beetles, lacewings and mites (Anderson 1982; Brassler 1930; Gillespie and McGregor 2000; Leigh et al. 2018; Lundgren 2009; Nomikou et al. 2003; Nyffeler et al. 2016; Pathak and Khan 1994; Patt et al. 2003; Torres and Boyd 2009; Triltsch 1997 and 1999). This behavior is one avenue that predators can take to supplement their diet to ensure they obtain the proper balance of nutrients. Relatively few studies have attempted to document the impact of zoophytophagy on the life history parameters of any organism or determine the driving factors, nutritional or otherwise, behind the behavior. Most researchers simply report that it occurs. For lady beetles, the few studies that investigate zoophytophagy take the form of determining the non-target effect of toxicants like Bacillus thuringiensis Berliner in genetically modified plants or systemic insecticides on larval development and survival (Ahmad et al. 2006; Bai et al. 2005; Moser et al. 2008; Moser and Obrycki 2009; Pilcher et al. 1997). Only Lundgren et al. (2011) and Moser et al. (2008) investigated the effects of foliage on life-history traits of lady beetles. However, these studies determined the influence of plant feeding on fitness proxies and did not measure actual beetle fitness or link beetles’ behavior to the need for a specific macro or micronutrient.
Recently, we showed that sevenspotted lady beetles (Coccinella septempunctata L.) reared on a diet of pea aphids (Acyrthosiphon pisum Harris) were unable to lay viable eggs due to insufficient sperm production by males (Ugine et al. 2019). We also showed that beetles readily engaged in herbivory and consumed fava bean foliage (Vicia faba L.) to obtain sterols (e.g. cholesterol). This behavior served to rescue sperm production and beetle fitness. Additionally, we reported that seven lady beetle species distributed across the three clades of the tribe Coccinellini (Escalona et al. 2017) all share this behavioral trait and employ it to obtain supplemental nutrients (sterols) to improve their fitness when they develop on sterol-poor aphid species (Ugine et al. 2020). Sterols are essential nutrients that have a number of essential functions in eukaryotes. They are the base molecule for insect molting hormones (Behmer and Nes 2003), a major component of plasma membranes (Carvalho et al. 2010; Tomazic et al. 2011), and an integral structural element of the sperm individualization complex that separates sister spermatids from one another (Ma et al. 2010). Arthropods are unable to produce sterols de novo and need to obtain them from their food (Jing and Behmer 2020).
There is a rich literature on the effect of aphid species on the fitness of a diverse array of lady beetles, with many examples of aphid species that do not support lady beetle growth and or reproduction (Hoděk and Evans 2012). A handful of studies have investigated tritrophic interactions between aphids, their host plant and predator fitness (Al-Zyoud et al. 2005; Du et al. 2004; Francis et al. 2001 and refs therein; Giles et al. 2002; Le Rü and Mitsipa 2000; White et al. 2017; Wu et al. 2010). While several of these reports link poor performance on the part of the predator to known plant defensive compounds or the relative abundance of amino acids in aphids, none resolve empirically what aspect of aphids’ composition changes to affect predator performance. Additionally, there is no literature on the palatability of different host plants to lady beetles or their reproductive rescue capacity when lady beetles are grown on sterol-poor prey.
We know from our previous work that pea aphids from fava beans do not provide sufficient quantities of sterols to lady beetles to maximize their fitness (Ugine et al. 2019, 2020). However, the mechanism underlying pea aphids’ low sterol content is unclear. This led us to test two broad hypotheses that we address in the current paper: 1) pea aphids are generally sterol deficient independent of their host plant, and 2) fava beans generate sterol-poor aphids regardless of aphid species. We addressed these hypotheses in a series of four experiments using C. septempunctata as our model lady beetle. This species was successful introduced into North America and is now ubiquitous. Additionally, it suffers a near total loss of fitness when fed a sterol-limited diet making it ideal for studying the effects of sterols on reproductive success (Ugine et al. 2019, 2020).
Methods and Materials
Plants
Fava beans (var. Windsor) were used to maintain aphid colonies; they were also used in experiments. Four beans were placed at the bottom of 10.2 cm diam. Pots containing LM-series professional growing media (Lambert; Quebec City, QC, Canada) and were irrigated daily with tap water. Barley seeds (Hordeum vulgare L.) for use in colonies and experiments were purchased from Howe Seeds (McLaughlin, SD, USA). Sixty to 80 barley seeds were placed on top of 5 cm of growing media in 10.2 cm diam. Pots and were covered with another 2 cm of media before being irrigated thoroughly with tap water. Sprouted barley seeds were moved into tray liners without holes for sub-irrigation. Alfalfa seeds (Medicago sativa L., var. N-R-GEE) were purchased from SeedWay (Hall, NY, USA), coated with rhizobia purchased at Agway/True Value of Ithaca (Ithaca, NY, USA), and planted singly into 10.2 cm diam. Pots containing the aforementioned potting mix. All plant species were initially grown in a common greenhouse maintained at 23 ± 2 °C, ambient relative humidity, and a 16:8 h L:D cycle, that was supplemented with artificial light as needed.
Insects
Adult C. septempunctata were collected locally on a farm in Harford, NY, USA and were used to initiate a colony. Adult lady beetles were maintained singly in 44 ml lidded clear plastic cups containing a 2 × 7 cm piece of paper towel. Five to seven holes were punched into lids using a metal dissecting probe for ventilation. Mated female beetles were transferred daily to new cups containing a fresh piece of paper towel, an excised fava bean leaf, and an ad libitum diet of mixed-aged pea aphids grown on and collected from fava bean plants. Cups containing eggs were maintained at 25 °C under a 16:8 h L:D cycle and were monitored daily for egg hatch. This data was used in the calculation of development times. Neonate larvae (<24 h old) were used to initiate all experiments.
