Abstract
The beech leaf-mining weevil, Orchestes fagi, is a common pest of European beech, Fagus sylvatica, and has recently become established in Nova Scotia, Canada where it similarly infests American beech, F. grandifolia. We collected volatile organic compounds (VOCs) emitted by F. grandifolia leaves at five developmental stages over one growing season and simultaneously analyzed them for volatile emissions and O. fagi antennal response using gas chromatography-electroantennographic detection (GC-EAD). Volatile profiles changed significantly throughout the growing season, shifting from primarily β-caryophyllene, methyl jasmonate, and simple monoterpene emissions to dominance of the bicyclic monoterpene sabinene during maturity. Two VOCs dominant during bud burst, (R)-(+)-limonene and geranyl-p-cymene, may be of biological relevance due to the highly specific oviposition period of O. fagi at this stage though antennal responses were inconclusive. Senescence showed a decrease in blend complexity with an increase in (Z)-3-hexenyl acetate and (Z)-3-hexen-1-ol as well as a resurgence of α-terpinene and geranyl-p-cymene. We present a novel electroantennal preparation for O. fagi. Antennae of both male and female O. fagi responded to the majority of detectable peaks for host volatiles presented via GC-EAD. Females displayed greater overall sensitivities and less specificity to host volatiles and it is hypothesized that this translates to more generalist olfaction than males. It is clear that olfactory cues are important physiologically though their implications on behaviour are still unknown. The results presented in this study provide a baseline and tools on which to connect the complex and highly time-specific phenology of both F. grandifolia and the destructive pest O. fagi through which olfactory-based lures can be investigated for monitoring systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The beech leaf-mining weevil (syn. Beech flea weevil), Orchestes fagi L. (previously Rhynchaenus fagi) (Coleoptera: Curculionidae: Curculioninae: Ramphini) is a common univoltine pest of European beech, Fagus sylvatica L. (Fagales: Fagaceae) in Europe (Alford 1995). Eggs are laid singly along the mid-rib on the underside of developing leaves at the time of budburst. Larval eclosion from eggs occurs within 8–10 days followed by 3 larval instars (Bale 1984; Nielsen 1966; Pullin 1985). Larvae feed and pupate within the internal tissues of the leaf creating characteristic linear blotch mines with a brown, scorched appearance (Nielsen 1966, 1978; Pullin 1985; Sweeney et al. 2012). Larval mortality is high (~ 85%) and has been linked to the natural lignification of beech leaf vascular tissue beginning as early as one week post-flush (Nielsen 1966, 1968; Nielsen and Ejlersen 1977; Watt and McFarlane 1992; Woodcock and Vanbergen 2008). Lignified tissues limit the ability of first instar larvae to feed and develop, effectively becoming ‘trapped’ by veins causing starvation. The egg-laying period is thus limited to approximately two weeks following bud burst with the majority of eggs laid within one week (Phillipson and Thompson 1983; Bale 1984). In Europe, adults emerge from pupal chambers and migrate from beech stands to feed on a large variety of non-host plants including apple (Malus spp.) and raspberry (Rubus spp.) and reject fully developed beech leaves (Bale and Luff 1978; Dieter 1964; Phillipson and Thompson 1983; Pajares et al. 1990). In North America, Moise et al. (2015) found that pre-overwintered adults engaged in little to no feeding on any plants, including beech. Newly emerged males are sexually mature before overwintering but females require reproductive diapause to complete ovariole development stimulated in early spring by lengthening photoperiod and subsequent feeding (Bale 1979). Adults overwinter within bark crevices and moss on beech as well as non-beech trees, e.g. spruce, throughout the forest floor in leaf litter and understory plants, and in adjacent evergreen stands (Bale 1981; Grimm 1973, 1990; Morrison et al. 2017; Nielsen 1970). Overwintering is broken in early spring and adults re-emerge to immediately feed on hawthorn (Crataegus spp.) and occasionally beet (Beta vulgaris L.) and other herbaceous plants such as raspberry (Rubus spp.) until beech bud burst (Bale and Luff 1978; Dieter 1964). Bale and Luff (1978) found young beech leaves to be preferred over all alternate food sources. It is clear that O. fagi follow a strict phenological timeline to coincide with the development of their host plant but it is currently unclear through what means this is regulated. It is the purpose of this study to examine potential chemical/olfactory relationships that may exist between these species.
Orchestes fagi was documented infesting American beech, Fagus grandifolia Ehrh. (Fagaceae), in Nova Scotia, Canada in 2012 but anecdotal reports of leaf damage indicate its presence up to 5 years prior to documentation (Sweeney et al. 2012). Fagus grandifolia is the only native beech tree in North America and is distributed throughout most of the eastern continent (Tubbs and Houston 1990). It is an ecologically important species, as beech nuts are a common food source for a variety of birds and mammals (Tubbs and Houston 1990). American beech was once a common lumber source for flooring and furniture but its commercial importance has declined due to heavy mortality from beech bark disease, caused by an invasive scale insect, Cryptococcus fagisuga Lind. (Hemiptera: Eriococcidae), which predisposes beech bark to infection by the fungal pathogens, Neonectria faginata (Lohman, Watson, and Ayers) (Hypocreales: Nectriaceae) and Neonectria ditissima (Tulasne and C. Tulasne) Samuels and Rossman (Davis and Meyer 2004; Ehrlich 1934; Houston 1994; Houston et al. 1979; Houston and O’Brien 1983; Jha et al. 2004; Tubbs and Houston 1990). Since the introduction of the scale insect to Halifax, Nova Scotia in1890, beech bark disease has spread about 15 km per year and often killed ≥50% of American beech >25 cm diameter at breast height in the first 10 years along the invasive front but many beech trees survive in the aftermath forest (Houston 1994; Morin et al. 2007). Orchestes fagi has been listed as a vector of the fungus N. galligena in Europe (Mihál et al. 2014) but it is unknown if a similar concern exists with the North American N. faginata. Infestation of American beech stands with O. fagi represents a potentially severe threat to American beech surviving in the aftermath of beech bark disease. Few documented European predator and parasitoid species are present in Nova Scotia (reviewed in Pawlowski 2017) allowing large populations of O. fagi to thrive unimpeded leading to almost complete defoliation of highly infested beech stands (personal observation, Halifax, NS). Subsequent defoliation year after year has led to high rates of beech mortality in infested areas (Sweeney et al. 2020). Heavy insect damage also renders beech susceptible to disease via the root fungus Armillaria sp. Staudt (Agaricales: Physalacriaceae) and other pathogens (Beaudet and Messier 2008; Houston 1994) as well as limiting beech nut production. The results of this study are part of an ongoing effort to produce effective tools for survey and monitoring of O. fagi in North America and limit its impact on F. grandifolia.
