Abstract
There are contrasting hypotheses regarding the role of plant volatiles in host plant location. We used the grape berry moth (GBM; Paralobesia viteana)-grape plant (Vitis spp.) complex as a model for studying the proximate mechanisms of long distance olfactory-mediated, host-plant location and selection by a specialist phytophagous insect. We used flight tunnel assays to observe GBM female in-flight responses to host (V. riparia) and non-host (apple, Malus domestica; and gray dogwood, Cornus racimosa,) odor sources in the form of plant shoots, extracts of shoots, and synthetic blends. Gas chromatography-electroantennographic detection and gas chromatography/mass spectrometry analyses were used to identify antennal-active volatile compounds. All antennal-active compounds found in grape shoots were also present in dogwood and apple shoots. Female GBM flew upwind to host and non-host extracts and synthetic blends at similar levels, suggesting discrimination is not occurring at long distance from the plant. Further, females did not land on sources releasing plant extracts and synthetic blends, suggesting not all landing cues were present. Additionally, mated and unmated moths displayed similar levels of upwind flight responses to all odor sources, supporting the idea that plant volatiles are not functioning solely as ovipositional cues. The results of this study support a hypothesis that GBM females are using volatile blends to locate a favorable habitat rather than a specific host plant, and that discrimination is occurring within the habitat, or even post-landing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An organism’s survival depends on the location of patchily distributed resources. For example, the distribution of plants is often mediated by patchy resources, such as sunlight or soil nutrient availability (Cole and Weltzin 2005; Galiano 1985), as well as competitive interactions between plants. Plants themselves are important resources to herbivores and pollinators, and can be difficult for herbivores to locate when patchily distributed (Miller and Strickler 1984). Phytophagous insects can use plants as food, courtship/mating locations, and oviposition sites (Dethier 1941; Landolt and Phillips 1997; Schoonhoven et al. 2005), and there is evidence that plant volatiles can play a critical role in the location of a host plant (Bruce et al. 2005; Bruce and Pickett 2011; Finch and Collier 2000; Fraenkel 1959). However, the precise role of these volatiles (and the behaviors they elicit) in the host-location process remains poorly understood, and there is much debate regarding the mechanisms of olfactory-mediated host plant location (Bruce et al. 2005; Finch and Collier 2000; Fraenkel 1959).
Four principal hypotheses describing host plant location are illustrated in Fig. 1. Favorable abiotic conditions initiate the host location process (Fig. 1a). Common to all of the hypotheses is the idea that detection of habitat cues (referred to as the ‘habitat odor hypothesis’) is proposed to be an important first step in host-selection (Webster and Cardé 2016; Fig. 1b). Because specific host plant(s) may be difficult to locate in a habitat where many plant species exist, an insect might first search for a favorable habitat that is associated with the host plant to increase the probability of finding a host (Fig. 1b; Bell 1990; Meiners 2015; Webster and Cardé 2016). Insects may also use nonspecific habitat cues such as visual (Döring 2014), or differences in CO2 (Faucher et al. 2013) and relative humidity (Janzen 1987), to aid in the location of a favorable habitat. Common plant volatiles in high quantities may also be important habitat cues. These volatiles are generally ubiquitous in nature and, therefore, may not provide cues for a specific host plant. For example, tobacco budworm moths, Chloridea virescens, displayed increased attraction to, and laid more eggs on, tobacco plants supplemented with synthetic Germacrene-D (a common plant volatile) compared to control plants that do not produce Germacrene-D (Mozuraitis et al. 2002). An insect might use any or all of these cues to locate a favorable habitat, and then search for and select a specific host plant using key volatiles.
Visual summary of hypotheses describing insect host location. A Insect and box indicates that all theories describe this process (Bruce et al. 2005; Fraenkel 1959; Finch and Collier 2000; Webster and Cardé 2016). B Boxes are supported by the habitat odor hypothesis (Webster and Cardé 2016). C Boxes/insects are supported by the appropriate/inappropriate landings hypothesis (Finch and Collier 2000). D Boxes/insects are supported by the specific blends of ubiquitous compounds hypothesis (Bruce et al. 2005), (E) boxes/insects are supported by the ‘token stimulus hypothesis’ (Fraenkel 1959), and (D + E) insects/lines are supported by both the ‘specific blends of ubiquitous compounds hypothesis’ and the token stimulus hypothesis (Fraenkel 1959; Bruce et al. 2005). Intermittent black dotted lines indicate an odor plume, and gray dotted lines indicate landing behavior. In all cases, the insect takes flight in response to abiotic conditions (1). Habitat cues (B) may enhance each set of behaviors (Webster and Cardé 2016). Oriented upwind flight (2) and landing (3) are elicited by either specific blends of volatile compounds (D), or species-specific compounds (E), or no oriented upwind flight occurs (2, C). Nonspecific plant volatiles stimulate the insect to land on the plant (3, C), and initiate post-landing assessments of the plant. Spiral flights (4, C) are performed to determine whether the plant is a host or a non-host. Asterisks indicate the point at which discrimination occurs
In the ‘appropriate/inappropriate landings hypothesis’, Finch and Collier (2000; Fig. 1C) proposed that flying insects use nonspecific plant volatiles to land on a potential host (Fig. 1C). The insect assesses the potential host based on a series of consecutive short flights, termed ‘spiral flights’ (Prokopy et al. 1983), on plant material (Fig. 1C-4). In their study system, the specialist cabbage root fly, Delia radicum, exhibited short (5–10 cm) spiral flights either from the plant to the substrate (the ground or paper), from the substrate to the plant, or to and from the same site (plant or substrate) prior to oviposition (Prokopy et al. 1983). Ninety percent of the observed females performed spiral flights with an average of 4 flights per female. Contacts with non-host material (inappropriate landings) prevented females from acquiring sufficient positive stimuli to lay eggs (Finch and Collier 2000). Additionally, the female required consecutive landings on host plant material (appropriate landings) to lay an egg. Thus, in this model, insects use appropriate/inappropriate landings to discriminate between host and non-host plants, with plant selection occurring post-landing (Fig. 1C-4*).
In a second hypothesis, commonly referred to as the ‘token stimulus hypothesis’, Fraenkel (1959) suggested that host plant choice is based solely on the presence of ‘odd compounds’ specific to a particular taxon of plants (Fig. 1e). According to this hypothesis, species-specific compounds stimulate an insect to fly upwind and land on the odor source (Fig. 1e). Thus, host-plant discrimination occurs at a distance through the detection of species-specific volatile compounds (Fig. 1e-2*). The best documented example to support this hypothesis is the aphid/mustard plant (Brassica spp.) complex. Aphids that specialize on mustard plants locate their host using isothiocyanates (Döring 2014; Pickett 1992; Webster et al. 2008), which are almost exclusively found in Brassica spp. (Ahuja et al. 2009). It should be noted that while there is strong evidence to support this hypothesis, these examples are limited, and do not provide a general mechanism for host-plant location.