Aphids
Fifty to one hundred mixed-aged pea aphids were added to pots containing four 1-day-old fava bean seedlings. Mixed-aged aphids were collected from plants 7–10 d later. Pots of beans were kept in an environmental growth chamber at 23 ± 2 °C under 16:8 h L:D. Pea aphids from this colony (the same clone) were also used to inoculate 2–3 month-old alfalfa plants, which were kept in an independent environmental growth chamber under identical conditions. Green peach aphids were collected from greenhouse-grown pansy plants (Viola tricolor var. hortensis DC.) and were used to inoculate fava bean seedlings. They were maintained on fava bean plants for 2–3 generations before being used for experiments, and the colony was maintained as described for pea aphids. Bird cherry-oat aphids, Rhopalosiphum padi L., were collected locally and maintained on 7–21 d old barley plants before being transferred to new 7 d-old plants.
All aphid colonies were kept in separate environmental growth chambers at 23 ± 2 °C and a photoperiod of 16:8 h (L:D), and were watered as-needed with tap water. Each aphid species was collected from plants for use in experiments by gently tapping the infested plants over a 1.9 L bucket whose walls were coated with Insect-a-Slip (BioQuip, Rancho Dominguez, CA, USA) to prevent aphids from escaping.
Experiment 1. Test of Aphid and Host Plant Effect in the Absence of Foliage
This experiment was designed to test two main hypotheses: 1) pea aphids are inherently poor-quality prey for C. septempunctata independent of their host plants, and 2) fava bean plants generate low quality aphids independent of aphid species. Neonate larvae from 5 to 10 females were pooled and arbitrarily assigned to one of four diet treatments. Larvae were maintained singly in 44 ml cups and provided an ad libitum diet of pea aphids reared on (1) fava bean or (2) alfalfa plants, or (3) green peach aphids from fava beans. Because both fava beans and alfalfa are legumes (Fabaceae), we tested the nutritional suitability of another common plant-aphid combination from a different plant family. For this, we reared (4) bird cherry-oat aphid, a common pest of cereals (Poaceae), on barley. In all four treatments lady beetles had access to aphids, but no plant foliage. We recorded the development time and survival of larvae to adulthood.
Beetles were sexed upon eclosion and mating pairs of beetles within a treatment were established 7 d post-eclosion. Female beetles were provided aphids (ad libitum) from their respective treatments and were transferred to new cups daily for seven consecutive days. We counted the number of eggs laid per female on each day. Cups containing eggs were maintained at 25 °C for 3–4 d, at which time the number of viable eggs was determined and recorded. Cups containing larval and adults were maintained in a climate-controlled incubator at 25° ± 2 °C with a 16:8 h L:D cycle and ambient relative humidity. The experiment was conducted four times with 4–14 mated females per treatment each time; there was a total of 18, 30, 32 and 45 replicate beetles reared on diets of (1) pea aphids/alfalfa, (2) green peach aphids/fava, (3) pea aphids/fava, and (4) bird-cherry oat aphids /barley, respectively.
Experiment 2. Test of Host Plant Effect with Foliage Present
Given the potential limitations of experiments conducted in small cups, we conducted an experiment with aphids on potted plants to test the effect of aphid species plus aphid host plant under more natural conditions; as in experiment 1, there were four treatments. Single fava bean seeds, 60–80 barley seeds, or 3–5 alfalfa seeds were potted into 10.2 cm diameter pots and allowed to grow for 7–10 days (fava beans and barley) and 5–6 weeks (alfalfa) in a greenhouse at 22–25 °C with supplemental lighting to maintain 16:8 h L:D. We added approximately100 mixed-aged pea aphids to pots of fava beans and alfalfa, 100 mixed-aged green peach aphids to pots containing fava bean seedlings, and 100–200 mixed-aged bird-cherry oat aphids to pots of barley seedlings. Neonate lady beetles (<24 h old) from 5 to 10 females were pooled and placed singly onto the potted plants. We then covered plants individually with micro-perforated plastic bread bags (160 holes per 6.45 cm2, Prism Pak Inc. Berwick, PA, USA). These bags fit around the plant pots tightly enough that no aphids or lady beetles could escape. They also provided enough ventilation such that there was little-to-no condensation within the bags. Bagged plants were placed into non-perforated trays for sub-irrigation with tap water and were maintained in the greenhouse. There were many aphids remaining on all plants at the end of larval development, indicating beetles had sufficient food. We set up 30 replicate pots for each aphid-plant combination on two independent occasions, except for pea aphids on alfalfa, which we set up on a single occasion with 40 replicate pots. Adult beetles were removed from their bags 7 d post-eclosion. We noted their development time in days and the beetles were sexed and mated within a treatment group. Because our goal in this study was to determine the nutritional value of aphids during the pre-reproductive period (i.e. do lady beetles consume enough food of sufficient quality to lay viable eggs, which we know is male-dependent), mated female beetles were transferred to 44 ml plastic cups and provided a diet of pea aphids from fava beans. Female beetles were transferred to new cups daily for seven consecutive days, and the number of eggs laid per female on each day was recorded. Cups containing eggs were maintained at 25 °C for 3–4 d, at which time we recorded the number of viable eggs. Cups containing adult females were maintained in a reach-in environmental incubator at 25° ± 2 °C with a 16:8 h L:D cycle and ambient relative humidity.