Olfaction of host and/or conspecific volatiles often plays a pivotal role in the behaviour and life history of an insect (Strausfeld 2012). Virtually all plant species produce volatile organic compounds (VOCs) as a means of communication and/or protection at some stage of development (Fall et al. 1999; Tholl et al. 2006). These natural volatile blends are often co-opted by infesting pest species in order to locate suitable hosts both long range and via contact chemoreception (Finch and Collier 2000). While the volatile profile of F. sylvatica has been widely studied (Dindorf et al. 2006; König et al. 1995; Moukhtar et al. 2005; Schuh et al. 1997; Tollsten and Müller 1996), little focus has been placed on its North American sister species F. grandifolia. Silk et al. (2017) found large quantities of 9-geranyl-p-cymene and 9-geranyl-α-terpinene emitted from F. grandifolia leaves at the time of budburst as well as lesser amounts of a variety of common green leaf volatiles and sesquiterpenes. Cross comparison between sister Fagus species is difficult as most volatile emission studies are conducted using adult leaves rather than at the time of budburst. Fully developed F. sylvatica leaves produce 9-geranyl-p-cymene and 9-geranyl-α-terpinene in much smaller concentrations (Dindorf et al. 2006; Gossner et al. 2014; Tollsten and Müller 1996) and emit instead large amounts of the bicyclic monoterpene sabinene.
The role of plant-produced VOCs in O. fagi host location and infestation is currently unclear. It is evident that activity on their beech hosts is time-sensitive and requires specific attraction to young bursting buds and flushing leaves. Silk et al. (2017) found that geranyl-p-cymene attracted male O. fagi in a Y-tube olfactometer as well as in the field but Goodwin et al. (2020) observed no attraction of O. fagi to geranyl-p-cymene in a field trapping bioassay. Our objectives were to elucidate the volatile profiles of F. grandifolia across a growing season using its sister species F. sylvatica as a reference and to examine O. fagi antennal response to compounds present within these blends. This information can be used to more efficiently select compounds to test in lab and field settings as potential lures for this invasive species by connecting the phenological sensitivity of O. fagi to the timeline of volatile production by its host. While this study is not inclusive of all VOCs produced by F. grandifolia, it represents a baseline of common Fagus emissions across a broad range of previously undocumented developmental stages.
Methods and Materials
Volatile Collections from Fagus grandifolia Ehrh
Volatile compounds emitted by F. grandifolia leaves were collected from five distinct developmental stages over the normal blooming season of 2016. Stages of development were selected based on visual and temporal characteristics: (1) closed and elongated bud; (2) newly bursting bud (i.e., leaves beginning to show, scales still intact); (3) newly emerged leaves (~ 1–2 weeks old); (4) fully developed leaves (> 1 month old); (5) senescing leaves (i.e. leaves beginning to change colour and/or wilt, > 4 months old) (Fig. 1). Branch samples containing no fewer than 20 leaves were collected from naturally occurring, mature F. grandifolia trees displaying no physical signs of damage (herbivory, fungi, etc.) growing along the Harriet Irving Botanical Gardens Woodland Trail, Acadia University, Wolfville, NS (45°05′12.5”N, 64°22′0.58”W). Due to the high variability in leaf dry weight across the growth season, leaf count was chosen as a standardizing method to better suit the goals of this study. Little, if any, herbivory was observed on the trees sampled and there were no signs of herbivory whatsoever on leaves of branches that were sampled. Cut ends of branches were immediately submerged in reverse osmosis water to reduce wound impact on volatile emissions (Fall et al. 1999). Branch samples were brought to an onsite phytotron at the K.C. Irving Environmental Science Centre (Acadia University, Wolfville, NS, Canada) and maintained at 21 °C and 75% relative humidity (RH). Branch sampling occurred once or twice weekly throughout the sampling period which limited access to short-lived developmental stages.
Five developmental stages of Fagus grandifolia leaves: (1) closed and elongating buds; (2) bud burst; (3) young, pre-lignified leaves (1–2 weeks post flush); (4) mature, lignified leaves (> 1 month post flush); (5) senescing leaves (>4 months post flush) (Image 5 adapted from Farmartin (2014)
Volatile organic compounds (VOCs) from different stages of beech leaf development were collected using dynamic headspace trapping (solid phase extraction) and analyzed using gas chromatography (GC) coupled with flame ionization detector (FID) or mass spectrometer (MS). Volatile collection technique was adapted from Tollsten and Müller (1996). Samples were individually inserted into a 45 × 55 cm Look® Oven Bag (Terinex Ltd., Bedford, England) and sealed around the lower branch. Charcoal filtered air was pumped into the headspace at a rate of 65 mL min−1 using a Portable Volatile Assay System (PVAS22, VAS volatile assay systems, Rensselaer, NY). The air was then passed to the outlet and over a new solid adsorbent volatile trap (HayeSep® Q porous polymer adsorbent, Sigma-Aldrich) which had been rinsed with hexane and baked at 200 °C for 2 h to remove contaminants. For each branch, volatiles were collected for a 4 h period between 900 and 1600 ADT for a total collection volume of approximately 15.6 L per branch sample. Due to the collection process, VOCs were collected from 1 to 2 samples at a given time. Collected compounds were eluted from the traps using 1 mL of hexane run through the trap in 150 μL aliquots under pressure of non-reactive, ultra high purity N2. Samples were frozen until use in chemical analysis and electrophysiological recordings.
GC-FID/-MS Analysis of Host Plant Volatiles
Volatile samples from all five F. grandifolia developmental stages were analysed on a Varian 450-gas chromatogram (GC-FID; Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a Stabilwax® Crossband® Carbowax® polyethylene glycol column (30 m × 0.25 mm × 0.25 μm; Chromatographic Specialties, Inc., Brockville, ON, Canada) and coupled with a flame ionization detector. Frozen samples were allowed to return to ambient room temperature and subsequently concentrated to 1 μL under a constant stream of ultra high purity N2 gas immediately prior to analysis. Volatile blend analyses were conducted according to methods adapted from Tollsten and Müller (1996) and Moukhtar et al. (2005). The oven temperature was held at 50 °C for 3 min; increased at 4 °C min−1 to 150 °C and held for 10 min; and then increased by 35 °C min−1 to 220 °C and held for 10 min. Samples were injected into a split/splitless injector port at 220 °C with the split closed for 1 min then at a split ratio of 20:1. Effluent was forced through the column using helium carrier gas to a glass two-way splitter where it was split to the flame ionization detector at 300 °C and into a heated EAD transfer line at 220 °C (Syntech, Kirchzarten, Germany). EAD effluent was delivered directly into a stream of charcoal-filtered humidified air directed at an antennal preparation. GC-FID signals were recorded using the Galaxie Chromatography Data System v. 1.9.302.952 (Agilent Technologies Inc., Santa Clara, CA) and expressed as μV min−1.
