Abstract
It is known that plant and associated bacteria coevolved, but just now the roles of chemical signaling compounds in these intricate relationships have been systematically studied. Many Gram-negative bacteria produce N-acyl-L-homoserine lactones (AHL), chemical signals used in quorum-sensing bacterial communications mechanisms. In recent years, it has been shown that these compounds may also influence the development of plants, acting as allelochemicals, in still not well understood eukaryot-prokaryot interactions. In the present work, a quorum-sensing molecule produced by the tomato associated bacterium Pseudomonas sp. was characterized and its effects on germination and growth of tomato seedlings were accessed. The chemical study of the bacterium extract led to the identification of N-3-oxo-dodecanoyl-L-homoserine lactone (1), using gas chromatography coupled to electron impact mass spectrometry (GC-MS), and ultra-high resolution Qq-time-of-flight mass spectrometry (UHR-QqTOF-MS) equipped with an electrospray ionization source (ESI). The synthetic compound was tested at different concentrations in tomato to evaluate its effects on seed germination and seedlings root growth. Inhibition of tomato seed germination and root growth were observed in the presence of micromolar concentrations of the compound 1. Scanning electron microscopy evidenced morphological alterations on roots in the presence of the compound, with reduction of growth, impaired root hairs development and cracks in the rhizodermis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tomatoes are an important part of the human diet and are commonly consumed in fresh or in a large variety of food products. Originally native from South America, it is now cultivated worldwide (Xu et al. 2018). In recent years, one of the main concerns in tomato cultivation is to understand the effects of compounds produced by environmental and rhizosphere microorganisms on the plant development (Zhang et al. 2010). The presence of bacteria in the rhizosphere can directly influence plant growth and root development through different mechanisms such as biological nitrogen fixation, phytohormones synthesis, biocontrol of pathogenic organisms, and synthesis of allelochemicals, which may benefit the plant growth according to the necessities of the bacteria (Scagliola et al. 2016; Babalola 2010; Bloemberg and Lugtenberg 2001; de Salamone et al. 2001).
In bacteria, many phenotypes are frequently regulated by a bacterial intercellular chemical communication mechanism known as quorum-sensing (Wei and Zhang 2006). It is an extracellular signaling process, which involves the production, release and detection of molecules called auto-inducers. In Gram-negative bacteria, the main signaling compounds are N-acyl-L-homoserine lactones (AHLs), which are biosynthesized from fatty acids and S-adenosyl-L-methionine as precursors (Federle and Bassler 2003).
Pseudomonas is a genus of Gram-negative bacteria commonly found in rhizosphere (Botelho and Mendonça-Hagler 2006). This genus currently comprises 255 species and several of them have the ability to colonize a wide range of plant niches. Among the most common auto-inducers produced by rhizosphere symbiotic pseudomonads are the N-hexanoyl-L-homoserine lactone (C6-L-HL), N-decanoyl-L-homoserine lactone (C10-L-HL), N-3-oxo-tetradecanoyl-L-homoserine lactone (3-oxo-C14-L-HL) and N-3-hydroxy-tetradecenoyl-L-homoserine lactone (3-OH-C14:1-L-HL) (Hartmann and Schikora 2012; Laue et al. 2000; Wei and Zhang 2006). An interesting example of quorum-sensing systems found in rhizosphere pseudomonads is presented by P. putida. This species, originally isolated from tomato rhizosphere, produces a wide spectrum of N-3-oxo-AHLs, with six to twelve carbon atoms in the acyl side chain, including N-3-oxo-dodecanoyl-L-homoserine lactone (3-oxo-C12-L-HL) (Arevalo-Ferro et al. 2005). In P. aureofaciens, the production of the antibiotic phenazine, an important compound for protection of wheat roots against phytopathogenic bacteria, is also controlled by quorum-sensing and AHL (Zhang and Pearson 2001; Wood and Pearson III 1996).