Experiment 3. Rescue Capacity of Different Plants on Beetle Development and Reproduction
Our previous studies demonstrated that lady beetles recognize fava bean foliage as food (Ugine et al. 2019, 2020), and that larvae and adults will consume fava bean foliage when provided a sterol-poor diet of pea aphids reared on fava beans. We tested the hypotheses that C. septempunctata larvae and adults recognize other species of plants as food and that their availability supports adult reproduction. Neonate C. septempunctata larvae (<24 h old) from 5 to 10 females were pooled and distributed arbitrarily into 44 ml lidded plastic cups containing a piece of paper towel (2 × 7 cm), an ad libitum diet of mixed-aged pea aphids from fava beans and: 1) no foliage (the control), 2) a fava bean leaf, 3) three trifoliate alfalfa leaves including the petiole, or 4) three 5 cm-long sections of barley leaves. Cups containing larvae were kept in a reach-in environmental cabinet at 25 ± 2 °C with a 16:8 h L:D cycle, and the temperature was monitored every 15 min using a Hobo electronic data logger (Onset, Bourne, MA, USA). Every 24 h larvae were scored as dead or alive, all live and dead aphids were removed and exchanged for freshly collected pea aphids, foliage was replaced, and 75 μl of tap water was applied to the paper towel to help maintain leaf freshness. We recorded the day that each adult eclosed and used this data to compare the development times among treatment groups.
Upon eclosion, adult beetles were provided foliage and aphids. Seven days post-eclosion, adult beetles were sexed and paired for mating (one male + one female) within a treatment. Once copulation had ceased, male and female beetles were separated and the males were discarded. Female beetles were provided a diet of pea aphids from fava beans, and transferred to new cups daily for 5 d without supplemental foliage. The number of eggs laid by each female was quantified daily and the eggs were held for 3–4 d at 25 °C to assess egg viability. The entire experiment (randomized complete block design with experimental date as blocks) was conducted on five separate occasions with between 5 and 12 individuals per treatment per occasion for a total of 35 (alfalfa), 34 (barley), 41 (fava) and 38 (no foliage; the control) replicates.
Experiment 4. Phloem, Leaf and Aphid Sterol Content
Given the importance of sterols for lady beetle sperm production, female viability, and beetles’ propensity to feed on foliage to obtain sterols, we profiled the sterol content of phloem and leaf tissue from (1) fava beans (800, 700 and 900 leaves per sample), (2) alfalfa (250, 800, 1000 leaves), and (3) barley (1000 leaves per sample). Additionally, we determined the sterol content of pea aphids from (1) fava bean and (2) alfalfa, (3) green peach aphids from fava bean, and (4) bird-cherry oat aphids from barley to assess how host plant sterol content affects aphid sterol content.
We collected phloem exudates from fava bean, alfalfa, and barley leaves using the EDTA phloem extraction protocol described in Karley et al. (2002) and Bouvaine et al. (2012). Leaves were excised from 2 to 3 wk. old plants and their petioles were immediately placed into 1 ml of 10 mM EDTA, pH 7.5. Leaves were kept in a high humidity chamber, in the dark, at 25 °C for 6 h. The leaves were then removed from the EDTA, the petioles were rinsed with water and the samples were stored at −20 °C prior to processing. We removed leaves from fava, alfalfa and barley and the sterol profile was characterized and quantified. Samples were homogenized with 1:1 chloroform:methanol. The samples were then incubated at room temperature for 24 h, evaporated and resuspended in 3 ml of hexane. The hexane fraction was washed with three volumes of 70% methanol:water-equilibrated hexane. The hexane sample was further processed and examined for sterol content. Following base saponification, free sterols and sterols freed following saponification were converted to TMSi derivatives and identification and quantification by GC–MS following previous methods described by Jing et al., 2013 and Ugine et al., 2019. The GC–MS ions from purified standards (Steraloids Inc. RI) were used in the identification of the sterols described in this study. We extracted three replicate samples of phloem and leaf tissue per host plant.
Finally, we analyzed the sterol content of (1) bird cherry-oat aphids grown on barley (four samples each 2.4–3.5 dry g), (2) green peach aphids grown of fava beans (three samples 1.1–3.3 dry g), (3) pea aphids grown on fava beans (two samples 2.5–3.7 dry g), and (4) pea aphids grown on alfalfa (11 samples 1.2–5.9 dry g). Aphids were lyophilized prior to extraction. The processing, extraction and cleanup procedures of freeze-dried aphids are described in detail in Ugine et al. (2019). Free sterols and sterols freed following saponification and hydrolysis were quantified by gas liquid chromatography-fid (GLC-fid) by comparison with authentic standards, and structures were verified by GCMS as described in Jing et al. (2013) and Ugine et al. (2019).
Statistical Analysis
All statistical analyses were conducted using the statistical packages JMP® Pro (version 11.0; SAS Institute, Carey, NC, USA) and SAS Pro software (Version 9.3, Carey, NC, USA). For all three experiments we conducted the same generalized series of analyses. The development time of beetles (in days) from neonate larvae to adulthood was analyzed using mixed-model analysis of variance (SAS: JMP Pro 11). We modeled the experimental repetition as a random block effect, and treatment as a fixed effect. Post-hoc multiple comparisons were performed using Tukey’s HSD (alpha =0.05). The survival of beetles to the adult stage and the proportion of female that laid any viable eggs during the test periods (female viability) were analyzed separately using logistic regression via the generalized linear model platform in JMP. We modeled our data using a binomial distribution with a logit link function and included the fixed effects block (experimental replicate) and treatment. Post-hoc multiple comparisons were performed using pre-planned contrasts, which were evaluated after controlling for multiple comparison via the Bonferroni correction. The total number of eggs laid by females in each experiment was square-root transformed to normalize the distribution and analyzed using mixed-model analysis of variance, with experimental block modeled as a random variable and treatment as a fixed effect. Post-hoc multiple comparisons were made using Tukey’s HSD (alpha = 0.05). The percentage of eggs that hatched as a function of the total laid was analyzed using PROC GLIMIX (SAS Pro 9.3). The response distribution was modeled as a binomial and we used a logit link function. Experimental block was modeled as a random variable and treatment was modeled as a fixed effect. Post-hoc multiple comparisons were performed using the lsmeans/pdiff adjust = Tukey command, to maintain a family-wise error rate at alpha = 0.05.