Compounds detected by the GC-FID were identified by comparison with external standards run on the same method. External standards were selected based on their presence within previously documented F. sylvatica volatile blends (Table 1). Peaks eluting at the same time as these standards were deemed to be these known compounds. Hexane blanks were run on identical methods to correct for contamination. Additionally, samples were run on a Scion 456 GC with SQ Mass Spectrometer (GC-MS; Bruker Daltonics Ltd., Coventry, UK) using the same GC-method detailed above. First and last masses scanned were 60–350. This was used to confirm known compounds by comparison with the National Institute of Standards and Technology (NIST) mass spectral library (version 2.0, USA) and external standards.
Insects
All O. fagi used in GC-EAD trials were collected near Ashburn Golf Club (AGC), NS, Canada (44.6453°N, 63.6361°W) in August and September 2016 by beating branches of American beech displaying characteristic damage. Since adult emergence from pupal cocoons midway through the season (late June - early July) is concurrent with mortality of adults from the previous generation (Bale 1981; Grimm 1973; Nielsen 1974a, 1974b), all adults collected were assumed to be pre-overwintered, i.e. they developed from egg to adult in 2016. Mating status could not be determined as female sexual development is incomplete prior to diapause but mating was still observed in the field (personal observation, Halifax, NS). Insects were returned to the lab and maintained at 5–10 °C and 80% RH. Sugar water was provided ad libitum. Upon collection, sexes were separated based on the curvature of the 5th abdominal sternite; males being concave laterally and females being convex throughout. Validation of this technique was confirmed in 2015 with a 98% success rate via dissection and comparison to curculionid genitalia documented by Aslam (1961). Individuals were maintained at ambient room temperature (18–21 °C) at 10:14 light:dark cycle for 24 h prior to experimental use.
Electroantennal Detection of Host Compounds
Adult O. fagi were inserted head first into the cut tip of a 100 μL plastic pipette tip until the head was exposed, lodging the pronotum and thus limiting movement of the beetle. A piece of Kimwipe® or cotton was inserted behind the beetle, blocking the open pipette tip and securing the beetle in place. The preparation was then mounted on a glass microscope slide using dental wax. A glass microscope cover slip was broken and a small shard (~ 1 mm × 4 mm) was placed on the wax under the exposed head of the beetle. Using forceps, both antennae were secured to the shard of coverslip using dental wax, ensuring that the club remained unencumbered. Due to poor conductivity in previous preparations attempted for O. fagi, a hole (~2 μm in diameter) was cut in the tip of the club using a modified cut sensillum technique (Hillier et al. 2006; Hillier and Kavanagh 2015; MacKay et al. 2015). A borosilicate capillary tube (1.0 mm × 0.5 mm; World Precision Instruments, Inc., Sarasota, FL, USA) was pulled to a 0.06 μm tip diameter with a 9–11 mm taper using a Co P-97 Flaming/Brown Micropipette Puller (Program 0: ramp 525, pull 150, velocity 75, time 250, pressure 500; Sutter Instruments, Novato, CA). The capillary was mounted on a piezoelectric crystal using a small amount of dental wax. The piezo-crystal was controlled using a manual switch attached to a function generator (INSTEK© GOS-620FG) and was placed directly adjacent to the distal segment of the antennal club using a manual micromanipulator (World Precision Instruments, Sarasota, FL). The capillary was made to resonate at high speed by alternating square wave sound frequencies (100 K signal amplification) from the generator through the piezo-crystal, effectively creating a “piezo-electric saw‟. Micromanipulation of the saw toward the antenna allowed for precise removal of small amounts of cuticle.
Electroantennal preparation was adapted from Leskey et al. (2009) and Ranger et al. (2012) due to the similarity in size of adult beetles; forked probes were also attempted according to Keesey et al. (2012) and Kendra et al. (2012), but this preparation did not yield consistently successful results with O. fagi. In preparation for mounting, the head of the insect was removed; ensuring the antennae remained intact, attached to the head, and embedded in wax. A 0.15 mm × 12 mm stainless steel insect pin (Minucie No. 15; Ento Sphinx®, Czech Republic) was embedded in wax along the proximal edge of the antennal club to secure the club in place. Additional borosilicate capillaries were pulled to a 1 μm tip diameter with a 3–4 mm taper (Program 1: ramp 515, pull 0, velocity 30, delay 1, pressure 500), filled with saline with sucrose solution (Insect Ringer; Kaissling 1974), and mounted over an Ag/AgCl wire for use as a reference electrode. The foramen of the head was attached to the reference electrode using conductive Signagel® (Parker Laboratories Inc., Fairfield, NJ). A portion of tungsten wire sharpened with KCl to an approximate tip diameter of 1 μm and rinsed with distilled water was used as a recording electrode. The recording electrode was mounted directly to a headstage probe through which the signal was amplified by an IDAC-2® amplifier and data acquisition system (Syntech, Kirchzarten, Germany) and recorded using Data acquisition for Gas Chromatograph with EAD software (GcEad 2014 v.1.2.5, Syntech). The tungsten electrode was coated lightly in Signagel® and inserted directly into the incised hole in the antennal club. The entire preparation was placed 10 mm from a continuous humidified air stream into which a transfer line from the GC was fed.
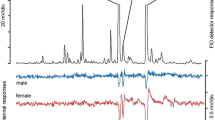
Effluent from F. grandifolia stage 1–5 volatile blend samples were simultaneously analysed by FID and presented to the antenna via electroantennal detection (EAD). Signals were recorded concurrently as mV amplitudes (Fig. 2).
Statistical Analyses
The area under recurring GC-FID peaks matching external standards was measured to determine relative concentration of compounds within American beech volatile blends. The mean area of each compound at each developmental stage was corrected using hexane blanks. Corrected means were converted to a proportion of total emissions based only on those selected from F. sylvatica blends rather than total emissions by F. grandifolia. This was done to eliminate unknown compounds from the collected samples and allowed comparison between sister species.