In a third hypothesis, Bruce et al. (2005) proposed that, in the majority of cases, insects use mixtures of compounds commonly found in the environment to locate their host plant (Fig. 1d). According to this hypothesis, specific blends of ubiquitous plant volatile compounds stimulate an insect to fly upwind and land on the odor source (Fig. 1d). Host plant discrimination occurs over a distance through the detection of these specific blends of ubiquitous volatile compounds (Fig. 1d-2*). Electrophysiological studies from insects across five orders have demonstrated the use of ubiquitous plant volatiles for host-plant location [reviewed in Bruce et al. 2005; see Table 1]. Importantly, if the behaviorally active compounds are common plant volatiles, then discrimination can be enhanced through the detection of incorrect blends containing compounds that antagonize upwind oriented flight.
There are documented cases of insects using antagonist compounds to discriminate between volatile mixtures produced by different plant species. Different host races of apple maggot flies, Rhagoletis pomonella, discriminate between host and non-host fruit. Apple and hawthorn race flies specializing on apple, Malus domestica, and hawthorn, Crataegus mollis, displayed maximal levels of upwind oriented flight to synthetic blends of natal host volatiles compared to blends of non-host volatiles (Linn et al. 2005). This blend discrimination is facilitated through the detection of compounds found in non-host blends (Linn et al. 2003b). For example, the addition of 3-methylbutan-1-ol (an essential component of the hawthorn blend) to the otherwise attractive apple blend reduced upwind flight of flies to the apple blend. The detection of compounds that antagonize oriented upwind flight may be an important evolutionary strategy to discriminate between similar odor blends.
However, not all insects in a population require specific volatile cues to initiate oriented upwind flight behavior. Evidence of a low but consistent, oriented upwind flight response to incorrect odor blends has been observed in both flies (Linn et al. 2003b; Nojima et al. 2003a; Powell et al. 2012) and moths (Droney et al. 2012; Linn et al. 2003a; Martin et al. 2016). Linn et al. (2003a) showed that 5–10% of European corn borer, Ostrinia nubilalis, male moths (both Z and E races) flew upwind to the dissimilar female-produced sex pheromone blend of a closely related species, the Asian corn borer, O. furnacalis. The O. nubilalis males that flew to the O. furnicalis blend also flew upwind to their respective female-produced sex pheromone blends, suggesting these males display a broad response to blends. Martin et al. (2016) showed that ~20% of E- strain O. nubilalis males fly upwind to a series of artificial combinations of O. nubilalis and O. furnacalis sex pheromone blends, suggesting that a portion of male moths can broadly respond, even to incorrect blends. Additionally, Karpati et al. (2013) found that after male O. nubilalis moths ‘lock on’ to an attractive odor plume, even previously unattractive pheromone blends elicit upwind flight behavior, suggesting further that moths are capable of broad pheromone responses. Apple maggot flies also displayed a broad response to natal and non-natal fruit blends. For example, 10–30% of apple host race flies that flew upwind to synthetic blends of apple also responded to synthetic blends of non-natal hawthorn, Crataegus spp., fruit odor and vice versa (Linn et al. 2003b). Broad responders could have significant evolutionary importance as a source of genetic variation that allows for sympatric speciation through shifts to new host plants (Clifford and Riffell 2013; Linn et al. 2005, 2012; Powell et al. 2012).
The grape berry moth (GBM), Paralobesia viteana, is a tortricid moth native to the eastern United States (Taschenberg et al. 1974), and is an important pest of cultivated grape (Williamson and Johnson 2005). The GBM is an ovipositional specialist, laying its eggs almost exclusively on grape clusters in the field but also on leaves under laboratory conditions (Clark and Dennehy 1988). In studies to determine whether a monitoring trap using plant volatiles could be developed for female GBM, Cha et al. (Cha et al. 2008a, b) showed that females displayed oriented flight toward host plant material in a flight tunnel. A blend of eleven behaviorally active compounds was identified, and two different 7-component subsets of the complete blend were found to elicit equivalent levels of behavior under the same conditions (Cha et al. 2008b, see Table 2). The identified compounds are common plant volatiles, supporting the ‘specific blends of common volatiles’ hypothesis for host location (Bruce et al. 2005).
Because of its narrow host range and responses to blends of ubiquitous volatiles, the GBM-grape plant complex represents an excellent system to test host plant discrimination hypotheses, especially the specific blend hypothesis proposed by Bruce et al. (2005) involving ubiquitous blends and antagonist compounds. The goal of this study was to determine whether GBM females discriminate host from non-host plants over a distance, and whether any discrimination involves the detection of antagonistic compounds from non-host plants that arrest long distance upwind flight. In previous work (Cha et al. 2008a), grape shoots of Vitis riparia elicited maximal levels of oriented upwind flight in flight tunnel assays compared to other grape tissue. Therefore, we used this as host material in this study. Apple, Malus domesticus, and gray dogwood, Cornus racemosa, were chosen as non-host plants because of their overlapping range and phenology with native grape. We used flight tunnel assays to record the insect’s behavioral responses to host and non-host plants. We collected volatiles from each plant, and used flight tunnel assays, gas chromatography-electroantennogram detection (GC-EAD) and gas chromatography/mass spectrometry (GC/MS) in an iterative process to identify a behaviorally active volatile blend for each plant. Contrary to our predictions for this specialist insect, we did not find evidence supporting long distance discrimination or antagonism but, rather, found that females responded equally well to the three plant species. We discuss the results in the context of the other two hypotheses, as well as the habitat location hypothesis, in order to understand the mechanism(s) of host plant location in phytophagous insects.
Methods and Materials
Insects
GBM were reared in cages placed in walk-in environmental chambers at 26 °C and 60% RH under a 16:8 L:D photoperiod. Adults were allowed to oviposit on seedless grapes (V. vinifera, red flame variety). Red flame variety was used for oviposition and larval development because this variety was readily available for purchase. First and second instars were transferred to a diet cup (30 ml, WinCup Inc.) and reared on semi-synthetic diet (Nagarkatti et al. 2000) that consisted of grapes, pinto beans, and commercially available tobacco hornworm diet (Bio-Serv, Flemington, NJ). For behavioral assays, unmated female moths were taken from cohorts set up by placing 10–15 female pupae (near eclosion) in a Plexiglass mating cage (30 cm H × 30 cm W × 30 cm D) and were provided with a 50% honey and water solution. Twenty male pupae were added to additional mating cages loaded with 10–15 female pupae, so as to provide mated females for bioassay. For all flight tunnel assays reported below, both unmated and mated females were tested to each treatment.
