Abstract
Volatiles emitted from unpollinated in situ flowers were collected from two male cultivars, ‘M33’, ‘M91’, and one female cultivar ‘Zesy002’ (Gold3) of kiwifruit (Actinidia chinensis var. chinensis). The samples were found to contain 48 compounds across the three cultivars with terpenes and straight chain alkenes dominating the headspace. Electrophysiological responses of honey bees (Apis mellifera) and bumble bees (Bombus terrestris) to the headspace of the kiwifruit flowers were recorded. Honey bees consistently responded to 11 floral volatiles from Gold3 pistillate flowers while bumble bees consistently responded to only five compounds from the pistillate flowers. Nonanal, 2-phenylethanol, 4-oxoisophorone and (3E,6E)-α-farnesene from pistillate flowers elicited responses from both bee species. Overall, honey bees were more sensitive to the straight chain hydrocarbons of the kiwifruit flowers than the bumble bees, which represented one of the main differences between the responses of the two bee species. The floral volatiles from staminate flowers of the male cultivars ‘M33’ and ‘M91’ varied greatly from those of the pistillate flowers of the female cultivar Gold3, with most of the bee active compounds significantly different from those in the Gold3 flower headspace. The total floral emissions of ‘M33’ flowers were significantly less than those of the Gold3 flowers, while the total floral emissions of the ‘M91’ flowers were significantly greater than those of the Gold3 flowers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since their release in the late 1990s, yellow-fleshed kiwifruit have become a high value crop, typically selling from 50% to 120% more than green-fleshed cultivars, and have a global production of over 250,000 tons (O’Rourke 2016). In 2010 Actinidia chinensis var. chinensis (Actinidiaceae) (A. Chev.) A. Chev. ‘Hort16A’ (the main yellow-fleshed cultivar grown at the time) was hit by the bacterial vine infection Pseudomonas syringae pv. actinidiae (Psa) (Costa and Ferguson 2015). ‘Hort16A’ was unable to tolerate Psa which led to the rise of a new Psa tolerant yellow-fleshed cultivar, Actinidia chinensis var. chinensis ‘Zesy002’; marketed as Zespri® SunGold Kiwifruit and is commonly known as Gold3. Aside from Psa, one of the challenges associated with kiwifruit production is attaining a suitable degree of pollination to reach optimum potential fruit size. Like all kiwifruit species Actinidia chinensis var. chinensis is dioecious, requiring pollen from male vines to be transferred to female vines for seed set to occur, where seed set is positively correlated with fruit weight (Seal et al. 2017). The main contributors to pollen transfer in kiwifruit cultivation are insect pollinators, wind, and artificial pollination (Craig et al. 1988).
Managed honey bee (Apis mellifera) colonies are the most common form of commercial kiwifruit pollination in New Zealand orchards (Goodwin et al. 2013) where the majority of Gold3 kiwifruit are grown. Honey bee colonies are typically brought into the orchard at the onset of flowering (Matheson 1991) and fed sugar syrup inside the hive to stimulate pollen foraging (Goodwin et al. 1991). Another insect pollinator of kiwifruit in New Zealand orchards is the bumble bee (Bombus spp.), in particular B. terrestris (Howlett et al. 2017), with hives commercially available. Several studies have investigated the potential of bumble bees (B. terrestris) as a commercial pollinator for kiwifruit (Pomeroy and Fisher 2002; Read et al. 1989). Bumble bees were found to forage in weather conditions that honey bees found adverse and also had more cross-over between pistillate and staminate flowers.
Pollinating insects, like all animals, use their senses to interact with their environment. In their role as pollinators, honey bees and bumble bees use both visual and olfactory cues to locate floral food sources (Kunze and Gumbert 2001; von Frisch 1974). Recently it has been reported that bumble bees also use electric fields to see whether or not a flower has already been visited (Clarke et al. 2013). Scent is an important cue to both species, particularly honey bees, which use scent to communicate about food sources within the hive (Díaz et al. 2007; Farina et al. 2005).