EAD responses were recorded as mV at each known retention time. Additional antennal responses were considered whenever the mV exceeded the normal fluctuations of the baseline (e.g. > 3 mV). Only those recordings with peaks above the baseline were considered for analysis. Results were pooled across growth stages to allow for statistical analysis of the full range of VOCs. Statistical analyses were completed using R v. 3.1.2 (R Core Team 2015). Known compounds were analysed by two-way ANOVA (generalized linear model method, α = 0.05, loglink, family = Poisson) to assess the effect of compound, sex, and their interaction on amplitude (i.e. mV) of response. Data were subsequently analyzed separately for males and females using one-way ANOVA.
Results
Volatile Profiles of Fagus grandifolia at Five Developmental Stages
A total of 36 volatile collections were taken; all samples were used in final analyses. All selected Fagus spp. volatiles were found in F. grandifolia in at least one stage of development except terpinolene and germacrene D (Table 1). Volatile profiles differed greatly between developmental stages with large fluctuations in all VOC emissions. Fagus grandifolia primarily emitted monoterpenes with fluctuations in sesquiterpene and alkyl aldehyde emissions over the growth season.
Stage 1 volatile profiles were characterised by emissions of β-caryophyllene and hexanal. Methyl jasmonate was produced in large quantities in only one sample and thus cannot be confirmed as a characteristic volatile. Stage 1 showed a distinct lack of myrcene and geranyl-p-cymene which were present in later stages of development. Emissions of the monoterpenes α-phellandrene, α-terpinene, α-terpineol, and (E,Z)-ocimene were greatest in this stage and were reduced to negligible levels as the leaves continued to develop (Table 1).
Stage 2 volatile profiles were characterized by emissions of methyl jasmonate, geranyl-p-cymene, and β-caryophyllene (Table 1). Emissions of the alkyl aldehyde hexanal decreased during this stage to a low baseline level held relatively constant over the remainder of the growing season. The cyclic monoterpene (R)-(+)-limonene was present at this stage in its highest relative concentration though fluctuations between samples limited confidence in its ubiquity.
Stage 3 volatile profiles were characterized by emissions of methyl jasmonate, sabinene, and β-caryophyllene. This stage was characteristic of low level emissions by all other compounds; the high levels of geranyl-p-cymene found in stage 2 ceased completely (Table 1). The monoterpene linalool was found only in this stage of development but was inconsistent and weak when present.
Stage 4 volatile profiles were characterized by emissions of sabinene, methyl jasmonate, α−/β-pinene, and (Z)-3-hexenyl acetate (Table 1). This stage showed a reduction in the previously high emissions of methyl jasmonate and β-caryophyllene in favour of the monoterpene sabinene. The pinene monoterpenes were emitted at highest levels during this developmental stage and the presence of camphene was recorded in very low concentrations.
Stage 5 volatile profiles were characterized by emissions of (Z)-3-hexenyl acetate, sabinene, and (Z)-3-hexen-1-ol as well as a resurgence in emissions of early stage compounds α-terpinene and geranyl-p-cymene (Table 1). Emissions of methyl jasmonate and β-caryophyllene ceased during this stage of development along with several other weakly emitted VOCs. Previously dominant sabinene emissions began to fall and were overtaken by the green leaf ester (Z)-3-hexenyl acetate as leaves senesced.
O. fagi electrophysiological response to F. grandifolia volatile blends. A total of 29 EAD recordings were taken. Sixteen recordings using alternate antennal preparations yielded no valuable results. Using the novel antennal preparation outlined in this paper, 8 out of 13 subsequent recordings yielded valuable results according to our criteria. Both male and female O. fagi displayed antennal sensitivities to most selected Fagus volatiles, Results from the two-way ANOVA showed a significant difference between sexes (F1, 140 = 5.74, P < 0.05) with females displaying greater mean amplitude of antennal response (Fig. 3) but effects of volatile compound (F17,140 = 0.49, P = 0.96) and sex*compound interaction (F17,140 = 0.65, P = 0.85) were not significant. When data were analyzed separately for males and females, no compound elicited a greater response than any other (males: F17,62 = 0.58, P = 0.89; females: F17,78 = 0.56, P = 0.91). Male antennal response was highly variable with some obvious responses to (R)-(+)-limonene, myrcene, and (Z)-3-hexenyl acetate but the only compound that elicited consistently strong responses was β-caryophyllene. Male antennae did not respond to sabinene or linalool and only responded weakly to geranyl-p-cymene. Females yielded more consistent electroantennal results with strong responses to most Fagus VOCs. In contrast to males, (R)-(+)-limonene elicited no antennal response in females whereas sabinene and (+)-3-carene elicited the most consistent and strong responses in females. Significant female electroantennal responses were recorded to unknown compounds eluting at 22.30 mins. and 23.28 mins.
Discussion
Each F. grandifolia developmental stage emitted unique volatile profiles either by blend composition or by relative level of emissions across the growing season. Little has been published on the volatile profiles of F. grandifolia or on developmental blend profiles in F. sylvatica, and thus comparison across and within species is difficult. Based on the published profiles, it can be assumed that most F. sylvatica studies were conducted on fully developed beech leaves (i.e., our stage 4). Evidence for this assumption derives from previous studies which found sabinene to be the major VOC emitted by F. sylvatica, comprising 74–94% of total emissions (Dindorf et al. 2006; König et al. 1995; Moukhtar et al. 2005; Schuh et al. 1997; Tollsten and Müller 1996). We observed high levels of sabinene only in mature leaves of F. grandifolia but never to the extent recorded in F. sylvatica, indicating that sabinene production is lower overall in the North American sister species. Terpenoid emissions are induced by enzymatic activities correlated with development and rate of biosynthesis (Fall et al. 1999; Schuh et al. 1997). It is unsurprising that the bicyclic monoterpene sabinene was the dominant volatile emitted by mature leaves of both F. grandifolia and F. sylvatica but whether the pattern of sabinene emission from F. sylvatica at other stages of leaf development mirrors that of F. grandifolia is unknown. Emission levels and blend profiles were relatively consistent between these congeners and this study provides useful baseline data from which to expand the volatile profiles of both species and allow comparison of leaf development within the Fagaceae and extending to other broad-leaved, deciduous trees.