Besides the above-mentioned examples of intraspecific bacterial quorum-sensing regulated processes, the AHL bacterial signaling compounds may also play a role in plant growth and development, in a poorly understood interspecific eukaryote-prokaryote chemical cross talk (Joseph and Phillips 2003; Schuhegger et al. 2006; Von Rad et al. 2008; Bai et al. 2012; Cha et al. 1998). The physiological effects and mechanisms of action of AHLs on plant development were previously investigated in Arabidopsis thaliana, where AHLs with acyl side chains ranging from 4 to 14 saturated carbon atoms were evaluated (Castro-Ortíz et al. 2008; Delatorre and Silva 2008). The use of micromolar concentrations of AHL in A. thaliana seeds led to a significant decrease in primary root growth, which was associated to interferences in ethylene phytohormone biosynthesis (Palmer et al. 2014). In addition to this, it has been shown that AHL may influence nodulation in Medicago truncatula, even in the absence of bacteria, and that this effect was not observed for M. sativa and Trifolium repens, indicating that the effects of AHL on plants may be species-specific (Palmer et al. 2016; Veliz-Vallejos et al. 2014). Schuhegger et al. (2006) demonstrated the induction of systemic resistance after application of micromolar concentrations of short-chain N-C6-L-HL and N-C4-L-HL (from Serratia liquefaciens) in tomato, which led to higher production of salicylic acid and expression of genes encoding pathogenesis-related proteins.
Another example of plant physiological response to the presence of AHL was reported recently by our research group. The phytochemical study of leaves and culms of Saccharum × officinarum led to the identification of N-3-oxo-octanoyl-L-HL, which was tested in sugarcane culms at micromolar concentrations. Changes in the biomass and length of germinated buds and sett roots were observed and scanning electron microscopy showed anato-morphological abnormalities in the cells of roots at both small and high concentrations of AHL (Olher et al. 2016).
Up to now, very little is known concerning to the effects of these molecules in plants. It is not clear if AHL compounds may stimulate or deter plant growth under different conditions and host-bacteria pairs. Indeed, the reduction of roots length in the presence of these compounds places doubts about the roles of these molecules and what benefits could arise for one of the evolved organisms from such interaction. In a series of papers, Schenck et al. (2012, 2014) and Schenk and Schikora (2015) demonstrated that AHL may cause reinforcement of roots cell walls of Arabidopsis plants, which could be associated for example to increased resistance to microorganisms. However, scarce information concerning to the effects of these compounds in other plant species, including crops, prevents more accurate discussions.
Taking into account the importance of chemical cross talk in plant-bacteria interactions and the recent developments in this research field, the aim of this study was to characterize the AHL produced by a bacterium of the genus Pseudomonas isolated from tomato (Solanum x lycopersicum L.) rhizosphere. Then, the effects of the identified compound on seeds germination and growth of tomato seedlings were also studied, including analyses by scanning electron microscopy. As far as we know, this is the first study of the effects of an AHL compound identified from a rhizosphere bacterium and its directly associated plant. Moreover, most of the literature found is related to experimental model plants, while little is found for commercially important legumes or crops, inside an agroecology perspective.
Material and Methods
General Experimental Procedures
The low resolution mass spectra were acquired on a Focus GC (Thermo Finnigan) gas chromatograph coupled to a DSQ II mass spectrometer (Thermo Finnigan) equipped with an electron impact ionization source (EI, 70 eV). Data acquisition was performed using the Xcalibur software. High purity helium (99.999%) (5.0) (White Martins) was used as drag gas with 1 mL min-1 flow. The analyses were performed with the injector working at 260 °C and 1:10 injection mode with filled injector liner. The capillary column used was DB-5 (30 m × 0.25 mm × 0.25 μm) (5% phenyl, 95% methyl polysiloxane) and the oven temperature program was 50 °C to 290 °C at 10 °C min-1, followed by isothermal at 290 °C for 20 min. Samples were prepared from 1 mg of each of the substances dissolved in 1 ml of chloroform (HPLC grade).