Plants Vitis riparia, a native host species of GBM in northeastern USA, was used as the host plant for these experiments, because this variety grew well under greenhouse conditions. Jonagold apple trees, M. domestica, and Gray Dogwood, C. racimosa, were purchased from a local nursery (Mayflowers, Canandaigua NY, US) and used as non-host plants for these experiments. All plants were maintained in a greenhouse as previously described (Cha et al. 2008a), with temperatures maintained between 21 and 26 °C. Supplemental light was provided to extend the day length to 16 hr.
Adsorbent Sampling
We used a push–pull collection system to collect headspace volatiles of live grape, apple, and gray dogwood plants. The system consisted of a custom-made, bell-shaped glass chamber (18 cm i.d., 10 l) with two air-in adapters (7 mm i.d.) on the top and four air-out adapters (7 mm i.d.) equally distributed on the bottom wall of the chamber. The glass chamber was placed on two pieces of Pyrex glass with a hole (2 cm) in the middle so that the vegetative portion of the plant could be sampled to accommodate a whole, live potted plant. After a plant was set up in the chamber, the chamber was flushed with filtered air (5 l.min−1) for 24 hr to replace the original air inside the chamber and to stabilize volatile emission from the plant, because we noticed that handling of the plant during set up temporarily induced release of green leaf volatiles. During the collection, flow meters were used to ensure that more filtered air was pushed into the chamber than pulled out through the charcoal filters, so as to eliminate possible contamination from outside air. Filtered clean air was pushed into the chamber at 5.0 l.min−1, and volatiles from the headspace of grape shoots were drawn by a vacuum pump onto four activated charcoal filters at 1.2 l.min−1/filter (ORBO32-small, Supelco Inc., Bellefonte, PA, USA). Adsorbent samplings were made over 4 d in the greenhouse (18:6 L:D). The chamber was washed with acetone, and new ORBO filters were used for each new plant. The volatiles were eluted with 300 μl hexane every 24 hr and combined in the same vial. The combined extract was concentrated to 1 ml under a gentle stream of nitrogen gas and kept in a freezer (−20 °C) before use in GC-EAD and GC-MS analyses, and flight tunnel assays.
Coupled GC-EAD Analysis
A Hewlett-Packard 5890 Series II GC, equipped with a DB-1 capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA), a DB-5 capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific), or a DB-Wax capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific) was used for GC-EAD. The oven temperature was 40 °C for 5 min, then increased at 15 °C.min−1 to 250 °C. Injector and detector temperatures were set at 280 °C and 270 °C, respectively. Splitless injection was used with nitrogen as carrier gas at 2 ml.min−1. The column effluent was split in a ratio of 1:1 to the flame ionization detector and the heated (270 °C) EAD port.
A whole head was removed from a 3-d-old virgin female GBM and mounted on a saline-filled micropipette in an acrylic holder as previously described (Cha et al. 2008b; Nojima et al. 2003b). Both antennae were positioned in the other saline-filled micropipette. We used an Ephrussi–Beadle Insect Ringer as saline (Ephrussi and Beadle 1936). The tips of both antennae were dipped in saline containing surfactant (0.02% Triton X-100) for easy manipulation. The antennal holder was placed inside a humidified cooling condenser maintained at 10 °C. A minimum of five different antennal pairs were used to analyze volatiles from plant shoot extracts. Synthetic blends were prepared according to the ratios in Table 1, and diluted with dichloromethane to 0.1 mg/ml.
Chemical Analysis
Extracts were analyzed using an Agilent 5890 gas chromatograph coupled to a 5973n mass selective detector running in EI mode at 70 eV. The GC was equipped with a DB-1 ms non-polar column (30 m × 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific) or a polar DB-Wax column (30 m × 0.25 mm ID, 0.25 μm film thickness; J&W Scientific). Helium was the carrier gas at a constant flow of 1.0 ml.min−1. The oven temperature program was 40 °C for 5 min, then 15 °C.min−1 to 250 °C, and held for 5 min. Volatile compounds were tentatively identified by mass spectral matches to library spectra and confirmed by retention time and mass spectral matches to available authentic standards.
Chemicals
(Z)-3-hexen-1-yl acetate, ethyl hexanoate, nonanal, racemic linalool, methyl salicylate, decanal, β-caryophyllene, and α-farnesene were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA), Alfa Aesar (Ward Hill, MA, USA), Fluka (Buchs, Switzerland) or TCI America (Portland, OR, USA). All except α-farnesene (a mixture of isomers) were > 97% purity. The 4,8-dimethyl-1,3(E),7-nonatriene was provided by the Chong lab (University of Waterloo, Ontario, CA). Germacrene-D was isolated from golden rod as 91% germacrene-D and 9% β-caryophyllene (by USDA Chemistry Research Unit, Gainesville, FL, USA).
Flight Tunnel
The flight tunnel was 2 m long X 0.6 m wide X 0.6 m high, with a fan installed at the upwind end to create a steady airflow into the tunnel and an exhaust hood at the downwind end to evacuate odor (Cha et al. 2008a, b). Wind speed was 0.25 m.s−1 at the wire stand where the moths were introduced into the tunnel. A pattern of dark green paper circles (10 cm diam.) was randomly presented both on a white background glass floor and on the glass ceiling below the light source to provide the insects with ample visual stimuli to fly upwind. During the experiments, the average temperature in the tunnel was 23.8 ± 0.07 °C, and the relative humidity 55.19 ± 0.33%. Female moths were placed in the flight tunnel room 1 hr prior to scotophase. Light intensity was reduced to 25 lx 30 min before dark, and remained at this intensity for the behavioral assays. Behavioral assays began 15 min. Prior to scotophase. These conditions promote high levels of GBM optomotor anemotaxis (Cha et al. 2008a).
The odor source was placed 30 cm from the upwind end of the tunnel. Four- to five-day old females were used in all flight tunnel assays. All insects were discarded after being assayed once. Female moths were placed in the flight tunnel individually in a metal screen release cage on a wire stand 1.5 m downwind of the source, and their behavior observed for 5 min. We recorded whether the insect flew out of the release cage, flew upwind (more than 10 cm of oriented flight toward the source), and landed on the source. Fisher’s exact test (P < 0.05) was used to compare the percent response of GBM females to the different odor sources. A G-test (P < 0.05) of independence was used to compare each odor source to the grape shoots and the expected response to a non-host plant. Based on our previous research, showing that levels of ‘broad response’ can vary from 5 to 30%, we selected an expected threshold of 10% for statistical comparisons.