Kiwifruit flower volatiles have been sporadically studied since the 1990s for the green-fleshed kiwifruit cultivars (Matich et al. 2003; Nieuwenhuizen et al. 2009; Samadi-Maybodi et al. 2002; Tatsuka et al. 1990; Twidle et al. 2015; Twidle et al. 2017). Many ubiquitous floral volatiles are reported in these studies, however Tatsuka et al. and Twidle et al. reported an abundance of unsaturated straight chain hydrocarbons, which are less common floral volatiles (Knudsen et al. 1993). These hydrocarbons have recently been identified in the green-fleshed kiwifruit cultivars as (8Z)-hexadecene, (6Z,9Z)-heptadecadiene, (3Z,6Z,9Z)-heptadecatriene, (8Z)-heptadecene and (9Z)-nonadecene with verification by synthesis (Twidle et al. 2015; Twidle et al. 2017). These compounds are more commonly encountered as insect produced communication compounds rather than floral volatiles as listed on The Pherobase (El-Sayed 2017), a website collating semiochemical occurrences in nature.
Gas chromatography coupled to electroantennogram detection (GC-EAD) has been used to examine the responses of many insects to different odour sources (Du and Millar 1999; Schiestl and Marion-Poll 2002; Struble and Arn 1984). Initial use focused mainly on insect pheromones (Struble and Arn 1984). However, more recent studies have seen this technique being used to find feeding attractants, and oviposition cues (Du and Millar 1999; Schiestl and Marion-Poll 2002). In the pollination field, GC-EAD has been used to identify the floral compounds of the crop recognised by their pollinators (Henning and Teuber 1992; Kobayashi et al. 2012; Thiery et al. 1990). This testing has also been applied to the flower headspace of the green-fleshed kiwifruit A. chinensis var. deliciosa (A. Chev.) A. Chev. ‘Hayward’ and its’ male polleniser ‘Chieftain’ with regard to honey bee response (Twidle et al. 2015). Here it was found that of the many volatiles in the flower headspace, the honey bee responded consistently to only six compounds.
The aim of this study was to provide information about the headspace of staminate and pistillate flowers of the different A. chinensis var. chinensis cultivars and to determine which volatiles the two main commercial pollinators, honey bees and bumble bees, respond to. This information will be of use to kiwifruit breeders when developing new cultivars and will provide valuable insight to the volatile cues used by commercial insect pollinators.
Methods and Materials
Insects
Honey bee (A. mellifera) pollen foragers were randomly collected from four hives at Plant & Food Research’s Lincoln, New Zealand, apiary (43°38.35’ S, 172°28.33′ E) as they returned to the hive. Bumble bee (B. terrestris) foragers were collected while foraging on clover fields (43°38.35’ S, 172°28.33′ E). Bumble bees came from one commercial hive and an unknown number of wild hives. Once captured, the bumble bees were anesthetised with CO2, then sexed, as unlike the honey bee, the male bumble bee will occasionally visit flowers too.
Volatile Collections
Dynamic headspace samples were collected from in situ flowers and leaves of kiwifruit vines during November 2015 from a commercial kiwifruit orchard in Riwaka, New Zealand (41°3.89’ S, 172°58.29′ E) following a previously published method (Twidle et al. 2017). Flower buds of A. chinensis var. chinesis cultivars ‘M33’ (n = 5), ‘M91’ (n = 5), and Gold3 (n = 6), were individually bagged in polyester oven bags 24 h before anthesis to prevent pollination. Once each flower was open, the polyester bag was removed and a two-part custom made glass chamber was carefully placed around the flower. The cylindrical glass chamber (radius = 20 mm, length = 60 mm) was wired onto the orchard canopy and carefully sealed with polytetrafluoroethylene tape to avoid any damage to the flower or vine. The glass chamber had a charcoal filter at the front and a polymer trap at the rear which was connected to a pump pulling 0.65 L/min of air through the system. Air entering the glass chamber passed through a charcoal filter and the volatiles were collected on 100 mg of Tenax#-GR 35/60 as air left the chamber. Before use, the glass chamber and charcoal filter were conditioned for 24 h at 150 °C and the Tenax was conditioned under nitrogen for 3 h at 250 °C. A leaf from the respective vine was set up in the same manner to the flowers above and collected from alongside the flowers as a control for each collection. The headspace was eluted from the Tenax using five 200 μL aliquots of distilled hexane and the resultant extract was stored at −80 °C until use.