Analyses were also performed in a ultra-high resolution Qq-time-of-flight mass spectrometer (UHR-QqTOF-MS) equipped with an electrospray ionization source (ESI) (Impact II, Bruker Daltonics Corporation, Germany). The samples were dissolved at approximately 5 ppm in 1:1 H2O:MeCN solution containing 0.1% formic acid and injected by direct infusion in a flow of 20 μL min−1. For the mass spectra the following instrumental parameters were used: 3 kV capillary voltage in positive ion mode, 20 V cone voltage, 120 °C source temperature, 0.5 L h−1 nebulization gas flow. Before each analysis the instrument was calibrated with 0.005% H3PO4 solution in H2O: MeCN 1:1, m/z 100 to 1000.
Bacterium Biological Material and Chemical Study
The bacterium Pseudomonas sp. strain 16S was isolated in a previous study from tomato horticultural soil under organic management, and presented growth-promoting activities, such as phosphate solubilization and siderophore production (Zuluaga 2016). The strain identification was carried out by phylogenetic positioning (16S rRNA) with MEGA v.7, using the Maximum Likelihood method based on the Jukes-Cantor model (Jukes and Cantor 1969). It was deposited at GenBank under the accession number KX884933.1.
For crude extract preparation, the bacterium was grown in a culture medium containing glycerol (3% w/v), sucrose (5% w/v), yeast extract (5% w/v), K2HPO4 (0.15 w/v), 0.1 mL/ solution of micronutrients (g L-1: H3BO3, 1.4; ZnSO4.7H2O, 1.2; MnSO4.H2O, 1.18; Na2MoO4.2H2O, 1.0; CuSO4.5H2O, 0.04), cultivated for 48 h, under low agitation (150 rpm) and 28 °C (Oliveira et al. 2017), prepared in 5 × 1 L of conditioned broth. The culture broth was extracted with ethyl acetate (3 × 250 mL, per liter), stirring for 20 min, followed by evaporation under reduced pressure. The residue was subsequently filtered on a silica gel 60 chromatographic column, eluted with n-hexane, ethyl acetate and methanol. The ethyl acetate fraction (1.2435 g) was chromatographed in silica gel 60 using mixtures of n-hexane, CH2Cl2, AcOEt and MeOH in increasing polarity, with 120 fractions (10 mL each) collected. Fractions were then analyzed by gas chromatography coupled to mass spectrometry. In the fraction eluted at CH2Cl2:AcOEt 4:1 polarity, the compound N-3-oxo-dodecanoyl-homoserine lactone was identified. The compound was further identified using ultra-high resolution Qq-time-of-flight mass spectrometry (UHR-QqTOF-MS, 10 and 35 eV).
Tomato Germination and Growth Biological Assays
Seeds of tomato hybrid (Solanum x lycopersicum L.) cv. “Santa Cruz Kada Gigante” (SCKG) (Topseed ®) were acquired locally from a commercial source. For bioassays, initially a stock solution was prepared with 500 mL of distilled water and 5 mg of commercially available N-3-oxo-dodecanoyl-L-homoserine lactone (> 98% purity, Sigma Aldrich), solubilized in 100 μL of DMSO. Then dilutions were performed to obtain the following concentrations of AHL: 10.0; 7.5; 5.0; 1.0; 0.5 and 0.1 mg L−1.
For seed germination, the germination boxes (Gerbox) contained two sheets of qualitative filter paper and 40 tomato seeds, irrigated with 12 mL of the AHL solutions in different concentrations. The treatments for seed germination and initial growth bioassays were composed of five replicates. Finally, 200 seeds were treated therefore for each different concentration of AHL. For control test, 100 μL of DMSO in 500 mL of distilled water was used (v/v). The Gerbox were sealed with transparent plastic film and exposed to UV light in a laminar flow chamber for 30 min, and then incubated for 10 days in a germination chamber adapted to 25 °C under 12 h light/dark photoperiod. The germinated seeds were counted daily. The data obtained from the tests were evaluated by Germination Percentage (%G), Mean Germination Time (MGT) and Germination Speed Index (GSI) according to Ferreira and Borghetti (2004).