Treatments
The behavioral responses (upwind flight and landing) of individual moths to plant odor sources (summarized in Table 2) were observed in the flight tunnel. Freshly cut plant shoots and rubber septa (Thomas Scientific, Swedesboro, NJ, USA) loaded with either synthetic blends or adsorbent extracts were used as odor sources in the flight tunnel. Two shoots were cut 15 cm in length and immediately placed in a 4 ml plastic tube filled with deionized water, as described in previous studies (Cha et al. 2008a, b), and discarded after one flight session. Responses of GBM females to grape shoots were used as positive controls in flight tunnel assays, and the responses of both mated and unmated females were tested. Expected response values for a non-host plant was set at 10% upwind flight (rather than 0%) based on the existence of broad responders found in previous work on apple maggot flies and European corn borers (Droney et al. 2012; Linn et al. 2003a, b, 2012; Martin et al. 2016; Nojima et al. 2003a; Powell et al. 2012). Septa were loaded with 300 μl of extract or blend (at 0.1 mg/ml) and placed in a fume hood for 1 hr to allow evaporation of the solvent. Septa were stored in a freezer (−20 °C) between tests. GC-EAD-active blends for each plant were prepared in ratios that corresponded to the ratios of compounds found in the corresponding plant extract.
Results
Analysis of GC-EAD-Active Compounds
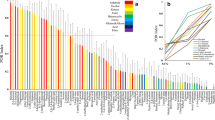
All of the previously identified EAD-active compounds in the grape volatile profile were also found in the dogwood and apple volatile profiles (Table 1; Fig. 2). (E)-β-Ocimene was not previously identified in the EAD-active grape volatile blend, but was EAD active and present in all three volatile blends in this study. (Z)-3-Hexan-1-ol and 1-methylcyclohexanol were EAD active and only found in the apple volatile blend.
Representative coupled gas chromatography-electroantennogram detection (GC-EAD) responses (using Paralobesia viteana female antennae) to plant extracts. EAD recordings are shown above the corresponding chromatograms. Numbered spikes displayed consistent antennal activity and were identified by gas chromatography/mass spectrometry (see Table 1)
GBM Response to Plant Shoots
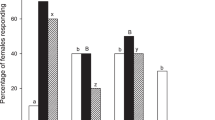
Of the GBM females (mated and unmated combined) tested, 59.1% flew upwind, and 37.5% landed to grape shoots (Fig. 3a; n = 296). The moths behaved similarly to dogwood shoots (n = 98), with 54.1% flying upwind (Fisher’s exact test, P = 0.35) and 35.7% landing (Fisher’s exact test P = 0.81). Female GBM (n = 148) flew upwind (44.6%) and landed (22.3%) in response to apple shoots, both lower percentages than to grape shoots (Fisher’s exact test, upwind flight P = 0.005; landing P = 0.01). A similar percentage (Fisher’s exact test P = 0.12) of females flew upwind to dogwood shoots as to apple shoots, but a higher percentage (Fisher’s exact test, P = 0.02) of females landed on dogwood shoots compared to apple shoots.
Female Paralobesia viteana responses to plants. a Flight tunnel response (%) to host and non-host plant shoots. Different letters (capital for upwind flight response, lower case for landing response) indicate differences (P < 0.05; Fisher’s exact test). The non-host shoots elicited a higher percentage of upwind flight and landing than we expected, indicated by the dotted line (P < 0.05; G-test). b Upwind flight of mated and unmated females to plant shoots. The same letter (capital for grape shoots, lower case for dogwood shoots, Greek for apple shoots) indicates similar response differences (P < 0.05; Fisher’s exact test)
Significantly higher percentages of female GBM flew upwind to and landed on both non-host plants than expected, using our 10% threshold for expected broad response individuals (G-test, dogwood: upwind flight P < 0.001, landing P = 0.002; apple: upwind flight P < 0.001, landing P = 0.004). When mated and unmated moths are considered separately, the results show similar percentages of both groups flew upwind to grape shoots (Fig. 3b; mated n = 143, 54.6%; unmated n = 153, 61.1%; Fisher’s exact test P = 0.29), dogwood shoots (mated n = 42, 54.8%; unmated n = 56, 53.6%; Fisher’s exact test P = 0.52), and apple shoots (n = 51, 50.1%; unmated n = 82, 44.5%; Fisher’s exact test P = 0.37).
GBM Response to Rubber Septa Releasing Adsorbent Extracts
Grape berry moth females (Fig. 4a; mated and unmated combined; n = 147) flew upwind 45.6 % of the time in response to grape extract, which was similar to the percentages to dogwood (n = 63, 50.8%, Fisher’s exact test P = 0.55), and apple extracts (n = 109, 45.0%, Fisher’s exact test P = 1). The upwind flight response to ogwood extract was not different from the response to apple extract (Fisher’s exact test, P = 0.53).
Female Paralobesia viteana responses to extracts. a Flight tunnel response (%) of to host and non-host extracts. The same letter indicates similar responses (P < 0.05; Fisher’s exact test). Females displayed similar upwind flight responses to all extracts. The non-host extracts elicited a higher percentage of upwind flight and landing than we expected (dotted line) (P < 0.05; G-test). b Upwind flight of mated and unmated females to extracts. The same letter (capital for grape extract, lower case for dogwood extract, Greek for apple extract) indicates similar responses (P < 0.05; Fisher’s exact test)
When considered separately, mated and unmated moths flew upwind in similar percentages to grape extract (Fig. 4b; mated n = 66, 40.9%; unmated n = 81, 49.4%; Fisher’s exact test P = 0.32), dogwood extract (mated n = 19, 47.4%; unmated n = 44, 52.3%; Fisher’s exact test P = 0.79), and apple extract (n = 51, 51.0%; unmated n = 58, 39.7%; Fisher’s exact test P = 0.25).
Female GBM moths did not land on rubber septum sources releasing grape and apple extract volatiles, and only 1.6% of females landed on the septum with dogwood extract.
GBM Response to Rubber Septa Sources Releasing Synthetic Blends
Female GBM (Fig. 5a; mated and unmated combined) flew upwind 51.6% of the time in response to the grape synthetic blend (n = 42), which similar to the percentages to the dogwood (n = 87, 52.9%, Fisher’s exact test P = 1), and the apple synthetic blends (n = 99, 48.5%, Fisher’s exact test P = 0.84). The upwind flight response to the dogwood synthetic blend was not different from the response to the apple blend (Fisher’s exact test, P = 0.56).