Gas Chromatography – Mass Spectrometry (GC-MS)
Headspace collections, reaction products and synthetic standards were analysed on either a Varian 3800 GC coupled to a Saturn 2200 MS or an Agilent 7890B GC coupled to an Agilent 5977A MSD. The flow rate of helium was 1 mL/min on the Varian system and 1.6 mL/min on the Agilent system; otherwise operating conditions for the instruments were the same. Samples were analysed on both DB-5 ms and DB-wax GC columns, while chiral compounds were additionally tested on a chiral CycloSil-β GC column. All three columns had the following dimensions: 30 m × 0.25 mm i.d. × 0.25 μm. All temperature programmes started at 40 °C (2 min hold), then were increased by 4 °C/min to 280 °C (DB-5 ms), 230 °C (DB-wax) and 220 °C (CycloSil-β) followed by a 10 min hold. The injector temperatures for the different column types were 250 °C (DB-5 ms), 230 °C (DB-wax) and 220 °C (CycloSil-β). All MS analysis was done using the electron impact mode at 70 eV.
Dimethyl Disulfide (DMDS) Derivatisation
One hundred microliters of pooled ‘M91’ headspace was blown down to dryness under argon. To this 20 μL of DMDS was added along with 10 μL of iodine in diethyl ether (60 mg/mL). This solution was sealed in a glass vial and heated to 40 °C. After 2 h, 50 μL of 5% Na2S2O3 (aq) was added to quench the reaction. The organic products were extracted with two 100 μL aliquots of hexane and dried (MgSO4).
Chemicals
Synthetic standards and reagents were obtain from Sigma Aldrich (St. Louis, MO, USA) unless otherwise stated. Chemical purity of the standard is listed (%): 6-methyl-5-hepten-2-one (99%), octanal (99%), (±)-linalool (97%), nonanal (95%), 2-phenylethanol (99%), 4-oxoisophorone (98%), 2,6,6-trimethyl-1,4-cyclohexanedione (98%) from Frinton Laboratories (Hainesport, NJ, USA), decanal (97%), 2-phenylethyl acetate (99%), tridecane (99%), cis-jasmone (99%) from Bedoukian (Danbury, CT, USA), tetradecane (99%), geranyl acetone (97%), pentadecane (99%), hexadecane (99%), heptadecane (99%), octadecane (99%), and nonadecane (99%). (+)-Linalool (78%) and (−)-linalool (71%) were gifts from Professor Rikard Unelius of Linnaeus University, Sweden. (−)-Germacrene D (92%), (±)-germacrene D (34%), (3E,6E)-α-farnesene (98%), (3Z,6E)-α-farnesene (99%), (8Z)-hexadecene (97%), (8Z)-heptadecene (99%), (6Z,9Z)-heptadecadiene (99%), (3Z,6Z,9Z)-heptadecatriene (99%), and (9Z)-nonadecene (94%) were gifts from Plant & Food Research collaborators.
Farnesal isomers (72%) were prepared by microscale pyridinium chlorochromate (PCC) oxidation of a commercial farnesol mixture. To 50 μg of the farnesol mixture in dichloromethane (DCM), approximately three equivalents of PCC in DCM were added. The mixture was left at room temperature for 2 h then the DCM was evaporated off and the remaining organic products were re-dissolved in 1 mL of hexane for GC-MS analysis.
Gas Chromatography – Electroantennogram Detection (GC-EAD)
Antennal depolarisations of honey bees and bumble bees in response to the floral volatiles of kiwifruit cultivars; ‘M33’, ‘M91’ and Gold3 were recorded using an Agilent 7890B GC coupled to a Syntech EAD recording unit. Captured honey bee pollen foragers were anesthetised with CO2, then the head was excised and mounted between silver electrodes housed in saline filled glass capillaries. For each kiwifruit cultivar seven honey bee head preparations (n = 7) were used, with the standard temperature programme described below. Unfortunately one area of the chromatogram did not separate well and gave ambiguous responses so another four head preparations (n = 4) were run for each cultivar on the slower oven ramp to determine the response at that location. The slower temperature programme could not be used to measure responses to all compounds, as the head mounts lost sensitivity later in the run with the slow programme. For the bumble bee, only antennal preparations (an excised antenna from a female forager) were used, as the head was too big to mount in the same manner as for the honey bee. For the ‘M91 and Gold3 cultivars six bumble bee antennal preparations (n = 6) were used with the standard temperature programme below. For the ‘M33’ cultivar seven bumble antennal preparations (n = 7) were used.