The relatively high emissions of the bicyclic sesquiterpene β-caryophyllene in early leaf development was surprising based on the low emissions previously recorded in F. sylvatica. This common essential oil component is present in many plant species including hops, Humulus lupulus L. (Cannabaceae), rosemary, Salvia rosmarinus Spenn. (Lamiaceae), and clove, Syzygium aromaticum (L.) Merrill and Perry (Myrtaceae), and denotes the characteristic scent of black pepper, Piper nigrum L. (Piperaceae), and Ashanti pepper, P. guineese Schumach. (Ghelardini et al. 2001; Jirovetz et al. 2002; Ormeño et al. 2008; Wang et al. 2008). It has been linked to defense mechanisms in the South Indian ginger, Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae), and as a contributing defensive compound in fragrant nutmeg, Myristica fragrans Houtt. (Myristicaceae) with both antimicrobial and antifungal activity (Gupta et al. 2013; Sabulal et al. 2006). It is possible that the release of such relatively large levels of β-caryophyllene by American beech presented here represents a systemic defensive response to beech bark disease fungal infestation. However, the antifungal effects of this compound on Neonectria spp. have not been tested. Ormeño et al. (2008) found an increase in β-caryophyllene emissions in siliceous soils typified by natural acidity and high mineral content comparable to soil types in Nova Scotia, Canada (Sangster et al. 2010). Either or both of these factors could help explain the high levels of β-caryophyllene, but perhaps not why its emission levels are particularly high only during stages 2–3 when buds are bursting and leaves are expanding.
The ester methyl jasmonate was found in higher levels in F. grandifolia leaves than expected. This compound is a well-known systemic defense compound, regulating internal and intraspecies signalling (Pichersky and Gershenzon 2002). Similar to β-caryophyllene, the apparent over-abundance of this compound in volatile profiles of American beech in comparison to European beech is surprising. Methyl jasmonate regulates plant growth under stress of high salinity in almond Prunus dulcis (Mill.) D.A. Webb (Rosaceae) and drought in bread wheat, Triticum aestivum L. (Poeaceae) (Javadipour et al. 2019; Tavallali and Karimi 2019). It is unclear if such environmental stresses may have affected F. grandifolia leaf volatile emissions during the described growing season, but no weather events of particular interest occurred during the study period. It is thus assumed that growing conditions during the course of this study were typical of the area and thus unlikely to account for upregulation of methyl jasmonate emissions. This compound is also a key cellular regulator mediating diverse developmental processes including germination, root growth, flowering, fruit ripening and carotenoid production (Cheong and Choi 2003; Luo et al. 2020). Due to the high levels of emissions during early development of leaves and tree growth, it is likely that methyl jasmonate is an important growth and development hormone in American beech. Whether or not this can be attributed to maintaining growth during environmental, phytophagous, or systemic stress is currently unclear.
American beech profiles showed an increase in the ester (Z)-3-hexenyl acetate emissions during senescence, and a general decrease in volatile blend complexity, similar to that found by Obando-Ulloa et al. (2009) in senescing leaves of muskmelon, Cucumis melo L. (Cucurbitaceae). We also observed an increase in emission of (Z)-3-hexen-1-ol from senescing American beech leaves, similar to marked increases in alcohol and simple aldehyde emissions from senescent leaves of maize, Zea mays L. (Poaceae), and the Brazillian tobacco plant, Nicotiana mutabilis Stehmann and Samir (Solanaceae) (Macnish et al. 2010; Mozaffar et al. 2018). However the apparent resurgence in the monoterpene α-terpinene and the alkylbenzene-linked monoterpene geranyl-p-cymene that we observed has not been observed in senescent leaves of other plants. It is unclear if this represents jettisoning of volatile pools during senescence or if their presence could be linked to biosynthetic pathways culminating in the aging process. The presence of high levels of geranyl-p-cymene in stage 5 leaves is especially surprising given that male adult O. fagi were found to be highly attracted to the compound (Silk et al. 2017) and yet displayed little attraction to adult beech leaves in lab or field settings (Bale and Luff 1978; Goodwin et al. 2020; Moise et al. 2015). It is unclear if this rejection of host tissue would extend from stage 4 leaves into stage 5; it would be interesting to examine this relationship given the apparent upsurge in previously attractive geranyl-p-cymene as leaves senesce.
Antennal responses by O. fagi confirmed differential relative sensitivities to host volatiles between the sexes previously documented in behavioural trials by Pawlowski (2014), Silk et al. (2017), and Goodwin et al. (2020). Contrary to expected from these behavioural trials, females tended to have greater antennal responses to almost all presented compounds indicating that female O. fagi likely utilize a broad range of volatiles for host selection whereas males may use few selective compounds of interest. Leskey et al. (2009) reported similar results in the plum curculio, Conotrachelus nenuphar Herbst (Curculionidae) however these results appear to be uncommon among weevils. Differing behavioural responses between sexes in a number of weevil species are well-documented (Cao et al. 2015; Dickens et al. 1991; Mutis et al. 2010; van Tol and Visser 2002; Toshova et al. 2010) yet rarely does this correlate to differential electroantennal sensitivities within the species. This lends credence to the hypothesis that chemical cues are an important factor in O. fagi behaviour with the sexes having different roles in host- and/or conspecific location. Further electroantennographic work and behavioral bioassays will be required to isolate compounds of particular interest for each sex and examine dose response to develop efficient lures for field use. Future examination of electroantennal response to VOCs at each developmental stage independently may allow for a more robust analysis of olfactory capabilities given the fluctuations in volatile emissions and their potential implications on dose-dependent antennal response. These results indicate that blended lures may be required to attract both male and female O. fagi.