Sterol amounts in phloem, foliage and aphid tissues were analyzed via ANOVA. We divided the phloem sterol amounts by the number of leaves that were extracted, foliage sterol amounts by their dry weight, and the aphid sterol amounts by the number of dry grams of aphid extracted to normalize the data. Post-hoc analyses were conducted using Tukey’s HSD.
Results
Experiment 1. Test of Aphid and Host Plant Effect in the Absence of Foliage
We observed a significant main effect of treatment on development times (F(3,120) = 11.9, P < 0.0001). However, there was no significant difference as a function of host plant (pea aphids grown on fava versus alfalfa; see Table 1), or effect of aphid species within a host plant (green peach versus pea aphids, both grown on fava beans; see Table 1). Beetles reared on pea aphids from fava beans versus alfalfa (host plant effect) eclosed after 14.5 and 14.2 d, respectively; beetles reared on green peach aphids from fava beans (aphid species effect) eclosed after 14.5 d. In contrast, beetles provided bird-cherry oat aphids took significantly longer to develop (15.0 d; Table 1). Immature survival did not differ among treatments (χ2 = 5.3, 3 df, P = 0.15, Table 1).
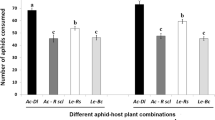
The seven-day fecundity differed significantly as a function of the aphid species/host plant treatment (F(3, 117) = 12.5, P < 0.001; Fig. 1a). Beetles receiving pea aphids from fava beans laid significantly fewer eggs than those receiving green peach aphids from fava beans (t-ratio − 5.5, 1 df, P < 0.0001). There was no difference in the total number of eggs laid by females receiving pea aphids from fava beans versus alfalfa (host plant effect: t-ratio − 0.6, 1 df, P = 0.54), and beetles provided bird-cherry oat aphid laid more eggs than beetles provided pea aphids from fava beans (t-ratio − 4.0, 1 df, P < 0.0001).
The effects of aphid species and aphid host plant on lady beetle performance in the absence of foliage (Experiment 1). Coccinella septempunctata larvae were provided pea aphids reared on fava bean or alfalfa (host pant effect), green peach aphids reared on fava bean (compared to pea aphids reared on fava beans to assess the aphid species effect), or bird-cherry oat aphids reared on barley – throughout development to adulthood. Bars represent the mean (±SE): a) eggs laid per 7 d; b) the proportion of females that laid any viable eggs; c) the proportion of viable eggs laid, excluding females that laid no viable eggs. Bars with different uppercase letters above them indicted significant differences (Tukey’s HSD)
There was also a significant effect of the aphid-host plant combination on the percentage of females that laid viable eggs during the 7 d test period (χ2 = 31.7, 3 df, P < 0.0001, Fig. 1b). First, we observed a significant host plant effect (χ2 = 4.8, 1 df, P = 0.03, Fig. 1b) when pea aphids were reared on fava versus alfalfa. Second, significantly more lady beetles produced viable eggs when they were provided green peach aphids from fava beans, compared to females fed pea aphids from fava beans (χ2 = 23.4, 1 df, P < 0.001). Female beetles fed bird-cherry oat aphids (from barley) produced significantly more viable eggs compared to beetles fed pea aphids from fava beans (χ2 = 17.5, 1 df, P < 0.0001). While 17.8% of females from the pea aphid fava bean treatment produced viable eggs, it is noteworthy that three quarters of these females laid ≤8 viable eggs during the 7d test period, and 75% of all the viable eggs were produced in the first clutch.
The proportion of eggs that were viable was affected by the host plant/aphid species combination (F(3,111) = 521.3, P < 0.0001; Fig. 1c) with all treatments differing significantly from each other with the exception of bird-cherry oat aphid from barley and pea aphids from alfalfa (bird-cherry oat aphid/barley = pea aphid/alfalfa > green peach aphid/fava > pea aphid/fava).
Experiment 2. Test of Host Plant Effects with Foliage Present
We found a significant effect of the aphid–host plant treatment on the development times of beetles (F(2,159) = 10.2, P < 0.0001). Beetles reared on fava bean plants infested with green peach aphids developed into adults significantly faster (ca. 1 d) compared to beetles reared on fava beans infested with pea aphids and barley infested with bird-cherry oat aphids (Table 1); unfortunately, we neglected to record development time data for beetles reared on alfalfa infested with pea aphids. Survival to adulthood was high in all treatments (87–97%) and did not vary with treatment (χ2 = 3.7, 3 df, P = 0.29; Table 1).
The seven-day fecundity was significantly affected by the plant-aphid treatment used to rear beetles (F(3,70) = 4.5, P = 0.006; Fig. 2b). Lady beetles from fava bean plants with pea aphids laid significantly more eggs (1.8 times more) compared to lady beetles from barley with bird-cherry oat aphids (Fig. 2a), but there were no other significant differences (Fig. 2a). However, we did not observe a significant treatment effect on the percentage of females that produced viable eggs (χ2 = 6.7, 3 df, P = 0.08, Fig. 2b). The proportion of eggs that hatched from eggs laid during the 7 d test period was significantly affected by the aphid-host plant treatment (F(3,69) = 243.9, P < 0.0001). This treatment effect was strong as all treatments differed significantly from each other (Fig. 2c).
Neonate C. septempunctata were reared to 7 d-old adults on potted fava bean plants infested with either green peach aphids or pea aphids, alfalfa infested with pea aphids, or barley infested with bird-cherry oat aphids (Experiment 2). Adult beetles were then mated within a treatment. Bars represent the mean (±SE): a) eggs laid per 7 d; b) the proportion of females that laid any viable eggs; c) the proportion of viable eggs laid, excluding females that laid no viable eggs. Bars with different uppercase letters above them indicted significant differences (Tukey’s HSD)
Experiment 3. Rescue Capacity of Different Plants on Beetle Development and Reproduction
The development time of lady beetles differed depending on which foliage was present (F(3,428) = 4.3, P = 0.006). Beetles reared with alfalfa leaves developed slower compared to beetles reared with fava bean foliage (Table 1), although only by one third of a day. The type of foliage present in an arena also affected the survival of larvae to adulthood (χ2 = 9.7, 3 df, P = 0.02), but in generally survival was high among all the different treatments and ranged from 74 to 87% (Table 1). Beetles that were reared with fava bean leaves suffered slightly higher rates of mortality (13% more) compared to the no-leaf treatment; the survival of beetles in the remaining treatments did not differ from each other (Table 1).