Individual flower headspaces were pooled for each cultivar type and concentrated ten times under a gentle stream of argon. Once the bee preparation was set up 1 μL of the pooled headspace of the cultivar to be tested was injected into the GC-EAD. The injector was set at 250 °C and the injection was splitless for 0.6 min. Helium was used as the carrier gas with a constant flow of 1.2 mL/min, and the GC was equipped with a DB-5 ms column with dimensions of 30 m × 0.25 mm i.d. × 0.25 μm. The transfer line from the GC to the head mount/antennal preparation was maintained at 250 °C for all runs. The lifetime of the head mount/antennal preparation was from 40 min to 60 min plus. Therefore the temperature programme of the GC oven was set accordingly, 40 °C (held for 2 min) then increased by 10 °C/min up to 280 °C. Where antennally active compounds did not separate well, a slower temperature programme was used, 40 °C (held for 2 min) then increased by 4 °C/min up to 280 °C, which gave separation of co-eluting compounds early in the run.
Statistical Analysis
The ion counts from the individual chromatograms for each flower; ‘M33’, ‘M91’ and Gold3 were compared using analysis of variance. Data were checked for variance homogeneity by plotting standardised residuals. Data were natural log-transformed before analysis to stabilise the variance. Ion counts for each of the compounds were analysed separately using Minitab 16. Results are presented as back-transformed means, mean Least Significant Ratios (LSRs) (i.e. back-transformed Least Significant Differences (LSD)) and overall P-values for F-tests comparing all three cultivars. The LSR is the smallest ratio between two means (larger mean/smaller mean) for the means to be significantly different at the 5% level (d.f. = 15). The mean LSR is presented because of the variation in numbers of replicates.
Results
Floral Volatiles
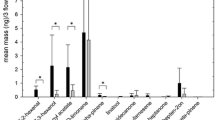
The analysis of the flower headspace of the three cultivars of A. chinensis var. chinensis yielded 48 compounds in total (Table 1). Hydrocarbons and terpenes dominated the headspace representing >74% in ‘M33’, > 95% in ‘M91’ and >92% in Gold3 of the total ion counts respectively. Dimethyl disulfide derivatisation was used to locate the double bond position in the monoenes. 9-Octadecene was identified by the M+ of m/z 304 and diagnostic fragment ion at m/z 173. All other straight chain alkenes were further identified to the correct geometric isomer by comparison with synthetic standards (Table 1).
The Gold3 flower headspace had 11 extra compounds compared to the ‘M33’flower, and six extra compounds compared to the ‘M91’flower. On the other hand the ‘M33’ flower had five compounds that were absent in the Gold3 flower headspace, while ‘M91’ had six compounds that were absent in the Gold3 headspace.
Honey Bee Antennal Responses
Honey bees responded to 18 compounds in the flower headspace of the kiwifruit cultivars (Table 2). Gold3 had 11 compounds in the headspace which elicited responses from the honey bee antennae: nonanal, 2-phenylethanol, 4-oxoisophorone, (+)-germacrene D, (3Z,6E)-α-farnesene, pentadecane, (3E,6E)-α-farnesene, (8Z)-hexadecene, hexadecane, (8Z)-heptadecene and farnesal isomer 2. ‘M33’ had eight compounds in the headspace of the flower eliciting responses from the honey bee antennae: 6-methyl-5-hepten-2-one, (−)-linalool, nonanal, 2-phenylethanol, 4-oxoisophorone, geranyl acetone, (3E,6E)-α-farnesene and (8Z)-heptadecene. The second male polleniser ‘M91’ had 14 antennally active compounds in the headspace: nonanal, 2-phenylethanol, 4-oxoisophorone, 2-phenylethyl acetate, (+)-germacrene D, (3Z,6E)-α-farnesene, pentadecane, (3E,6E)-α-farnesene, (8Z)-hexadecene, hexadecane, (6Z,9Z)-heptadecadiene, (8Z)-heptadecene, heptadecane and (9Z)-nonadecene. 2-Phenylethanol gave the largest antennal responses across all samples. Some example honey bee GC-EAD responses are presented in Fig. 1.