Female Paralobesia viteana responses to synthetic blends. a Flight tunnel response (%) of to host and non-host synthetic blends. The same letter indicates similar responses (P < 0.05; Fisher’s exact test). Females displayed similar upwind flight responses to all synthetic blends. The non-host blends elicited a higher percentage of upwind flight and landing than we expected as indicated by the dotted line (P < 0.05; G-test). b Upwind flight of mated and unmated females to synthetic blends. The same letter (capital for grape blend, lower case for dogwood blend, Greek for apple blend) indicates similar responses (P < 0.05; Fisher’s exact test)
As observed for the extracts, females did not land on rubber septum sources releasing any of the three synthetic blends. When considered separately, mated and unmated moths flew upwind in similar percentages to the grape (mated n = 24, 50.0%; unmated n = 7, 57.1%; Fisher’s exact test P = 1), dogwood (mated n = 44, 61.4%; unmated n = 43, 44.2%; Fisher’s exact test P = 0.20), and apple synthetic blends (n = 53, 50.1%; unmated n = 46, 45.7%; Fisher’s exact test P = 0.69).
Discussion
Host plant location by phytophagous insects involves a cascade of behaviors including oriented upwind flight, landing, and host acceptance, ultimately culminating with feeding, or in the case of female moths, oviposition or release of sex pheromone (Fig. 1; Landolt and Phillips 1997; Visser 1986, 1988). We used the GBM-grape plant complex as a model for understanding the proximate olfactory mechanisms for host plant location by a specialist phytophagous insect over distance. Female GBM displayed higher levels of upwind flight to non-host odor sources (Figs. 3, 4 and 5) than we expected from the specific blend of ubiquitous odors hypothesis (Bruce et al. 2005). The similar levels of upwind oriented flight to host grape and non-host gray dogwood and apple support the conclusion that the moths are not discriminating between host and non-host plants over a distance. Mated and unmated females oriented at similar levels to all odor sources (Figs. 3, 4 and 5b), further suggesting that plant volatiles are not being used as a specific long-range cue.
Phytophagous insects have diverse uses for their host plants. Much of the literature has focused on host plant location for the purpose of feeding or oviposition (Bruce et al. 2005; Bruce and Pickett 2011; Finch and Collier 2000; Webster and Cardé 2016), and have, therefore, focused on mated females. However, unmated moths may already be on a host plant before releasing sex pheromone, making host location by mated moths less relevant (Shorey 1974). In that case, unmated female moths may display oriented upwind flight to their host plants for courtship/mating purposes, and should, therefore, be considered in behavioral assays to understand mechanisms for host plant location. For example, host plant volatiles stimulate female ermine moths, Yponomeuta spp., corn earworm moths, Helicoverpa zea, and cabbage looper moths, Trichoplusia ni, to release pheromone (Hendrikse and Vos-Bünnemeyer 1987; Landolt et al. 1994; Raina et al. 1992). Mated and unmated GBM females displayed similar responses to host plant volatiles, suggesting unmated moths could use the host plant as a courtship/mating site in addition to an oviposition site.
In our initial assays with plant shoots, female GBM displayed higher levels of upwind flight and landing to non-host apple shoots than expected (Fig. 3a), but at a lower percentage compared to grape, which might suggest that, based only on responses to plant material, antagonist compounds in apple might be present. However, this difference can also be explained by a difference in concentration or release rate among the plants. The length of plant shoots used in flight tunnel assays was controlled among plant species, but each species might be releasing volatiles at different rates, resulting in small (~15%) differences in behavior. The moths displayed similar percentages of upwind flight when the concentration of the volatiles was controlled (in the extracts and synthetic blends; Figs. 4a and 5a), supporting the idea that the difference could be mediated by differences in relative release rates between grape and apple shoots. Additionally, it is important to note that it is not known how the release rates from the rubber septa compare to release rates from the plants. Importantly, however, all odor sources elicited similar levels of upwind flight behavior, indicating stimulatory quantities of volatiles were being released by the odor sources.
All of the EAD-active compounds in the grape volatile profile were also found in the dogwood and apple volatile profiles (Table 1; Fig. 2). The non-host plants contained volatile compounds not previously identified in the grape blend, but these compounds were not antagonistic, as a higher percentage of moths flew upwind to synthetic blends containing these compounds than expected. The redundancy of the compounds in all three volatile profiles supports the observed flight tunnel behavior, and the conclusion that female GBM are not using blends of ubiquitous volatiles to discriminate between host and non-host plants over a distance.
The lack of observed discrimination among blends in the no-choice assays is not necessarily surprising because the composition of all three blends is similar. Blend preferences could be observed using choice tests. Additional testing could also explore the plasticity of the oriented upwind flight response using artificial blend manipulation. Artificial blends were manipulated in previous work exploring the importance of key compounds by changing the presence/absence of the compounds (Cha et al. 2008b). However, further testing of artificial blends should be conducted to mimic and change non-host blends. For example, the apple blend contained ~2x more methyl salicylate and ~4x more (E)-β-Ocimene than the grape blend (Table 1). Future studies could increase the relative amounts of these compounds to observe whether a threshold of these compounds exists that fails to elicit upwind oriented flight.
Hypotheses for Volatiles and Host Plant Location at a Distance
The results of this study do not support the token stimulus (Fig. 1e; Fraenkel 1959) or the ‘ratio specific blends’ hypotheses (Fig. 1d; Bruce et al. 2005). Bruce et al. (2005) suggested that, in general, insects use blends of ubiquitous plant volatiles rather than species-specific compounds, as Fraenkel had suggested (Fraenkel 1959). Furthermore, Bruce et al. (2005) argued that insects would be tuned to specific ratios of ubiquitous compounds that comprise an appropriate volatile blend (Bruce et al. 2005; Bruce and Pickett 2011). In previous work, GBM females displayed lower levels of upwind flight when certain key EAD-active volatiles were removed from the complete blend (Cha et al. 2008b). Additionally, GBM females also displayed lower levels of upwind flight when the ratios of key EAD-active volatiles were individually doubled, or adjusted to match ratios emitted by grape plants damaged by Japanese beetles, Popillia japonica, (Cha et al. 2011). The results of these studies indicate GBM females are sensitive to the specific composition of blends, suggesting relative insensitivity to modest differences in ratios. Moreover, the studies also reported higher levels of upwind flight than would be expected if these ratios were the result of an adaptive mechanism for host plant discrimination (antagonism).