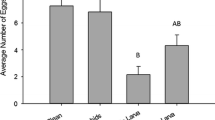
The plant species used to supplement a diet of pea aphids reared on fava bean had a significant effect on the total number of eggs laid during the test period (F(3, 141.5) = 3.6, P = 0.02). Post-hoc comparisons showed that beetles provided fava bean leaves laid significantly more eggs (1.9 times more) than beetles receiving barley leaf supplements, and there were no differences among the number of eggs laid by females receiving barley, alfalfa or the no-leaf control (Fig. 3a). We also found a significant treatment effect on the percentage of females that laid viable eggs (χ2 = 30.7, 3 df, P < 0.0001, Fig. 3b). A significantly greater percentage of female beetles that received fava bean as a leaf supplement laid viable eggs compared to females that received alfalfa as a supplement (χ2 = 10.0, 1 df, P = 0.001), barley as a supplement (χ2 = 8.6, 1 df, P = 0.003), or no leaf supplement (χ2 = 28.1, 1 df, P < 0.0001). Lady beetle larvae, especially fourth instar and adult beetles, readily feed on the midrib and edges of fava bean leaves, and sometimes chewed holes directly through the leaf. It is interesting to note that while we did not observe feeding damage on barley or alfalfa leaves, more females (ca. 10% more) that received these two plants laid viable eggs compared to the no-leaf control group (χ2 = 5.1, 1 df, P = 0.02). Finally, females provided fava bean leaves laid a significantly higher percentage of eggs that hatched compared to the other treatments (F(3,137) = 403.9, P < 0.0001), and each treatment differed significantly from each other (Fig. 3c).
Neonate C. septempunctata were reared on a diet of pea aphids from fava beans and provided no leaf supplement, or a barley, fava or alfalfa leaf supplement throughout development and for 7 d into adulthood (Experiment 3). Bars represent the mean (±SE): a) number of eggs laid during the first 5 d of oviposition; b) the proportion of females that laid any viable eggs; c) the proportion of viable eggs laid, excluding females that laid no viable eggs. Bars with different uppercase letters above them indicted significant differences (Tukey’s HSD)
Experiment 4. Phloem, Leaf and Aphid Sterol Content
We found significant differences in the amount of sterol in the phloem of the different plants (F(2,6) = 108.9, P < 0.0001; Fig. 4a). Alfalfa phloem contained more total sterol per leaf compared to fava and barley leaves, which did not differ from each other. The alfalfa sterol profile was dominated by 22-dihydrospinasterol and cholesterol, which comprised 88% of the total sterol pool. Fava bean and barley phloem contained a majority of sitosterol and stigmasterol, which comprised 84% and 94% of the total sterol pool per plant species, respectively. Similarly, we found a significant effect of plant species on the sterol content of foliage (F(2,6) = 9.6, P = 0.01). Fava foliage contained significantly more sterol per dry gram of tissue than barley and alfalfa, which did not different from each other (Fig. 4b). Alfalfa foliage had small but appreciable amounts of delta-7 (∆7) sterols (7-campesterol, 22-dihydrospinasterol, spinasterol), whereas fava and barley foliage only maintained ∆5 sterols (campesterol, cholesterol sitosterol, stigmasterol).
Sterol content of leaves and phloem collected from fava beans, alfalfa and barley (Experiment 4). Panel a) shows the total sterol pool and the mean (±SE) μg of the different sterol species found in fava bean, alfalfa and barley phloem (on a per leaf basis). Panel b) shows the total sterol pool and the mean (±SE) μg of sterol species found in leaves (per dry gram of tissue) from the same three plant species
We found that green peach aphids reared on fava beans had significantly higher levels of sterols compared to pea aphids reared on fava beans (Fig. 5). Additionally, pea aphids reared on alfalfa acquired more total sterols than the same clone reared on fava beans (Fig. 5). Generally, pea aphids and bird-cherry oat aphids had very low tissue sterol levels.
Discussion
Overall, we found that the host plant on which aphids develop affected the performance and fitness of C. septempunctata. When we provided lady beetles pea aphids reared on fava beans versus alfalfa in the absence of foliage, fewer females laid viable eggs and fewer fertile eggs were laid. Additionally, pea aphids reared on alfalfa contained 22-times more sterol compared to pea aphids reared on fava beans. This result is consistent with our previous findings that the sterol content of aphids dictates beetle fitness (Ugine et al. 2019, 2020). Our hypothesis that fava beans generally produce low-quality aphids across aphid species was not supported. Green peach aphids from fava beans supported reproduction, whereas pea aphids from fava beans did not. This is explained by the higher sterol content of green peach aphids compared to pea aphids reared on fava beans; they contained 125-times more sterol when both were reared on fava beans.
When we provided sterol-deficient lady beetles alfalfa or barley foliage, we found that they were poor supplements compared to fava bean foliage. Both alfalfa and barley foliage contain large amounts of common phytosterols that can rescue the fitness of sevenspotted lady beetles (Ugine et al. 2019), yet alfalfa and barley supplements only yielded a modest 10% increase in the percentage of female that laid any viable eggs compared to controls. We believe this was largely due to lower palatability of alfalfa and barley foliage. We did not observe any feeding damage on these tissues, whereas fava bean leaves typically had visible amounts of herbivory along the edges of leaves and along the abaxial leaf midrib. Our findings that alfalfa and barley leaf supplements do not result in a large percentage of females laying viable eggs, or a large increase in the absolute number of viable eggs laid, coupled with the lack of observable feeding damage suggests that C. septempunctata does not feed on foliage indiscriminately.