Bumble Bee Antennal Responses
Bumble bees responded to 12 compounds in the flower headspace of the kiwifruit cultivars (Table 3). The Gold3 headspace had only five compounds which elicited responses from the bumble bee antennae: nonanal, 2-phenylethanol, 4-oxoisophorone, tetradecane and (3E,6E)-α-farnesene. There were no responses seen to the unsaturated straight chain hydrocarbons. The ‘M33’ headspace had eight compounds in the headspace of the flower that elicited responses from the bumble bee antennae: 6-methyl-5-hepten-2-one, (−)-linalool, nonanal, 2-phenylethanol, 4-oxoisophorone, tetradecane, geranyl acetone, and (3E,6E)-α-farnesene. Again, there were no responses to the unsaturated straight chain hydrocarbons. The second male polleniser ‘M91’ had nine antennally active compounds in the headspace: nonanal, 2-phenylethanol, 4-oxoisophorone, 2-phenylethyl acetate, tetradecane, (3E,6E)-α-farnesene, (8Z)-hexadecene, (6Z,9Z)-heptadecadiene and (8Z)-heptadecene. The ‘M91’ cultivar was the most prolific volatile producer of the kiwifruit flowers (Table 4) and its increased hydrocarbon production saw three responses to hydrocarbons that had been active in the honey bee, albeit as smaller responses. 2-Phenylethanol also gave the largest antennal responses from the bumble bees. Some example bumble bee GC-EAD responses are presented in Fig. 1.
Statistical Analysis of Floral Emissions and Bee Perceived Compounds
Overall Gold3 produced more than four times the amount of floral volatiles than ‘M33’ while ‘M91’ produced one and half times more than Gold3 (Table 4). The male polleniser ‘M33’ produced similar amounts of nonanal and 4-oxoisophorone to Gold3. ‘M33’ produced significantly less of all the other bee perceived compounds compared to Gold3 except for 6-methyl-5-hepten-2-one and (−)-linalool where it produced more. The ‘M91’ polleniser produced similar amounts of 6-methyl-5-hepten-2-one, 2-phenylethanol, 4-oxoisophorone, (+)-germacrene D, (3Z,6E)-α-farnesene and (3E,6E)-α-farnesene to the Gold3 female while producing significantly more of all other bee perceived compounds, except for farnesal isomer 2 which it did not produce.
Discussion
There was large variation in the floral volatiles emitted across the kiwifruit cultivars. There were clear qualitative differences between Gold3 and the male pollenisers (Table 1). This is a stark contrast to the cultivars of A. chinensis var. deliciosa previously reported which showed great similarity both qualitatively and quantitatively (Twidle et al. 2017). The quantitative flower emissions presented for the A. chinensis var. chinensis cultivars in both Tables 1 and 4 again show large variation. They have less hydrocarbons and a more diverse headspace than the previously reported A. chinensis var. deliciosa cultivars. These differences help to explain the variety of responses seen to the same compounds in the various cultivars during the GC-EAD testing.
The ‘M33’ flower headspace contains all of the compounds from the Gold3 headspace that elicited a response from honey bees (Table 4). Yet, honey bees did not respond to (+)-germacrene D, (3Z,6E)-α-farnesene, pentadecane, (8Z)-hexadecene, hexadecane or farnesal isomer 2 in the ‘M33’ headspace (Table 2). It was a different case with the ‘M91’ headspace. Here, the ‘M91’ polleniser contained all of the honey bee perceived compounds from Gold3 except farnesal isomer 2, and the honey bees responded to all of those compounds. Thus the quantity of floral volatile emissions had a profound effect on the EAD response of the honey bees, with many of the bee active compounds below detection threshold in the ‘M33’ headspace. The effect these differences between staminate and pistillate flowers have on pollination remains unknown.