A number of antennally active compounds were found in F. grandifolia samples that did not correlate to the known F. sylvatica external standards and could not be identified. Future GC-MS analysis of these blends will provide more robust understanding of the compounds present within volatile blend profiles and their olfactory relevance to O. fagi. This study provides a novel electroantennal preparation which yielded stable signals from O. fagi, a technique which will be invaluable for future study of this invasive species. Our results clearly indicate the importance of olfactory cues in O. fagi though the roles of individual compounds or blends in the host selection behavior of O. fagi needs much additional research. Low antennal response in male O. fagi to geranyl-p-cymene does not seem to correlate to observed behavioural responses in a Y-tube olfactometer (Silk et al. 2017) but this behaviour did not translate to increased attraction in the field (Goodwin et al. 2020). Weevils tend to use complex volatile blends for host and/or conspecific location (Bartelt 1999) and thus it is likely that multiple antennally active compounds act synergistically and will be required in the composition of an efficient lure. At this stage, it is difficult to assess which compounds may be of most relevance due to apparent ubiquity of response to host volatiles. Therefore, examination of F. grandifolia volatile profiles at each stage of development in the context of O. fagi phenology and electrophysiology can provide some insight. The relatively strong but inconsistent antennal response of male O. fagi to (R)-(+)-limonene, present primarily during the most biologically active stage of bud burst, may indicate its importance in host location and colonization by males. This compound has been shown to be attractive to male and female Aegorhinus superciliosus Guérin-Méneville (Cyclominae) in both field and lab settings (Mutis et al. 2010) and requires further examination for its behavioural activity in O. fagi. The assortment of monoterpenes present primarily in stage 1 leaves may also provide potential chemical cues to foraging O. fagi because mating pairs are often observed within beech buds prior to budburst (personal observation, Halifax, NS). The dominance of sabinene in stage 4 leaves did not reflect significant antennal sensitivities in O. fagi. This further suggests that attraction to young leaves and almost complete rejection of adult leaves documented by Bale and Luff (1978) and Moise et al. (2015) is a chemically-driven response. Using the tools and chemical/electrophysiological baselines provided in this study, further research can be conducted to elucidate host and mate location mechanisms in this species and to improve tools for detection and monitoring of this highly damaging invasive pest. Future research should explore Fagus volatile blend analyses to broaden the scope of potential synergists, electroantennal response comparisons of pre- and post-overwintered O. fagi, and lure development for field and lab trials.
References
Alford DV (1995). A color atlas of pests of ornamental trees, shrubs and flowers. Halsted Press, New York
Aslam NA (1961) An assessment of some internal characters in the higher classification of the Curculionidae S.L. (Coleoptera). Trans R Ent Soc Lond 113:417–480
Bale JS (1979) The occurrence of an adult reproductive diapause in the univoltine life cycle of the beech leaf mining weevil, Rhynchaenus fagi L. Int J Invert Rep 1:57–66
Bale JS (1981) Seasonal distribution and migratory behaviour of the beech leaf mining weevil, Rhynchaenus fagi L. Ecol Entomol 6:109–118
Bale JS (1984) Bud burst and success of the beech weevil, Rhynchaenus fagi: feeding and oviposition. Ecol Entomol 9:139–148
Bale JS, Luff ML (1978) The food plants and feeding preference of the beech leaf mining weevil, Rhynchaenus fagi L. Ecol Entomol 3:245–249
Bartelt RJ (1999) Weevils. In: Hardie J, Minks AK (eds) Pheromones of non-lepidopteran insects associated with agricultural plants. CABI, Wallingford, pp 91–112
Beaudet M, Messier C (2008) Beech regeneration of seed and root sucker origin: a comparison of morphology, growth, survival, and response to defoliation. Forest Ecol Manag 255:3659–3666
Cao QJ, Yu J, Ran YL, Chi DF (2015) Effects of plant volatiles on electrophysiological and behavioural responses of Cryptorhynchus lapathi. Ent Exp App 156:105–116
Cheong JJ, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19:409–413
Davis C, Meyer T (2004) Field guide to tree diseases of Ontario. Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre, Sault Ste. Marie, ON. NODA/NFP Tech Rep TR-46:92–93
Dickens JC, Prestwich GD, Sun WC (1991) Behavioral and neurosensory responses of the boll weevil, Anthonomus grandis Boh. (Coleoptera: Curculionidae), to fluorinated analogs of aldehyde components of its pheromone. J Chem Ecol 17:1007–1020
Dieter VA (1964) Beitrag zur epidemiologie und biologie des Buchenspringrüßlers Rhynchaenus (Orchestes) fagi L. an Obstgewächsen. Anz Schädl 37:161–163
Dindorf T, Kuhn U, Ganzeveld L, Schebeske G, Ciccioli P, Holzke C, Kölbe R, Seufert G, Kesselmeier J (2006) Significant light and temperature dependent monoterpenes emissions from European beech (Fagus sylvatica L.) and their potential impact on the European volatile organic compound budget. J Geosphy Res 111:1–15
Ehrlich J (1934) The beech bark disease. A Nectria disease of Fagus, following Cryptoccus fagi (Baer.). Can J Res 10:593–692
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci 101:1781–1785
Fall R, Karl T, Hansel A, Jordan A, Lindinger W (1999) Volatile organic compounds emitted after leaf-wounding: on-line analysis by proton-transfer-reaction mass spectrometry. J Geol Res 104:15963–15974
Farmartin (2014) American beech foliage during autumn in the woodlands along the West Branch Shabakunk Creek in Ewing, New Jersey. In: Wikimedia Commons. https://commons.wikimedia.org/w/index.php?curid=36975055#filehistory. Accessed 8 September 2020
Finch S, Collier RH (2000) Host-plant selection by insects – a theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. Entomol Exp Appl 96:91–102
Ghelardini C, Galeotti N, Mannelli LDC, Mazzanti G, Bartolini A (2001) Local anaesthetic activity of β-caryophyllene. Il Farmaco 56:387–389