We know from laboratory studies that sterol-deficient beetles across the Coccinellini readily consume fava bean leaves to obtain supplemental sterols, and that this behavior allows male lady beetles to mature more sperm compared to non-supplemented individuals (Ugine et al. 2019, 2020). Our data support the hypothesis that beetles are selective in what plants they choose to consume. It’s interesting that on the one hand, pea aphids from fava beans are a poor host for numerous lady beetle species (Ugine et al. 2020), but on the other hand the same host plant is a favored supplement that restores beetle fitness. Lundgren et al. (2011) showed that pinto bean foliage, Phaseolous vulgaris (var. pinto), is readily consumed by Coleomegilla maculata. Additionally, some Coccinellids in the genus Epilachna like the Mexican bean beetle (Epilachna varivestis Mulsant) are destructive pests that specialize on legumes. A simple testable hypothesis is that legumes are favored foliage-based source of sterols for lady beetles, although our data from alfalfa does not support that hypothesis. Understanding what plant traits lady beetles use to assess foliage as a resource and what sensory modalities are employed to make assessments are important next steps in determining the evolution of omnivory in this system. Additionally, while we have shown that seven different species consume fava foliage in response to sterol-limitation (Ugine et al. 2020), it is possible that many lady beetle species meet their sterol requirement by consuming other tissues, including pollen, fungi, and alternative non-aphid prey.
Beetles reared on aphid-infested barley, alfalfa and fava bean plants performed much better than the beetles we reared in small cups and provided foliage supplements; a larger percentage of females laid viable eggs and more of the eggs they laid were viable. This was a curious result given that we were not able to see visible damage to alfalfa and barley leaves when they were provided to beetles during development. We can think of two possible explanations for this result. First, when beetles are reared on whole plants they have a wider variety of plant tissues and ages of tissues to choose from compared to what was available in the small cup assay. It could be that while the beetles did not feed upon excised foliage in cups, they did feed upon plant tissues when they were grown on whole intact plants. Because we did not assess plant damage on whole plants, we cannot verify this hypothesis. Second, when aphids are collected off plants and placed into cups as food for beetles, the aphids are no longer feeding on phloem. Therefore, after a short period of time we assume that their guts are emptied of phloem. Aphids have specialized guts that are designed to quickly remove excess water and concentration nutrients (Cohen 2015), and sterols are also excreted in aphids’ honeydew (Bouvaine et al. 2012). If lady beetles obtain their requisite dietary sterols from the gut contents of aphids, then removing aphids from their host plants could deprive beetles of an important source of sterols.
The pattern of sterol accumulation by the different aphid species was variable and we saw significant effects of aphids’ host plant on sterol abundance. As we have observed in the past, pea aphids that develop on fava beans sequester low levels of sterol, about 0.034 μg/dry gram of aphid in this study. This is in stark contrast to the large amount of sterols sequestered by the same clone of pea aphid grown on alfalfa, about 0.745 μg/dry gram of aphid. Additionally, we saw a very large difference in sterol accumulation between pea and green peach aphids reared on fava beans. This is interesting because each aphid species consumed phloem from the same host species. Green peach aphids appear to selectively concentrate specific sterols in their tissues (e.g. campesterol and cholesterol), whereas pea aphids seemingly excrete or metabolize the majority of the sterols they consume. Fava bean phloem contained a majority of sitosterol followed by campesterol, and these sterol species were also the most abundant in green peach aphids, perhaps suggesting the active accumulation of sterols. This is in contrast to what we observed with pea aphids reared on alfalfa. The phloem of alfalfa was dominated by 22-dihydrospinasterol, which contains a single double bond at carbon seven (∆7) in the sterol nucleus. Sterols with a double bond at this position can be problematic for other insect taxa and can cause delays in development and increase mortality (Behmer and Grebenok 1998; Behmer et al., 1999). Interestingly, pea aphids accumulated a proportionally small amount of this sterol, although they did sequester some spinasterol, another ∆7 sterol. However, the negative effect of the ∆7 sterols on lady beetles did not result in a fitness reduction. It is unclear from our data whether green peach aphids have an overall greater sterol need than pea aphids, or whether they passively accumulate whatever sterols they encounter. Similarly, it is unclear whether pea aphids actively excrete sterols to maintain low sterol levels as a novel form of defense against predators. Janson et al. (2009) reported that two congeneric aphid species in the genus Uroleucon accumulated different sterol profiles from Solidago altissima. One species maintained a majority of cholesterol and minor amount of spinasterol, whereas the second species maintained more spinasterol than any other single sterol.
Viewing coccinellids as obligate omnivores forces us to rethink their interaction with plants and even their more familiar role as predators. All of these mechanisms and behaviors will not only potentially influence local plant-predator-prey interactions, but combined they will potentially influence coccinellid distribution and abundance in the landscape. Given that lady beetles can use foliage as a source of supplemental nutrients, their distribution at the scale of the individual beetle may be influenced by vegetation in addition to prey presence and abundance. Our results indicate that the quality of aphids for lady beetles is determined by at least two factors. First, in the instance of pea aphids, the sterol content of their host plant appeared to determine aphids’ sterol content and beetle fitness. Second, there was an effect of aphid species whereby green peach aphids sequestered a much larger amount of sterol compared to pea aphids reared on the same plant. Our results demonstrate two key findings: 1) host plant mediated effects on prey quality can drive omnivory, and 2) diet mixing to redress micronutrient deficits benefits growth and reproduction.