In the case of the bumble bee, the difference in responses to the cultivars of the A. chinensis var. chinensis kiwifruit flowers is also explained by considering both the qualitative and quantitative volatile emissions of the different cultivars. It can be seen that the ‘M33’ and ‘M91’ flower headspaces contain all of the compounds from the Gold3 headspace that elicited a response from bumble bees. However, bumble bees also responded to compounds from both ‘M33’ and ‘M91’ flowers that were absent or in low amounts in Gold3 flowers. (−)-Linalool and geranyl acetone were unique to ‘M33’ flower headspace, while 6-methyl-5-hepten-2-one was produced in much larger amounts by ‘M33’ flowers, and all three compounds elicited responses from the bumble bee antennae for that headspace sample. On the other hand, 2-phenylacetate was unique to ‘M91’ flowers which also produced much more (8Z)-hexadecene, (6Z,9Z)-heptadecadiene and (8Z)-heptadecene than the other cultivars and had the sole responses to these compounds amongst the A. chinensis var. chinensis cultivars. Bumble bees responded more strongly to the typical floral compounds such as 2-phenylethanol and 4-oxoisophorone than the hydrocarbon compounds (Table 3, Fig. 1). This was one of the main differences between the response of the bumble bee and honey bee, with honey bees being more sensitive towards the straight chain hydrocarbons than the bumble bees. The only exception to this was tetradecane, where bumble bees were more sensitive than the honey bees, giving consistent small responses to the saturated hydrocarbon. Tetradecane is well reported as both a plant volatile and insect communication compound on The Pherobase (El-Sayed 2017).
Hydrocarbons are widely used as communication compounds by insects, from type II moth sex pheromones (Ando et al. 2004) to cuticular hydrocarbons for nestmate recognition in social insects (Howard and Blomquist 2005). Social insects also use hydrocarbons to inform each other of their tasks/roles within the colony (Greene and Gordon 2003). Apis mellifera and B. terrestris both use hydrocarbons for nestmate recognition (Dani et al. 2005; Martin et al. 2010), and are likely to use hydrocarbons in additional communication channels similar to other social insects.
The difference in responses to kiwifruit flower scents between honey bees and bumble bees can probably be attributed, at least in part, to these hydrocarbon communication channels. It is likely that the responses seen by honey bees to the uncommon floral hydrocarbons are not a standard floral response, like those seen for the common floral volatiles such as 2-phenylethanol. Instead the strong response of the honey bees to the hydrocarbons in the headspace of the kiwifruit flowers is probably because the kiwifruit flower hydrocarbons are already used by honey bees as communication compounds. So far only the hydrocarbon (8Z)-heptadecene has been linked with honey bees, found on both workers (Del Piccolo et al. 2010) and larvae (Nazzi et al. 2002), yet it is likely other hydrocarbons in the kiwifruit flower are chemical communication compounds too. What was surprising, was that (9Z)-nonadecene elicited no antennal response from B. terrestris, when it is a known pheromone component for the closely related B. hortorum (Appelgren et al. 1991). Often when closely related insect species share habitats they find the pheromones of those related species repellent (Birch and Haynes 1982), hence an EAD response was expected from B. terrestris to (9Z)-nonadecene. The lack of response could also be due to low concentration in the headspace, since the pheromone titres of the B. hortorum are unknown. Apart from the hydrocarbons, the honey bees and bumble bees responded very similarly to the typical floral volatiles such as nonanal, 2-phenylethanol, 4-oxoisophorone, and (3E,6E)-α-farnesene, which was expected since both species are generalist pollinators.
In the case of kiwifruit flowers, the scent profiles of the A. chinensis var. deliciosa cultivars are very similar (Twidle et al. 2017). This means there is more chance of floral cross-over by experienced foragers and for an even sex-split between pistillate and staminate flowers visits by naïve foragers as they learn the scent of the new food source associated with the waggle dance of their returning forager sister. The scent profiles of the A. chinensis var. chinensis cultivars are very different from each other and do not lend themselves as well to the advantages of honey bee pollination, yet yellow-fleshed cultivars are still well pollinated.
The pollination requirements of the A. chinensis var. chinensis cultivar Gold3 are well below those of the A. chinensis var. deliciosa ‘Zes007’ (Green11) and ‘Hayward’. The green-fleshed kiwifruit cultivars such as ‘Hayward’ typically require 1300–1600 seeds to achieve full pollination, which equates to about 40 bee visits per flower (Goodwin and Haine 1995). Gold3 fruit on the other hand requires only 600 seeds to achieve full pollination, which equates to only six bee visits per flower (pers. comm. Mark Goodwin, PFR).