Goodwin JTL, Pawlowski SPP, Mayo PD, Silk PJ, Sweeney JD, Hillier NK (2020) Influence of trap colour, type, deployment height, and a host volatile on monitoring Orchestes fagi (Coleoptera: Curculionidae), in Nova Scotia, Canada. Can Entomol 152:98–109
Gossner MM, Weisser WW, Gershenzon J, Unsicker SB (2014) Insect attraction to herbivore-induced beech volatiles under different forest management regimes. Oecologia 176:569–580
Grimm R (1973) Food and energy turnover of phytophagous insects in beech forests. I Oecologia 11:187–262
Grimm R (1990) Flugverhalten und wirtdfindung des Buchenspringrüßlers Rhynchaenus fagi L. (Col.: Curculionidae) beim einflug in den buchenwald nach der überwinterung. Mitt Dtsch Ges Allg Angew Ent 7:395–404
Gupta AD, Bansal VK, Babu V, Maithil N (2013) Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J Gen Eng Biotech 11:25–31
Hillier NK, Kavanagh RMB (2015) Differential octopaminergic modulation of olfactory receptor neuron responses to sex pheromones in Heliothis virescens. PLoS One 10:e0143179
Hillier NK, Kleineidam C, Vickers NJ (2006) Physiology and glomerular projections of olfactory receptor neurons on the antennae of female Heliothis virescens (Lepidoptera: Noctuidae) responsive to behaviourally relevant odors. J Comp Physiol A 192:199–219
Houston DR (1994) Major new tree disease epidemics: beech bark disease. Annu Rev Phytopathol 32:75–87
Houston DR, O’Brien, JT (1983). Beech bark disease. Forest insect & disease leaflet 75, U.S. Department of Agriculture Forest Service
Houston DR, Parker EJ, Lonsdale D (1979) Beech bark disease: patterns of spread and development of the initiating agent Cryptococcus fagisuga. Can J For Res 9:336–344
Javadipour Z, Balouchi H, Dehnavi MM, Yadavi A (2019) Roles of methyl jasmonate in improving growth and yield of two varieties of bread wheat (Triticum aestivum) under different irrigation regimes. Agr Water Manage 222:336–345
Jha S, Harcombe PA, Fulton MR, Elsik IS (2004) Potential causes of decline in American beech (Fagus grandifolia Ehrh.) in weir woods, Texas. Tex J Sci 56:285–298
Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M (2002) Aroma compound analysis of Piper nigrum and Piper guineese essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J Chromatogr A 976:265–275
Kaissling KE (1974) Sensory transduction in insect olfactory receptors. In: Jaenicke L (ed) Biochemistry of sensory functions. Springer, Heidelberg, pp 243–273
Keesey IW, Barrett BA, Lin CH, Lerch RN (2012) Electroantennographic responses of the small chestnut weevil Curculio sayi (Coleoptera: Curculionidae) to volatile organic compounds identified from chestnut reproductive plant tissue. Environ Entomol 41:933–940
Kendra PE, Montgomery WS, Niogret J, Deyrup MA, Guillén L, Epsky ND (2012) Xyleborus glabratus, X. affinis, and X. ferrugineus (Coleoptera: Curculionidae: Scolytinae): Electorantennogram responses to host-based attractants and temporal patterns in host-seeking flight. Environ Entomol 41:1597–1605
König G, Brunda M, Puxbaum H, Hewitt CN, Duckham SC (1995) Relative contribution of oxygenated hydrocarbons to the total biogenic VOC emissions of selected mid-European agricultural and natural plant species. Atmos Environ 29:861–874
Leskey TC, Wright SE, Anger W, Chouinard G, Cormier D, Pichette A, Zhang A (2009) Electroantennogram technique for Conotrachelus nenuphar (Coleoptera: Curculionidae). Environ Entomol 38:870–878
Luo H, He W, Li D, Bao Y, Riaz A, Xiao Y, Song J, Liu C (2020) Effect of methyl jasmonate on carotenoids biosynthesis in germinated maize kernels. Food Chem 307:125525
MacKay CA, Sweeney JD, Hillier NK (2015) Olfactory receptor neuron responses of a longhorned beetle, Tetropium fuscum (Fabr.) (Coleoptera: Cerambycidae), to pheromone, host, and non-host volatiles. J Insect Physiol 83:65–73
Macnish AJ, Jiang CZ, Negre-Zakharov F, Reid MS (2010) Physiological and molecular changes during opening and senescence of Nicotiana mutabilis flowers. Plant Sci 179:267–272
Mihál I, Cicák A, Tsakov H (2014) Selected biotic vectors transmitting beech bark necrotic disease in central and South-Eastern Europe. Folia Oecologica 41:62–74
Moise ERD, Forbes GB, Morrison A, Sweeney JD, Hillier NK, John RC (2015) Evidence for a substantial host-use bottleneck following the invasion of an exotic, polyphagous weevil. Ecol Entomol 40:796–804
Morin RS, Liebhold AM, Tobin PC, Gottschalk KW, Luzader E (2007) Spread of beech bark disease in the eastern United States and its relationship to the regional forest composition. Can J For Res 37:726–736
Morrison A, Sweeney J, Hughes C, Johns R (2017) Hitching a ride: firewood as a potential pathway for range expansion of an exotic beech leaf-mining weevil, Orchestes fagi (Coleoptera: Curculionidae). Can Entomol 149:129–137
Moukhtar S, Bessagnet B, Rouil L, Simon V (2005) Monoterpene emissions from beech (Fagus sylvatica) in a French forest and impact on secondary pollutants formation at regional scale. Atmos Environ 39:3535–3547
Mozaffar A, Schoon N, Bachy A, Digrado A, Heinesch B, Aubinet M, Fauconnier ML, Delaplace P, du Jardin P, Amelynck C (2018) Biogenic volatile organic compound emissions from senescent maize leaves and a comparison with other leaf developmental stages. Atmos Environ 176:71–81
Mutis A, Parra L, Manosalva L, Palma R, Candia O, Lizama M, Pardo F, Perich F, Quiroz A (2010) Electroantennographic and behavioral responses of adults of raspberry weevil Aegorhinus superciliosus (Coleoptera: Curculionidae) to odors released from conspecific females. Environ Entomol 39:127–1282
Nielsen O (1966) Studies on the fauna of beech foliage 1. Contribution to the biology of the early stages of the beech weevil (Rhynchaenus (Orchestes) fagi L.), (Coleoptera: Curculionidae). Natura Jutl 12:162–181
Nielsen O (1968) Studies on the fauna of beech foliage 2. Observations on the mortality and mortality factors of the beech weevil [Rhynchaenus (Orchestes) fagi L.] (Coleoptera: Curculionidae. Natura Jutlandica 14:1011–1021
Nielsen O (1970) Observations on the hibernation of the beech weevil (Rhynchaenus fagi L.) in Denmark. Ent Scand 1:223–226
Nielsen BO (1974a) A record of insect activity on beech stems (Fagus sylvatica L.) by means of arboreal photoecloectors. Ent Meddr 42:1–18
Nielsen BO (1974b) A study on the weevil fauna (Curculionidae) in a Danish beech forest. Ent Medd 42:169–188
Nielsen BO, Ejlersen A (1977) The distribution pattern of herbivory in a beech canopy. Ecol Entomol 2:293–299