References
Ahmad A, Wilde GE, Whitworth RJ, Zolnerowich G (2006) Effect of corn hybrids expressing the coleopteran-specific Cry3Bb1 protein for corn rootworm control on aboveground insect predators. J Econ Entomol 99:1085–1095
Agrawal AA, Kobayashi C, Thaler JS (1999) Influence of prey availability and induced host-plant resistance on omnivory by western flower thrips. Ecology 80:518–523
Al-Zyoud F, Tort N, Sengonca C (2005) Influence of host plant species of Bemisia tabaci (Genn.)(Hom., Aleyrodidae) on some of the biological and ecological characteristics of the entomophagous Serangium parcesetosum Sicard (Col., Coccinellidae). J Pest Sci 78(1):2530
Anderson JME (1982) Seasonal habitat utilization and food of the ladybirds Scymnodes lividigaster (Mulsant) and Leptothea galbula (Mulsant) (Coleoptera: Coccinellidae). Aust J Zool 30:59–70
Bai YY, Jiang MX, Cheng JA (2005) Effects of transgenic cry1Ab rice pollen on fitness of Propylea japonica (Thunberg). J Pest Sci 78:123–128
Behmer ST, Elias DO, Bernays EA (1999) Post-ingestive feedbacks and associative learning regulate the intake of unsuitable sterols in a generalist grasshopper. J Exp Biol 202:739–748
Behmer S, Grebenok R (1998) Impact of dietary sterols on life-history traits of a caterpillar. Physiol Entomol 23(2):165–175
Behmer ST, Nes D (2003) Insect sterol nutrition and physiology: a global overview. Adv Insect Physiol 31:1–72
Behmer ST, Raubenheimer D, Simpson SJ (2001) Frequency-dependent food selection in locusts: a geometric analysis of the role of nutrient balancing. AnimBehav 61(5):995–1005
Behmer ST, Simpson SJ, Raubenheimer D (2002) Herbivore foraging in chemically heterogeneous environments: nutrients and secondary metabolites. Ecology 83(9):2489–2501
Bouvaine S, Behmer ST, Lin GG, Faure M, Grebenok RJ, Douglas AE (2012) The physiology of sterol nutrition in the pea aphid Acrythosiphon pisum. J Insect Physiol 13:1383–1389
Brassler K (1930) Ist Coccinella septempunctata L. wirklich nur Blattlausfresser? Zeitschrift für Pflanzenkrankheit, Pflanzenpathologie, und Pflanzenschutz 40: 511–513
Carvalho M, Schwudke D, Sampaio JL, Palm W, Riezman I, Dey G, Kurzchalia TV (2010) Survival strategies of a sterol auxotroph. Development 137(21):3675–3685
Cohen AC (2015) Insect diets: science and technology. CRC press
Coll M, Guershon M (2002) Omnivory in terrestrial arthropods: mixed plant and prey diets. Ann Rev Entomol 47:267–297
Coogan SC, Raubenheimer D, Stenhouse GB, Nielsen SE (2014) Macronutrient optimization and seasonal diet mixing in a large omnivore, the grizzly bear: a geometric analysis. PLoS One 9(5):e97968
Du L, Ge F, Zhu S, Parajulee MN (2004) Effect of cotton cultivar on development and reproduction of Aphis gossypii (Homoptera: Aphididae) and its predator Propylaea japonica (Coleoptera: Coccinellidae). J Econ Entomol 97(4):1278–1283
Escalona HE, Zwick A, Li HS, Li J, Wang X, Pang H, Hartley D, Jermiin LS, Nedvěd O, Misof B, Niehuis O, Ślipiński A, Tomaszewska W (2017) Molecular phylogeny reveals food plasticity in the evolution of true ladybird beetles (Coleoptera: Coccinellidae: Coccinellini). BMC Evol Biol 17(1):151. https://doi.org/10.1186/s12862-017-1002-3
Eubanks MD, Denno RF (1999) The ecological consequences of variation in plants and prey for an omnivorous insect. Ecology 80:1253–1266
Francis F, Haubruge E, Hastir P, Gaspar C (2001) Effect of aphid host plant on development and reproduction of the third trophic level, the predator Adalia bipunctata (Coleoptera: Coccinellidae). Environ Entomol 30:947–952
Giles KL, Madden RD, Stockland R, Payton ME, Dillwith JW (2002) Host plants affect predator fitness via the nutritional value of herbivore prey: investigation of a plant-aphid-ladybeetle system. BioControl 47(1):1–21
Gillespie DR, McGregor RR (2000) The functions of plant feeding in the omnivorous predator Dicyphyus Hesperus: water places limits on predation. Ecol Entomol 25:380–386
Hailey A, Chidavaenzi RL, Loveridge JP (1998) Diet mixing in the omnivorous tortoise Kinixys spekii. Funct Ecol 12:373–385
Hoděk I, Evans TW. (2012). Food relationships, 141–274. Ecology and behaviour of the ladybird beetles (Coccinellidae). Blackwell Publishing Ltd., Oxford, United Kingdom
Janson EM, Grebenok RJ, Behmer ST, Abbot P (2009) Same host-plant, different sterols: variation in sterol metabolism in an insect herbivore community. J Chem Ecol 35(11):1309–1319
Jing X, Behmer ST (2020) Insect sterol nutrition: physiological mechanisms, ecology, and applications. Annual review of entomology 65:251–271
Jing X, Grebenok R, Behmer S (2013) Sterol/steroid metabolism and absorption in a generalist and specialist caterpillar: effects of dietary sterol/steroid structure, mixture and ratio. Insect Bioch Mol Biol 43:580–587
Joern A, Provin T, Behmer ST (2012) Not just the usual suspects: insect herbivore populations and communities are associated with multiple plant nutrients. Ecology 93(5):1002–1015
Karley AJ, Douglas AE, Parker WE (2002) Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J Exp Biol 205:3009–3018
Leigh SC, Papastamatiou YP, German DP (2018) Seagrass digestion by a notorious ‘carnivore’. Proc Royal Soc B: Biol Sci 285(1886):20181583
Lundgren JG (2009) Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol Cont 51:294–305
Lundgren JG, Moser SE, Hellmich RL, Seagraves MP (2011) The effects of diet on herbivory by a predaceous lady beetle. Bio Sci Tech 21(1):71–74
Ma Z, Liu Z, Huang X (2010) OSBP- and FAN-mediated sterol requirement for spermatogenesis in Drosophila. Development 137:3775–3784
Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ (2005) Nutrient specific foraging in invertebrate predators. Science 307:111–113
Moser SE, Harwood JD, Obrycki JJ (2008) Larval feeding on Bt hybrid and non-Bt corn seedlings by Harmonia axyridis (Coleoptera: Coccinellidae) and Coleomegilla maculata (Coleoptera: Coccinellidae). Environ Entomol 37(2):525–533
Moser SE, Obrycki JJ (2009) Non-target effects of neonicotinoid seed treatments: mortality of coccinellid larvae related to zoophytophagy. Biol Cont 48:487–492
Nyffeler M, Olson EJ, Symondson WO (2016) Plant-eating by spiders. JoA 44:15–27
Nomikou M, Janssen A, Sabelis MW (2003) Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp Appl Acarol 31:52–26
Pathak MD, Khan ZR (1994) Insect pests of rice. Los Baños, Laguna. 89 pp.