The A. chinensis var. deliciosa cultivars have been developed and selected by growers and breeders since the early part of the twentieth century (Schroeder and Fletcher 1967). During this time the main goal of kiwifruit breeders for male cultivars has been to align the flowering period of male vines with that of the female vines and to achieve maximum pollination rates (Ferguson et al. 1990). However, during the course of this selection process, floral odour and its effect on pollinators has generally been overlooked. Only male cultivars that have the correct flowering period and give good yields of fruit on the female vines are continued. While the observed and selected trait is the timing of flower anthesis, the volatile profile of the flower is also likely to be a factor and an unseen contributor to successful pollination of the A. chinensis var. deliciosa cultivars. Since A. chinensis var. deliciosa kiwifruit flowers require about 40 bee visits to reach suitable fruit size, it is probable that any factor which could aid in bee visits between flowers would be beneficial for pollination. Therefore, the breeding selection process over the last 60+ years has likely resulted in some serendipitous alignment of the volatiles from the pistillate and staminate flowers, where well performing male lines are selected based on their pollination performance which will include a contribution from the floral odour.
Gold3 on the other hand is a new cultivar developed during the twenty first century as replacement for ‘Hort16A’ with serendipitous Psa tolerance. With less time and lower selection pressure on seed set, and hence bee visits, these cultivars have not yet developed a similar odour profile amongst pistillate and staminate flowers. However, improving the similarity of the volatile profiles between these flowers of the A. chinensis var. chinensis cultivars could greatly enhance pollination rates and seed set, producing consistently larger fruit. Orchard scale trials are now underway to test the effect differences in flower volatiles have on pollination and fruit size.
References
Ando T, S-i I, Yamamoto M (2004) Lepidopteran sex pheromones. In: Schulz S (ed) The chemistry of pheromones and other Semiochemicals I. Springer, Berlin Heidelberg, Berlin, pp 51–96
Appelgren M, Bergstrom G, Svensson BG, Cederberg B (1991) Marking pheromones of megabombus bumble bee males. Acta Chem Scand 45:972–974. https://doi.org/10.3891/acta.chem.scand.45-0972
Birch MC, Haynes KF (1982) Insect pheromones. Edward Arnold, London
Clarke D, Whitney H, Sutton G, Robert D (2013) Detection and learning of floral electric fields by bumblebees. Science 340:66–69. https://doi.org/10.1126/science.1230883
Costa G, Ferguson AR (2015) Bacterial canker of kiwifruit: response to a threat. Acta Hortic (1095):27-40. https://doi.org/10.17660/ActaHortic.2015.1095.2
Craig JL, Stewart AM, Pomeroy N, Heath ACG, Goodwin RM, Craig JL, Stewart AM (1988) A review of kiwifruit pollination - where to next. N Z J Exp Agric 16:385–399
Dani FR, Jones GR, Corsi S, Beard R, Pradella D, Turillazzi S (2005) Nestmate recognition cues in the honey bee: differential importance of Cuticular alkanes and alkenes. Chem Senses 30:477–489. https://doi.org/10.1093/chemse/bji040
Del Piccolo F, Nazzi F, Della Vedova G, Milani N (2010) Selection of Apis mellifera workers by the parasitic mite Varroa destructor using host cuticular hydrocarbons. Parasitology 137:967–973. https://doi.org/10.1017/s0031182009991867
Díaz P, Grüter C, Farina W (2007) Floral scents affect the distribution of hive bees around dancers. Behav Ecol Sociobiol 61:1589–1597. https://doi.org/10.1007/s00265-007-0391-5
Du Y-j, Millar JG (1999) Electroantennogram and oviposition bioassay responses of Culex quinquefasciatus and Culex tarsalis (Diptera: Culicidae) to Chemicals in Odors from Bermuda grass infusions. J Med Entomol 36(2):158–166. https://doi.org/10.1093/jmedent/36.2.158
El-Sayed AM (2017) The Pherobase: Database of Insect Pheromones and Semiochemicals. <http://www.pherobase.com> 2003-2017 - The Pherobase - Ashraf M. El-Sayed