The initial growth bioassays were performed on 12 cm Petri dishes containing two sheets of qualitative filter paper. Seeds of tomato, previously germinated (± 2 mm of root gravitropic curvature), irrigated with 15 mL of the solutions of AHL or blank solution were also added. The AHL solutions were prepared in the same concentrations and conditions for the germinations assays. Each Petri dish contained 15 pre-germinated seeds and the experiment was performed with five replicates. The dishes were sealed with transparent plastic film and exposed to UV light in a laminar flow chamber for 30 min. The incubation was done for 7 days in a germination chamber adapted to 25 °C under light/dark photoperiod 12 h. Growth was evaluated by measuring the root length and the hypocotyl length of 5 seedlings of each replicate (25 in total) with the help of graph paper. The results were evaluated using analysis of variance compared with Dunn’s test (p < 0.05), using the GraphPad Prism 7.0 program. The statistical results of the bioassays are expressed in comparison to the control of the tests (mean ± standard error of mean).
Scanning Electron Microscopy
SEM analyses were carried out for tomato seedlings treated with 3-oxo-C12-L-HL at 1.0; 5.0; 7.5; 10.0 mg L−1 concentrations, for both germinations and seedlings growth bioassays performed. The samples were fixed in Eppendorf tubes with Karnovsky solution (2.5% glutaraldehyde and 2.0% paraformaldehyde in 0.05 M cacodylate buffer, pH 7.2). The samples were dehydrated using ethanol (10% - 100%) to the critical point, at temperature of 9 °C and 55 bar of CO2. After drying, the samples were metalized using high-purity gold before SEM analyses performed on Quanta 250 equipment (FEI Company).
Results
Strain Identification
The Pseudomonas sp. was identified by the global alignment of its partial 16S rRNA gene sequence to all Pseudomonas type strains sequences retrieved from GenBank (Fig. 1 and Supplementary Material S1). The respective GenBank access numbers are presented in the brackets.
Chemical Study of Cultures Broth
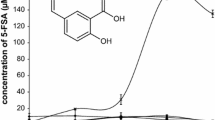
The chemical study of cultures broth of this bacterial strain revealed the presence of N-3-oxo-dodecanoyl-L-homoserine lactone (1) (Fig. 2), as a minor component in a complex fraction, identified by gas chromatography coupled to mass spectrometry detector, in electron impact mode (70 eV). Due to the small amount present, the chromatogram was acquired in selected ion mode, and a peak with retention time of 16.59 min with coherent fragmentation pattern was identified (Supplementary Material S2).
The low resolution mass spectrum (Supplementary Material S3) exhibited fragmentation peaks at m/z 185 and 143, coming from the lateral chain McLafferty rearrangements of β-keto group and amide carbonyl group, respectively (Cataldi et al. 2004, 2009). The molecular ion at m/z 297 was also observed, in small intensity. In the ultra-high resolution Qq-time-of-flight mass spectrum (Supplementary Material S4), the protonated molecular ion at m/z 298.2999 was observed (10 eV), together with the typical fragment at m/z 102.0544, which is the protonated homoserine lactone moiety (positive mode, 35 eV, Supplementary Material S5). The fragment at m/z 185.0749 (10 eV) was also observed. According to these data the compound was identified as N-3-oxo-dodecanoyl-L-HL (1).
Germination and Growth Biological Assays
Biological assays with the commercially available compound 1 were performed with seeds and seedlings of tomato (Solanum x lycorpersicum cv. SCKG). The parameters analyzed were seed germination percentage (%G), mean germination time (MGT) and germination speed index (GSI). The results may be seen in Fig. 3.
Germination profile of tomato seeds (S. x lycorpersicum cv. SCKG) under different concentrations of compound 1 (n = 200 replicates). Seeds in the presence of control solution (DMSO in water) or different concentrations of compound 1 were incubated for 10 days in a germination chamber adapted to 25 °C under 12 h light/dark photoperiod. (A) Germination percentage (%G), (B) mean germination time (MGT), and (C) germination speed index (GSI). Higher concentrations of compound 1 statistically reduced the germination percentage. *Mean ± SEM differs significantly from the control assay (Dunn’s test, p < 0.05)
Analyzing the germination profile in different concentrations of compound 1, a significant decrease in germination percentage from 80% to 58% was observed with the increase of the concentration of compound 1 up to 7.5 mg L−1. The germination speed index, presented a smaller value of 4.19 in the concentration of 7.5 mg L−1. Therefore, statistically significant changes in the germination profile were observed in bioassays with compound 1 at higher concentrations. Representative picture of seeds used in this experiment may be seen in Fig. 4.