Patt JM, Wainright SC, Hamilton GC, Whittinghill D, Bosley K, Dietrick J, Lashomb JH (2003) Assimilation of carbon and nitrogen from pollen and nectar by a predaceous larva and its effects on growth and development. Ecol Entomol 28(6):717–728
Pilcher CD, Obrycki JJ, Rice ME, Lewis LC (1997) Preimaginal development, survival, and field abundance of insect predators on transgenic Bacillus thuringiensis corn. Environ Entomol 26:446–454
Le Rü B, Mitsipa A (2000) Influence of the host plant of the cassava mealybug Phenacoccus manihoti on life-history parameters of the predator Exochomus flaviventris. Entomol Exp Appl 95(2):209–212
Sanchez JA (2008) Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric For Entomol 10:75–80
Simpson SJ, Raubenheimer D (2015) Nutritional physiology: sex elicits a taste for salt in drosophila. Curr Biol 25(20):R980–R982
Singer MS, Bernays EA, Carriere Y (2002) The interplay between nutrient balancing and toxin dilution in foraging by a generalist insect herbivore. Anim Behav 64:629–643
Singer MS, Bernays EA (2003) Understanding omnivory needs a behavioral perspective. Ecology 84:2532–2537
Toft S (2005) The quality of aphids as food for generalist predators: implications for natural control of aphids. Eur J Entomol 102:371–383
Tomazic L, Najle SR, Nusblat AD, Uttaro AD, Nudel CB (2011) A novel sterol desaturase-like protein promoting dealkylation of phytosterols in Tetrahymena thermophile. Eukaryot Cell 10:423–434
Torres JB, Boyd DW (2009) Zoophytophagy in predatory Hemiptera. Braz Arch Biol Technol 52:1199–1208
Triltsch H (1997) Gut contents in field sampled adults of Coccinella septempunctata (Col.: Cocinellidae). Entomophaga 42:125–131
Triltsh H (1999) Food remains in the guts of Coccinella septempunctata (Coleoptera: Coccinellidae) adults and larvae. Eur J Entomol 96(4):355–364
Unsicker SB, Oswald A, Köhler G, Weisser WW (2008) Complementarity effects through dietary mixing enhance the performance of a generalist insect herbivore. Oecologia 156(2):313–324
Ugine TA, Krasnoff SB, Grebenok RJ, Behmer ST, Losey JE (2019) Prey nutrient content creates omnivores out of predators. Ecol Lett 22:275–283
Ugine TA, Nagar A, Grebenok RJ, Behmer ST, Losey JE (2020) Herbivory improves the fitness of predatory beetles. J Anim Ecol 89:2473–2484
White JA, McCord JS, Jackson KA, Dehnel AC, Lenhart PA (2017) Differential aphid toxicity to ladybeetles is not a function of host plant or facultative bacterial symbionts. Funct Ecol 31(2):334–339
Wu XH, Zhou XR, Pang BP (2010) Influence of five host plants of Aphis gossypii glover on some population parameters of Hippodamia variegata (Goeze). J Pest Sci 83(2):77–83
Acknowledgments
We thank Dr. Sana Gardescu and the two anonymous reviewers for their helpful comments on earlier versions of this manuscript. We thank Kathryn Chinn for technical assistance. This work was supported by grants from the National Science Foundation (NSF-DRL-1114525 awarded to JEL.), and the United States Department of Agriculture (NIFA-AFRI-2016-67013-24762 awarded to STB. and RJG).
Data Accessibility
We confirm that the data supporting the results will be archived in Dryad and the data DOI will be included at the end of the article. Data in support of this manuscript can be found at: https://doi.org/10.5061/dryad.hdr7sqvh6
Author information
Authors and Affiliations
Contributions
TAU conceived the project, developed the hypotheses and designed and conducted the experiments with HKG. TAU, RJG and NH developed the methods. TAU, HKG, RJG and NH collected the data. TAU, RJG, STB and JEL analyzed the data, prepared the figures and tables and wrote the manuscript.
Corresponding author
Supplementary Information
ESM 1
(MOV 17384 kb)
Rights and permissions
About this article
Cite this article
Ugine, T.A., Gill, H.K., Hernandez, N. et al. Predator Performance and Fitness Is Dictated by Herbivore Prey Type Plus Indirect Effects of their Host Plant. J Chem Ecol 47, 877–888 (2021). https://doi.org/10.1007/s10886-021-01251-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01251-4