Nielsen BO (1978) Food resource partition in the beech leaf‐feeding guild. Ecol Entomol 3:193–201
Obando-Ulloa JM, Nicolai B, Lammertyn J, Bueso MC, Monforte AJ, Fernández-Trujillo JP (2009) Aroma volatiles associated with the senescence of climacteric or non-climacteric melon fruit. Postharvest Biol Technol 52:146–155
Ormeño E, Baldy V, Ballini C, Fernandez C (2008) Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: effect on soil nutrients. J Chem Ecol 34:1219–1229
Pajares JA, Allue M, Hernandez E (1990) Rhynchaenus fagi L., un curcliónido minador foliar del haya. Bol San Veg Plaga 16:411–418
Pawlowski SP (2014). Chemical ecology of the invasive beech leaf mining weevil (Orchestes fagi L.) in Nova Scotia, Canada. B.Sc. Honours Thesis, Acadia University, April 2014
Pawlowski, SP (2017). Comparative analyses on the phenology, electrophysiology, and chemistry of the beech leaf-mining weevil (Orchestes fagi L.) and its novel host American beech (Fagus grandifolia Ehrh.). Master’s thesis. Acadia University
Phillipson J, Thompson DJ (1983) Phenology and intensity of phyllophage attack on Fagus sylvatica in Wytham woods, Oxford. Ecol Entomol 8:315–330
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Pullin AS (1985) A simple life table study based on development and mortality in the beech leaf mining weevil Rhynchaenus fagi L. J Biol Educ 19:152–156
R Core Team (2015). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available from: http://www.R-project.org/
Ranger CM, Reding ME, Schultz PB, Oliver JB (2012) Ambrosia beetle (Coleoptera: Curculionidae) responses to volatile emissions associated with ethanol-injected Magnolia virginiana. Environ Entomol 41:636–647
Sabulal B, Dan M, John A, Kurup R, Pradeep NS, Valsamma RK, George V (2006) Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: chemical characterization of antimicrobial activity. Phytochemistry 67:2469–2473
Sangster A, Singh S, Sooksom R (2010). Climate and soil considerations: resource kit for Nova Scotia farmers. Nova Scotia Department of Agriculture. https://novascotia.ca/thinkfarm/documents/fsheets/02-climate-soil-considerations.pdf. Accessed 4 December 2019
Schuh G, Heiden AC, Hoofman T, Khal J, Rockel P, Rudolph J, Wildt J (1997) Emissions of volatile organic compounds from sunflower and beech: dependence on temperature and light intensity. J Atmos Chem 27:219–318
Silk PJ, Mayo PD, LeClair G, Brophy M, Pawlowski S, MacKay C, Hillier NK, Hughes C, Sweeney JD (2017) Semiochemical attractants for the beech leaf-mining weevil, Orchestes fagi. Ent Exp App 164:102–112
Strausfeld NJ (2012) Arthropod brains: evolution, functional elegance, and historical significance. Harvard University Press, Cambridge, Massachusetts
Sweeney J, Anderson RS, Webster RP, Neville R (2012) First records of Orchestes fagi (L.) (Coleoptera: Curculionidae: Curculioninae) in North America, with a checklist of the north American Ramphini. Coleopt Bull 66:297–304
Sweeney J, Hughes C, Zhang H, Hillier NK, Morrison A, Johns R (2020) Impact of the invasive beech leaf-mining weevil, Orchestes fagi, on American beech in Nova Scotia, Canada. Front For Global Change 3:1–11
Tavallali V, Karimi S (2019) Methyl jasmonate enhances salt tolerance of root stalks by regulating endogenous phytohormones, antioxidant activity and gas exchange. J Plant Physiol 234-235:98–105
Tholl D, Boland W, Hansel A, Loreto F, Röse USR, Schnitzler JP (2006) Practical approaches to plant volatile analysis. Plant J 45:540–560
Tollsten L, Müller PM (1996) Volatile organic compounds emitted from beech leaves. Phytochemistry 43:759–762
Toshova TB, Velchev DI, Subchev MA, Tóth M, Vuts J, Pickett JA, Dewhirst SY (2010) Electrophysiological responses and field attraction of the grey corn weevil, Tanymecus (Episomecus) dilaticollis Gyllenhall (Coleoptera: Curculionidae) to synthetic plant volatiles. Chemoecology 20:199–206
Tubbs CH, Houston DR (1990). American beech (Fagus grandifolia Ehrh.). In: Silvics of North America (Burns RM, Honkala BH: technical coordinators). USDA Forest Service agriculture handbook 654, USDA Forest Service, Washington, DC, USA, pp 654-667. Available from: https://www.srs.fs.usda.gov/pubs/misc/ag_654/volume_2/vol2_table_of_contents.htm. Accessed 8 Sept 2020
van Tol RWHM, Visser JH (2002) Olfactory antennal responses of the vine weevil Otiorhynchus sulcatus to plant volatiles. Ent Exp App 102:49–64
Wang G, Tian L, Aziz N, Broun P, Dai X, He J, King A, Zhao PX, Dixon RA (2008) Terpene biosynthesis in glandular trichomes of hops. Plant Physiol 148:1254–1266
Watt AD, McFarlane AM (1992) Does damage-mediated intergenerational conflict occur in the beech leaf-mining weevil? Oikos 63:171–174
Woodcock BA, Vanbergen AJ (2008) Parasitism of the beech leaf-miner weevil in a woodland: patch size, edge effects and parasitoid species identity. Insect Conserv Diver 1:180–188
Acknowledgements
We would like to thank Drs. Peter Silk and Peter Mayo for providing chemical inventory and to Natural Resources Canada, SERG-International, Atlantic Canada Opportunities Agency – Atlantic Innovation Fund, and Acadia University for funding. All experiments reported here comply with the laws of Canada.
Availability of Data and Material
Data are not openly accessible.
Code Availability
Not applicable.
Funding
Funding for this research was provided by grants and contracts from Natural Resources Canada, SERG-International grant, Atlantic Canada Opportunities Agency Atlantic Innovation Fund (197853), and Acadia University.
Author information
Authors and Affiliations
Contributions
All authors participated in designing research. SP conducted experiments, analyzed results, and wrote manuscript. NKH and JS edited manuscript. All authors read and approved manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
Nothing to disclose.
Ethics Approval
No ethics approval required, this research did not involve studies with human participants or animals.
Consent to Participate
No consent to report.
Consent for Publication
No consent to report.
Rights and permissions
About this article
Cite this article
Pawlowski, S.P., Sweeney, J.D. & Hillier, N.K. Electrophysiological Responses of the Beech Leaf-Mining Weevil, Orchestes fagi, to Seasonally-Variant Volatile Organic Compounds Emitted by American Beech, Fagus grandifolia. J Chem Ecol 46, 935–946 (2020). https://doi.org/10.1007/s10886-020-01216-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01216-z