The scanning electron microscopy analyses of tomato seedlings (Fig. 5) showed that highest concentrations of compound 1 (7.5 and 10.0 mg mL−1) clearly prejudiced the root growth, leading to thickened and underdeveloped roots in comparison to the control (Fig. 5c and d). Figure 5d shows the full extent of the tomato seedling at a concentration of 10 mg mL−1 in the same SEM scale as the other ones, highlighting the size difference between tomato seedlings.
Scanning electron microscopy (500 μm) analyses of tomato seedlings germinated in the presence of control solution and treated with compound 1 at 1.0 mg L−1 (a), 5.0 mg L−1 (b), 7.5 mg L−1 (c), and 10.0 mg L−1 (d) concentrations. Shorter, underdeveloped roots were observed in the presence of compound 1 at higher concentrations
The initial growth behavior of tomato seedlings subjected to different concentrations of compound 1 was also studied (Fig. 6). A general trend of decreased root length with the increase of concentration of compound 1 was observed. Higher concentrations of the compound (7.5 and 10 mg L−1) led to statistically significant decrease in the root length of the seedlings. Hypocotyl growth behavior was similar at concentrations of 0.1 to 1.0 mg L−1 of compound 1, with an average of 2.73 to 3.18 cm, and were slightly smaller than to the control.
Growth of tomato seedlings (S. x lycorpersicum cv. SCKG) in the presence of control solution and different concentrations of compound 1 (n = 25 replicates). Seeds were previously germinated and exposed to the testing solutions, incubated for 7 days at 25 °C under light/dark photoperiod of 12 h. Higher concentrations of the compound led to reduced roots length. *Mean ± SEM differs significantly from the control assay (Dunn’s test, p < 0.05)
Scanning electron microscopy was also used to analyze roots after seedling growth assays with different concentrations of compound 1 (Fig. 7). For the roots treated with this compound in lower concentrations, the cells were regular, flat and entirety, while roots of plants treated with compound 1 at 7.5 mg L−1 and 10 mg L−1 showed wrinkled cells, with cracks in the root dermis and few root hairs.
Discussion
The Pseudomonas sp. species studied herein was previously identified as a tomato associated bacterium (Zuluaga 2016). For strain identification, a phylogenetic tree was built with 15 type strains of representative type species from Pseudomonas putida group (Gomila et al. 2015) and the strain 16S (shown in bold) used in this study. The Jukes-Cantor method was applied to construct distance matrices followed by generation of dendrogram by neighbor-joining (Jukes and Cantor 1969). The outgroup is represented by Cellvibrio japonicus Ueda107, and bootstraps values higher than 50% after 1000 replicates are indicated at branches. Highest sequence identity of the isolated strain was observed with the species P. entomophila and P. mosselii (98.4% identity), as observed in Fig. 1.
Acyl-homoserine lactones have long been recognized as chemical signaling compounds in bacteria. Up to now, this class of compounds is reported only in Gram-negative bacteria, while Gram-positive employ mainly oligopeptides in chemical communication processes (Whitehead et al. 2001). Besides of typically controlling the expression of several phenotypes for producing bacteria, it has been recognized that these compounds may influence the ability of the organisms to adapt themselves in natural ecosystems, including host interactions. Herein, the strain Pseudomonas sp. 16S isolated from the tomato rhizosphere produced N-3-oxo-dodecanoyl-L-homoserine lactone 1. This compound was already described for other pseudomonads, specially the important human pathogen P. aeruginosa, which uses it for regulation of expression of several phenotypes like motility, biofilm formation, and iron scavenging (Lee and Zhang 2015). Compound 1 and other structurally related AHL were also described for plant-associated pseudomonads (Loh et al. 2002).