Our results support the ‘habitat odor’ hypothesis for host plant location (Fig. 1b), by suggesting that host volatiles provide a cue to a suitable habitat where a specific plant can be selected (Webster and Cardé 2016). Their hypothesis, in fact, goes further and suggests that insects can use a number of habitat cues, such as common volatile compounds, CO2 and/or humidity gradients, as well as visual cues, to maximize the likelihood of encountering specific host plant cues (Fig. 1; Webster and Cardé 2016). According to the hypothesis, habitat odor cues differ from host cues in that they are generally not species-specific, are released in large quantities, can be detected at long distances, and are associated with host-specific cues (Webster and Cardé 2016). Habitat cues can attract insects to an area associated with the host (habitat), and once in the habitat, insects may use additional, species-specific cues to locate the host. For example, European grapevine moths, Lobesia botrana, have a wild host, V. vinifera, and a recently colonized host, Daphne gnidium (Thiéry and Moreau 2005). The volatile profiles of each plant were analyzed, and blends of common and unique (to each plant) EAD-active green leaf volatiles (GLVs) were prepared. A low percentage of gravid females flew upwind to synthetic blends of the EAD-active GLVs specific for each plant, as well as to a blend of only the common GLVs (Tasin et al. 2009). However, the upwind flight behavior was recovered when the common GLVs were added to each plant-specific blend, suggesting both common and plant-specific GLVs are used to locate a host.
Hawkmoth pollinators may use floral CO2 and humidity gradients to select an appropriate nectar source (Contreras et al. 2013; von Arx et al. 2012). White-lined sphinx moths, Hyles lineatea, consistently approached and probed flowers with elevated humidity more than those at ambient humidity, suggesting the moths use small differences in relative humidity to select a host. Furthermore, tobacco hornworm moths, Manduca sexta, displayed high levels of upwind flight in response to small differences in relative humidity (Wolfin et al. 2018). Additionally, hawkmoths spent more time on the side of the flight tunnel with elevated humidity compared to the side of the tunnel with ambient relative humidity, suggesting humidity may also be an important upwind flight cue.
It is important to note that the hypotheses discussed in this paper are largely not mutually exclusive. An insect may use habitat cues to locate a favorable habitat then, once in the favorable habitat, use some combination of mechanisms represented by the token stimulus hypothesis, specific blends of ubiquitous compounds hypothesis, and/or the appropriate/inappropriate landings hypothesis. For example, hawkmoths use differences in relative humidity to locate potential host plants in more humid habitats (Janzen 1987). However, hawkmoths also use floral scent to discriminate between nectar sources (Brantjes 1973). Therefore, it is likely that insects may employ one or more of these hypotheses to locate a suitable host.
Using habitat odors to locate a host would be particularly effective in the GBM-grape plant complex given the life histories of the GBM and V. riparia. Vitis riparia is native to North America from Canada to Texas, and the Rocky Mountains to the Atlantic Ocean (Keller 2015). It is a woody plant that climbs on trees and shrubs along riverbanks (Keller 2015). Female GBM could use habitat cues from either riverbanks (humidity) or surrounding flora to increase the probability of detecting host plant cues and locating a host. Grapevines share range and phenology with wild apple trees and gray dogwood shrubs, and may climb on them in wild habitats. This association between host and non-host plants may explain the observed orientation and landing behavior of GBM to the non-host plants in this study, and supports the use of habitat cues to locate a host plant (Webster and Cardé 2016).
Landing Response
Higher percentages of female GBM landed on the non-host plants than we expected from the hypothesis that this specialist species should detect antagonistic non-host compounds as an adaptive mechanism for host plant discrimination (Linn et al. 2003b; Nojima et al. 2003a; Powell et al. 2012). The fact that the moths landed on non-host plants is further evidence that GBM females are not using a ‘token stimulus’ (Fraenkel 1959), or specific blends of volatile compounds (Bruce et al. 2005) to locate a host. However, the results support the ‘appropriate/inappropriate landings’ hypothesis (Finch and Collier 2000). In this hypothesis, a flying insect is stimulated to land through the detection of nonspecific plant volatiles (Fig. 1c). Upon landing, the insect performs multiple post-landing assessments of a plant to discriminate between host and non-host. Post-landing behaviors were beyond the scope of the current study, and require additional behavioral assays to characterize.
Habitat cues may also be necessary to elicit GBM landing (Finch and Collier 2000). The ‘appropriate/inappropriate landings hypothesis’ (Finch and Collier 2000) suggested that nonspecific plant cues, such as common GLVs, stimulate insects to land on a nearby green surface, while host plant discrimination and acceptance is mediated by post-landing behaviors. For example, when presented with host plants paired with non-host plants, and host plants paired with green paper, cabbage root flies, Delia radicum, landed more on non-host substrates than expected (Kostal and Finch 1994). Additionally, D. radicum did not display an ovipositional preference between artificial plants baited with host odors compared with unbaited artificial plants (Prokopy et al. 1983). These studies suggest that volatiles are a nonspecific landing cue, and host plant discrimination occurs post-landing.
Finch and Collier (2000) suggested that insects use contact chemoreceptors on their tarsi to assess a host plant. The small cabbage white butterfly, Pieris rapae, uses tarsal chemoreceptors to detect glucosinolates and cardenolides that act as deterrents or stimulants for oviposition (Roessingh et al. 1992; Stadler et al. 1995). Blaney and Simmonds (1990) observed behavioral and electrophysiological responses of tarsal chemoreceptors in Spodoptera littoralis, Spodoptera frugiperda, C. virescens, and Helicoverpa armigera. All four moth species detect sugars, amino acids, and allelochemicals using tarsal receptors, and have varying levels of sensitivities to each. These sugars, amino acids, and allelochemicals could stimulate important behaviors such as feeding and oviposition (Roessingh et al. 1992; Stadler et al. 1995). Because GBM females landed on host-and non-host plants, contact chemoreception might be involved in host plant selection.
In the current study, female GBM did not land on a rubber septum source in response to extracts or synthetic blends of host and non-host plants. If all of the necessary host plant cues were present, we expected similar percentages of moths to land on the septa as on the corresponding plant shoots. However, this lack of landing on the septa indicates GBM may require additional cues to land. Cabbage moths, Mamestra brassicae, flew upwind to extracts of host plant volatiles in a flight tunnel (Rojas and Wyatt 1999). However, the moths did not land on the odor source unless a visual cue was also present. The observed landing response here is consistent with previous studies on GBM females (Cha et al. 2008b). Carbon dioxide or humidity gradients in the presence of olfactory cues may also affect landing behavior (Contreras et al. 2013; von Arx et al. 2012).
Conclusions
Many of the previous studies on insect host location over distance have focused on oriented upwind flight as a key discriminatory behavior (Bruce et al. 2005; Bruce and Pickett 2011). In the present study, similar percentages of GBM females flew upwind to host and non-host sources, suggesting discrimination is not occurring over distance. This result supports the idea that phytophagous insects may fly upwind to locate a favorable habitat rather than a host plant, and that discrimination may occur within the habitat, or even post-landing (Finch and Collier 2000; Webster and Cardé 2016). GBM females did not land on extract/synthetic odor sources in this study, and additional studies are needed to explore the cues that stimulate landing.