Once compound 1 was identified, its ability to interfere in tomato seeds germination and growth was also assessed. The germination and growth biological assays were done with the same tomato variety used to confirm the growth promoting activity of the Pseudomonas sp. 16S strain. According to germination of tomato seeds assays, small concentrations of compound 1 did not alter significantly the roots length and several germination parameters. Nevertheless, higher concentrations of compound 1 led to lower germination percentages, for example, the application of compound 1 at a concentration of 7.5 mg/L reduced tomato germination percentage by 58%.
The germination speed index (GSI) (Maguire 1962) has been the most used test to evaluate the speed of germination. The calculation uses the sum of the average daily germination and, the higher the index, the higher the seed germination speed. At a concentration of 7.5 mg L−1, germination percentage and germination speed index of tomato seedlings decreased. Scanning electron microscopy for tomato seedlings at this concentration (Fig. 5b) corroborate these results, showing shorter and underdeveloped roots in comparison to the control, indicating that high concentrations of compound 1 are toxic for germination. As previously reported (Palmer et al. 2014), AHL can induce the production of ethylene, and can therefore inhibit the development of roots.
The difference in the growth behavior of tomato seedlings in the presence of different concentrations of compound 1 corroborates the results observed in the germination assays (Fig. 5), where higher concentrations seem to inhibit root development. Scanning electron microscopy showed a clear indication of abnormal development for the roots at after seedlings growth assays, as observed in Fig. 7. In the bioassay with compound 1 at concentration 5 mg L−1 the cells were regular, and with the increase in concentration the roots showed cells imperfections. In a series of papers, Schenck et al. (2012, 2014, 2015) demonstrated that AHL may cause reinforcement in Arabidopsis roots cells walls. A similar behavior was observed in the present study under SEM analyses (Fig. 7a), where the roots of tomato seedlings grown in the presence of compound 1 at 5.0 mg L−1 showed a much more woody aspect than the control. Hypothetically, the reinforcement of plant cell walls may be a direct response of the plant to the presence of a bacterial signaling compound, which therefore is used as an allelochemical signal by the plant defense system to prevent its infection and colonization by microorganisms. It is know that Arabidopsis plants develop more resistant roots in the presence of AHL (Schenk et al. 2012, 2014 Schenk and Schikora 2015; Schikora et al. 2011), which could be associated for example to increased resistance to microorganisms. On the other hand, cracks in the roots tissues could create niches able to facilitate the growth of beneficial bacteria, or even facilitate the entrance of phytopathogens. In this way, the possible benefits or detriments of the biological action of such molecules for plants or microorganisms in rhizosphere remain as an issue for future works.
Herein, it was demonstrated for the first time that an AHL produced by a bacterium may change the seed germination and growth pattern of roots of its direct host plant. Several benefits have been arising recently from agroecology studies, and we believe the correct understanding of how rhizosphere bacterial compounds influence plant growth is crucial not only for model plants but also crops.
References
Arevalo-Ferro C, Reil G, Görg A, Eberl L, Riedel K (2005) Biofilm formation of Pseudomonas putida IsoF: the role of quorum sensing as assessed by proteomics. Syst Appl Microbiol 28(2):87–114. https://doi.org/10.1016/j.syapm.2004.10.005
Babalola OO (2010) Beneficial bacteria of agricultural importance. Biotechnol Lett 32:1559–1570. https://doi.org/10.1007/s10529-010-0347-0
Bai X, Todd CD, Desikan R, Yongping Y, Hu X (2012) N-3-oxo-decanoyl-L-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide-dependent cyclic GMP signaling in mung bean. Plant Physiol 158:725–736. https://doi.org/10.1104/pp.111.185769
Bloemberg GV, Lugtenberg BJJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Botelho GR, Mendonça-Hagler LC (2006) Fluorescent pseudomonads associated with the rhizosphere of crops - an overview. Braz J Microbiol 3:401–416. https://doi.org/10.1590/S1517-83822006000400001
Castro-Ortíz R, Trujillo-Martínez M, Bucio-López J (2008) N-acyl-L-homoserine lactones: a class of bacterial quorum sensing signals alter post-embryonic root development in Arabidopsis thaliana plant. Plant Cell Environ 31:1497–1509
Cataldi TRI, Bianco G, Abate S (2009) Accurate mass analysis of -acyl-homoserine-lactones and cognate lactone-opened compounds in bacterial isolates of PAO1 by LC-ESI-LTQ-FTICR-MS. Int J Mass Spectrom 44(2):182–192
Cataldi TRI, Bianco G, Frommberger M, Schmitt-Kopplin P (2004) Direct analysis of selected N-acyl-L-homoserine lactones by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 18(12):1341–1344. https://doi.org/10.1002/rcm.1480
Cha C, Gao P, Chen YC, Shaw PD, Farrand SK (1998) Production of acyl-homoserine lactone quorum sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact 11:1119–1129
Delatorre CA, Silva AA (2008) Arabidopsis thaliana: a small plant a big role. Rev Ciên Agrár 31:58–67
de Salamone IG, Hynes RK, Nelson LM (2001) Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can J Microbiol 47:404–411. https://doi.org/10.1139/cjm-47-5-404
Federle MJ, Bassler BL (2003) Interspecies communication in bacteria. J Clin Invest 112:1291–1298
Ferreira AG, Borghetti F (2004) Germinação: do básico ao aplicado. Porto Alegre, Artmed
Gomila M, Peña A, Mulet M, Lalucat J, GarcÃa-Valdés E (2015) Phylogenomics and systematics in Pseudomonas. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00214
Hartmann A, Schikora A (2012) Quorum sensing of bacteria and trans-Kingdom interactions of N-Acyl homoserine lactones with eukaryotes. J Chem Ecol 38(6):704–713
Joseph C, Phillips D (2003) Metabolites from soil bacteria affect plant water relations. Plant Physiol Biochem 41:189–192
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Laue BE, Jiang Y, Chhabra SR, Jacob S, Stewart GS, Hardman A, Downie JA, O'Gara F, Williams P (2000) The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl)homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology 146:2469–2480. https://doi.org/10.1099/00221287-146-10-2469
Lee J, Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6(1):26–41. https://doi.org/10.1007/s13238-014-0100-x
Loh J, Pierson EA, Pierson LS III, Stacey G, Chatterjee A (2002) Quorum sensing in plant associated bacteria. Curr Op Plant Biol 5(4):285–290. https://doi.org/10.1016/S1369-5266(02)00274-1
Maguire JD (1962) Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Science, Madison 2:176–177. https://doi.org/10.2135/cropsci1962.0011183X000200020033x
Olher VG, Ferreira NP, Souza AG, Chiavelli LUR, Teixeira AF, Santos WD, Santin SM, Ferrarese Filho O, Silva CC, Pomini AM (2016) Acyl-homoserine lactone from Saccharum × officinarum with stereochemistry-dependent growth regulatory activity. J Nat Prod 79(5):1316–1321. https://doi.org/10.1021/acs.jnatprod.5b01075
Oliveira ALM, Santos OJAP, Marcelino PRF, Milani KML, Zuluaga MYA, Zucareli C, Gonçalves LSA (2017) Maize inoculation with Azospirillum brasillense Ab-V5 cells enriched with exopolysaccharides and polyhydroxybutyrate results in high productivity under low N fertilizer input. Front Microbiol 8:1837
Palmer AG, Senechal AC, Mukherjee A, Ané JM, Blackwell HE (2014) Plant responses to bacterial N-acyl-L-homoserine lactones are dependent on enzymatic degradation to L-homoserine. ACS Chem Biol 9:1834–1845. https://doi.org/10.1021/cb500191a