References
Ahuja I, Rohloff J, Bones AM (2009) Defence mechanisms of brassicaceae: implications for plant-insect interactions and potential for integrated pest management. Sustain Agric 30:623–670. https://doi.org/10.1007/978-94-007-0394-0_28
Bell WJ (1990) Searching behavior patterns in insects. Annu Rev Entomol 35:447–467. https://doi.org/10.1146/annurev.en.35.010190.002311
Blaney WM, Simmonds MSJ (1990) A behavioural and electrophysiological study of the role of tarsal chemoreceptors in feeding by adults of Spodoptera, Heliothis virescens and Helicoverpa armigera. J Insect Physiol 36. https://doi.org/10.1016/0022-1910(90)90048-K
Brantjes NBM (1973) Sphingophilous flowers, function of their scent. In: Brantjes NBM, Linskens HF (eds) Pollination and dispersal. University of Nijmegan, Nijmegan, Netherlands, pp 27–46
Bruce TJ, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects-finding the right mix. Phytochemistry 72:1605–1611. https://doi.org/10.1016/j.phytochem.2011.04.011
Bruce T, Wadhams L, Woodcock C (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274. https://doi.org/10.1016/j.tplants.2005.04.003
Cha DH, Hesler SP, Moser CL, Nojima S, Linn, Charles E. Jr, Roelofs WL, Loeb GM (2008a) Flight tunnel responses of female grape berry moth (Paralobesia viteana) to host plants. J Chem Ecol 34:622–627. https://doi.org/10.1007/s10886-008-9474-7
Cha DH, Nojima S, Hesler SP, Zhang A, Linn CE Jr, Roelofs WL, Loeb GM (2008b) Identification and field evaluation of grape shoot volatiles attractive to female grape berry moth (Paralobesia viteana). J Chem Ecol 34:1180–1189. https://doi.org/10.1007/s10886-008-9517-0
Cha DH, Linn CE, Teal PE et al (2011) Eavesdropping on plant volatiles by a specialist moth: significance of ratio and concentration. PLoS One 6:e17033. https://doi.org/10.1371/journal.pone.0017033
Clark LG, Dennehy TJ (1988) Oviposition behavior of grape berry moth. Entomol Exp Appl 47:223–230. https://doi.org/10.1111/j.1570-7458.1988.tb01140.x
Clifford MR, Riffell JA (2013) Mixture and odorant processing in the olfactory systems of insects: a comparative perspective. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 199:911–928. https://doi.org/10.1007/s00359-013-0818-6
Cole PG, Weltzin JF (2005) Light limitation creates patchy distribution of an invasive grass in eastern deciduous forests. Biol Invasions 7:477–488. https://doi.org/10.1007/s10530-004-5171-9
Contreras HL, Goyret J, von Arx M, Pierce CT, Bronstein JL, Raguso RA, Davidowitz G (2013) The effect of ambient humidity on the foraging behavior of the hawkmoth Manduca sexta. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 199:1053–1063. https://doi.org/10.1007/s00359-013-0829-3
Dethier V (1941) The function of the antennal receptors in lepidopterous larvae. Biol Bull 80:403–414
Döring TF (2014) How aphids find their host plants, and how they don’t. Ann Appl Biol 165:3–26. https://doi.org/10.1111/aab.12142
Droney DC, Musto CJ, Mancuso K, Roelofs WL, Linn CE Jr (2012) The response to selection for broad male response to female sex pheromone and its implications for divergence in close-range mating behavior in the European corn borer moth, Ostrinia nubilalis. J Chem Ecol 38:1504–1512. https://doi.org/10.1007/s10886-012-0208-5
Ephrussi B, Beadle GW (1936) A technique of transplantation for Drosophila. Am Soc Nat 13:229–246
Faucher CP, Hilker M, de Bruyne M (2013) Interactions of carbon dioxide and food Odours in Drosophila: olfactory hedonics and sensory neuron properties. PLoS One 8. https://doi.org/10.1371/journal.pone.0056361
Finch S, Collier RH (2000) Host-plant selection by insects - a theory based on “appropriate/inappropriate landings” by pest insects of cruciferous plants. Entomol Exp Appl 96:91–102. https://doi.org/10.1046/j.1570-7458.2000.00684.x
Fraenkel GS (1959) The raison d ’ Etre substances of secondary plant substances. Science (80- ) 129:1466–1470
Galiano EF (1985) The small-scale pattern of Cynodon dactylon in Mediterranean pastures. Vegetatio 63:121–127. https://doi.org/10.1007/BF00044062
Hendrikse A, Vos-Bünnemeyer E (1987) Role of host-plant stimuli in sexual behaviour of small ermine moths (Yponomeuta). Ecol Entomol 12:363–371. https://doi.org/10.1111/j.1365-2311.1987.tb01017.x
Janzen DH (1987) How moths pass the dry season in a Costa Rican dry Forest. Insect Sci Its Appl 8:489–500. https://doi.org/10.1017/S1742758400022530
Karpati Z, Tasin M, Carde RT, Dekker T (2013) Early quality assessment lessens pheromone specificity in a moth. Proc Natl Acad Sci 110:7377–7382. https://doi.org/10.1073/pnas.1216145110
Keller M (2015) Botany and anatomy. In: The science of grapevines, 2nd edn, pp 1–57
Kostal VI, Finch S (1994) Influence of background on host-plant selection and subsequent oviposition by the cabbage root fly (Delia radicum). Entomol Exp Appl 70:153–163
Landolt PJ, Phillips TW (1997) Host plant influences on sex pheromone behavior of phytophagous insects. Annu Rev Entomol 42:371–391. https://doi.org/10.1146/annurev.ento.42.1.371
Landolt PJ, Heath RR, Millar JG, Davis-Hernandez KM, Dueben BD, Ward KE (1994) Effects of host plant, Gossypium hirsutum, on sexual attraction of cabbage looper moths, Trichoplusia (Hubner) (Lepidoptera: Noctuidae). J Chem Ecol 20:2959–2974
Linn C, O’Connor M, Roelofs W (2003a) Silent genes and rare males: a fresh look at pheromone blend response specificity in the European corn borer moth, Ostrinia nubilalis. J Insect Sci 3:15. https://doi.org/10.1093/jis/3.1.15
Linn CE, Feder JL, Nojima S et al (2003b) Fruit odor discrimination and sympatric host race formation in Rhagoletis. Proc Natl Acad Sci U S A 100:11490–11493. https://doi.org/10.1073/pnas.1635049100
Linn CE, Dambroski H, Nojima S et al (2005) Variability in response specificity of apple, hawthorn, and flowering dogwood-infesting Rhagoletis flies to host fruit volatile blends: implications for sympatric host shifts. Entomol Exp Appl 116:55–64. https://doi.org/10.1111/j.1570-7458.2005.00310.x