Palmer AG, Mukherjee A, Stacy DM, Lazar S, Ané JM, Blackwell HE (2016) Interkingdom responses to bacterial quorum sensing signals regulate frequency and rate of nodulation in legume-rhizobia symbiosis. Chembiochem 17:2199–2205
Scagliola M, Pii Y, Mimmo T, Cesco S, Ricciuti P, Crecchio C (2016) Characterization of plant growth promoting traits of bacterial isolates from the rhizosphere of barley (Hordeum vulgare L.) and tomato (Solanum lycopersicon L.) grown under Fe sufficiency and deficiency. Plant Physiol Biochem 107:187–196. https://doi.org/10.1016/j.plaphy.2016.06.002
Schenk ST, Schikora A (2015) AHL-priming functions via oxylipin and salicylic acid. Front Plant Sci 5. https://doi.org/10.3389/fpls.2014.00784
Schenk ST, Stein E, Kogel KH, Schikora A (2012) Arabidopsis growth and defense are modulated by bacterial quorum sensing molecules. Plant Signal Behav 7:178–181. https://doi.org/10.4161/psb.18789
Schenk ST, Hernandez-Reyes C, Samans B, Stein E, Neumann C, Schikora M, Reichelt M, Mithöfer A, Becker A, Kogel K, Schikora A (2014) N-acyl-homoserine lactone primes plants for cell wall reinforcement and induces resistance to bacterial pathogens via the salicylic acid/oxylipin pathway. Plant Cell 26:2708–2723. https://doi.org/10.1105/tpc.114.126763
Schikora A, Schenk ST, Stein E, Molitor A, Zuccaro A, Kogel KH (2011) N-acyl-homoserine lactone confers resistance towards biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol 157:1407–1418. https://doi.org/10.1104/pp.111.180604
Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, Hutzler P, Schmid M, van Breusegem F, Eberl L, Hartmann A, Langebartels C (2006) Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29:909–918. https://doi.org/10.1111/j.1365-3040.2005.01471.x
Veliz-Vallejos DF, van Noorden GE, Yuan M, Mathesius U (2014) A Sinorhizobium meliloti-specific N-acyl homoserine lactone quorum-sensing signal increases nodule numbers in Medicago truncatula independent of autoregulation. Front Plant Sci 5:551–564. https://doi.org/10.3389/fpls.2014.00551
Von Rad UV, Klein I, Kottova J, Zazimalova E, Fekete A, Hartmann A, Opplin-Schimitt P, Durner J (2008) Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine-lactone, a bacterial quorum sensing molecule produced in the rhizosphere. Planta 229:73–85
Wei H-L, Zhang L-Q (2006) Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 89(2):267–280
Whitehead NA, Barnard AML, Slater H, Simpson NJL, Salmond GPC (2001) Quorum sensing in gram negative bacteria. FEMS Microbiol Rev 25:365–404
Wood DW, Pierson LS III (1996) The PhI gene of Pseudomonas aureofaciens 30-84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168:49–53
Xu Q, Adyatni I, Reuhs B (2018) Effect of processing methods on the quality of tomato products. Food Nutr Sci 9:86–98. https://doi.org/10.4236/fns.2018.92007
Zhang Z, Pierson LS (2001) A second quorum-sensing system regulates cell surface properties but not phenazine antibiotic production inPseudomonas aureofaciens. Appl Environ Microbiol 67(9):4305–4315
Zhang F, Shen J, Zhang J, Zuo Y, Li L, Chen X (2010) Rhizosphere processes and management for improving nutrient use efficiency and crop productivity: implications for China. Adv Agron 107:1–32. https://doi.org/10.1016/S0065-2113(10)07001-X
Zuluaga MYA (2016) Caracterização bioquímica e molecular de bactérias diazotróficas isoladas de tomate (Solanum lycopersicum) e lulo (Solanum quitoense): influência do efeito rizosfera. Dissertation Master’s Degree in Biotechnology. State University of Londrina, Londrina, Paraná, Brazil. (http://www.bibliotecadigital.uel.br/document/?code=vtls000210152).
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (Capes), and Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Paraná (1.094.4056; conv. 02/2017).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 73 kb)
Rights and permissions
About this article
Cite this article
Ferreira, N.P., Ximenez, G.R., Chiavelli, L.U.R. et al. Acyl-Homoserine Lactone from Plant-Associated Pseudomonas sp. Influences Solanum lycopersicum Germination and Root Growth. J Chem Ecol 46, 699–706 (2020). https://doi.org/10.1007/s10886-020-01186-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01186-2