Linn CE, Yee WL, Sim SB et al (2012) Behavioral evidence for fruit odor discrimination and sympatric host races of Rhagoletis Pomonella flies in the Western United States. Evolution (N Y) 66:3632–3641. https://doi.org/10.1111/j.1558-5646.2012.01719.x
Martin N, Moore K, Musto CJ, Linn CE (2016) Flight tunnel response of male European corn borer moths to cross-specific mixtures of European and Asian corn borer sex pheromones: evidence supporting a critical stage in evolution of a new communication system. J Chem Ecol 42:51–54. https://doi.org/10.1007/s10886-015-0656-9
Meiners T (2015) Chemical ecology and evolution of plant-insect interactions: a multitrophic perspective. Curr Opin Insect Sci 8:22–28. https://doi.org/10.1016/j.cois.2015.02.003
Miller JR, Strickler KL (1984) Finding and Accepting Host Plants. In: Bell WJ, Cardé RT (eds) Chemical Ecology of Insects. Springer, Boston, MA
Mozuraitis R, Stranden M, Ramirez MI, Borg-Karlson AK, Mustaparta H (2002) (-)-Germacrene D increases attraction and oviposition by the tobacco budworm moth Heliothis virescens. Chem Senses 27:505–509. https://doi.org/10.1093/chemse/27.6.505
Nagarkatti S, Muza A, Saunders M (2000) Meridic diet for Endopiza viteana (Lepidoptera: Tortricidae). Can Entomol 132:259–261. https://doi.org/10.4039/Ent132259-2
Nojima S, Linn CE, Morris B (2003a) Identification of host fruit volatiles from hawthorn (Crataegus spp.) attractive to hawthorn-origin Rhagoletis pomonella flies. J Chem Ecol 29:321–336
Nojima S, Linn CE, Morris B et al (2003b) Identification of host fruit volatiles from flowering dogwood (Cornus florida) attractive to dogwood-origin Rhagoletis pomonella flies. J Chem Ecol 29:321–336
Pickett J (1992) The chemical ecology of aphids. Annu Rev Entomol 37:67–90. https://doi.org/10.1146/annurev.ento.37.1.67
Powell THQ, Cha DH, Linn CE, Feder JL (2012) On the scent of standing variation for speciation: Beehavioral evidence for native sympatric host races of Rhagoletis pomonella (Diptera: tephritidae) in the southern United States. Evolution (N Y) 66:1215–1221. https://doi.org/10.1111/j.1558-5646.2012.01625.x
Prokopy R, Collier R, Finch S (1983) Visual detection of host plants by cabbage root flies. Entomol Exp Appl 34:85–89
Raina AK, Kingan TG, Mattoo AK (1992) Chemical signals from host plant and sexual behavior in a moth. Science (80- ) 255:592–594
Roessingh P, Städler E, Fenwick GR et al (1992) Oviposition and tarsal chemoreceptors of the cabbage root fly are stimulated by glucosinlolates and host plant-extracts. Entomol Exp Appl 65:267–282. https://doi.org/10.1111/j.1570-7458.1992.tb00680.x
Rojas JC, Wyatt TD (1999) Role of visual cues and interaction with host odour during the host-finding behaviour of the cabbage moth. Entomol Exp Appl 91:59–65. https://doi.org/10.1023/A:1003605125191
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology, 2nd edn. Oxford University Press, Oxford
Shorey HH (1974) Environmental and physiological control of insect sex pheromone behaviour. In: Birch MC (ed) Pheromones. North-Holland Publishing Company, New York, pp 68–20
Stadler E, Renwick JAA, Radke CD, Sachdev-Gupta K (1995) Tarsal contact chemoreceptor response to glucosinolates and cardenolides mediating oviposition in Pieris rapae. Physiol Entomol 20:175–187. https://doi.org/10.1111/j.1365-3032.1995.tb00814.x
Taschenberg E, Cardé R, Hill A et al (1974) Sex pheromone trapping of the grape berry moth. Environ Entomol 3:1973–1975
Tasin M, Bäckman A, Anfora G (2009) Attraction of female grapevine moth to common and specific olfactory cues from 2 host plants. Chem Senses 35:57–64. https://doi.org/10.1093/chemse/bjp082
Thiéry D, Moreau J (2005) Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 143:548–557. https://doi.org/10.1007/s00442-005-0022-7
Visser J (1986) Host odor perception in Phytophagous insects. Annu Rev Entomol 31:121–144. https://doi.org/10.1146/annurev.ento.31.1.121
Visser J (1988) Host-plant finding by insects: orientation, sensory input and search patterns. J Insect Physiol 34:259–268
von Arx M, Goyret J, Davidowitz G, Raguso R (2012) Floral humidity as a reliable sensory cue for profitability assessment by nectar-foraging hawkmoths. Proc Natl Acad Sci U S A 109:9471–9476. https://doi.org/10.1073/pnas.1121624109
Webster B, Cardé RT (2016) Use of habitat odour by host-seeking insects. Biol Rev 92:1241–1249. https://doi.org/10.1111/brv.12281
Webster B, Bruce T, Dufour S, Birkemeyer C, Birkett M, Hardie J, Pickett J (2008) Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. J Chem Ecol 34:1153–1161. https://doi.org/10.1007/s10886-008-9510-7
Williamson J, Johnson D (2005) Effects of grape berry moth management practices and landscape on arthropod diversity in grape vineyards in the southern United States. Horttechnology 15:232–238
Wolfin MS, Raguso RA, Davidowitz G, Goyret J (2018) Context-dependency of in-flight responses by Manduca sexta moths to ambient differences in relative humidity. J Exp Biol. https://doi.org/10.1242/jeb.177774
Acknowledgements
We thank Shinyoung Park, Callie Musto, and Stephen Hesler for help maintaining the greenhouse, GBM colonies, and for setting up cohorts for flight tunnel tests. We thank Stephen Parry at the Cornell University Statistical Consulting Unit for his statistical guidance. Sara Volo, Yuxi Liu, and Jonathan Thrall were undergraduates at Hobart and William Smith Colleges, Geneva, NY participating in a Summer Scholars Program supported by funding from the David and Brenda Rickey Foundation. We also thank Paul Robbins for his support, advice, and optimism regarding GC-EAD problem solving. We thank the Chong Lab for providing the dimethyl-1,3(E),7-nonatriene. The research was supported by a USDA-AFRI proposal # 2012-67013-19364, and a USDA Federal Formula Fund Initiative #2014-15-154.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolfin, M.S., Chilson, R.R., Thrall, J. et al. Proximate Mechanisms of Host Plant Location by a Specialist Phytophagous Insect, the Grape Berry Moth, Paralobesia Viteana. J Chem Ecol 45, 946–958 (2019). https://doi.org/10.1007/s10886-019-01112-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-019-01112-1