Farina WM, Grüter C, Díaz PC (2005) Social learning of floral odours inside the honeybee hive. Proc R Soc B Biol Sci 272:1923–1928. https://doi.org/10.1098/rspb.2005.3172
Ferguson AR, Seal AG, Davison RM (1990) Cultivar improvement, genetics and breeding of kiwifruit. Acta Hortic (282):335-348. https://doi.org/10.17660/ActaHortic.1990.282.43
Goodwin M, Haine H (1995) How many bee visits to fully pollinate kiwifruit? N Z Kiwifruit 111:5
Goodwin RM, Ten Houten A, Perry JH (1991) Feeding sugar syrup to honey bee colonies to improve kiwifruit pollen collection: a review. Acta Hortic (288):265-269. https://doi.org/10.17660/ActaHortic.1991.288.40
Goodwin RM, McBrydie HM, Taylor MA (2013) Wind and honey bee pollination of kiwifruit (Actinidia chinensis 'HORT16A'). N Z J Bot 51:229–240. https://doi.org/10.1080/0028825x.2013.806934
Greene MJ, Gordon DM (2003) Social insects: Cuticular hydrocarbons inform task decisions. Nature 423:32–32
Henning JA, Teuber LR (1992) Combined gas chromatography-electroantennogram characterization of alfalfa floral volatiles recognized by honey bees (hymenoptera: Apidae). J Econ Entomol 85:226–232
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393. https://doi.org/10.1146/annurev.ento.50.071803.130359
Howlett BG, Read SFJ, Jesson LK, Benoist A, Evans LE, Pattemore DE (2017) Diurnal insect visitation patterns to ‘Hayward’ kiwifruit flowers in New Zealand. N Z Plant Prot 70:52–57
Knudsen JT, Tollsten L, Bergström LG (1993) Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry 33:253–280. https://doi.org/10.1016/0031-9422(93)85502-I
Kobayashi K, Arai M, Tanaka A, Matsuyama S, Honda H, Ohsawa R (2012) Variation in floral scent compounds recognized by honeybees in Brassicaceae crop species. Breed Sci 62:293–302. https://doi.org/10.1270/jsbbs.62.293
Kunze J, Gumbert A (2001) The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav Ecol 12:447–456. https://doi.org/10.1093/beheco/12.4.447
Martin SJ, Carruthers JM, Williams PH, Drijfhout FP (2010) Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J Chem Ecol 36:855–863. https://doi.org/10.1007/s10886-010-9805-3
Matheson AG (1991) Managing honey bee pollination of kiwifruit (Actinidia deliciosa) in New Zealand - a review. Acta Hortic (288):213–219
Matich AJ, Young H, Allen JM, Wang MY, Fielder S, McNeilage MA, MacRae EA (2003) Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry 63:285–301. https://doi.org/10.1016/s0031-9422(03)00142-0
Nazzi F, Milani N, Vedova GD (2002) (Z)-8-heptadecene from infested cells reduces the reproduction of Varroa destructor under laboratory conditions. J Chem Ecol 28:2181–2190
Nieuwenhuizen NJ, Wang MY, Matich AJ, Green SA, Chen XY, Yauk YK, Beuning LL, Nagegowda DA, Dudareva N, Atkinson RG (2009) Two terpene synthases are responsible for the major sesquiterpenes emitted from the flowers of kiwifruit (Actinidia deliciosa). J Exp Bot 60:3203–3219. https://doi.org/10.1093/jxb/erp162
O'Rourke AD (2016) World kiwifruit review. Belrose, Inc., Pullman
Pomeroy N, Fisher RM (2002) Pollination of kiwifruit (Actinidia deliciosa) by bumble bees (Bombus terrestris): effects of bee density and patterns of flower visitation. N Z Entomol 25:41–49
Read PEC, Donovan BJ, Griffin RP (1989) Use of bumble bees, Bombus terrestris, as pollinators of kiwifruit and lucerne in New Zealand. N Z Entomol 12:19–23
Samadi-Maybodi A, Shariat MR, Zarei M, Rezai MB (2002) Headspace analysis of the male and female flowers of kiwifruit grown in Iran. J Essent Oil Res 14:414–415
Schiestl FP, Marion-Poll F (2002) Detection of physiologically active flower volatiles using gas chromatography coupled with Electroantennography. In: Jackson JF, Linskens HF (eds) Analysis of taste and aroma. Springer Berlin Heidelberg, Berlin
Schroeder CA, Fletcher WA (1967) The Chinese gooseberry (Actinidia chinensis) in New Zealand. Econ Bot 21:81–92
Seal AG, Clark CJ, Sharrock KR, de Silva HN, Jaksons P, Wood ME (2017) Choice of pollen donor affects weight but not composition of Actinidia chinensis Var. chinensis ‘Zesy002’ (Gold3) kiwifruit. N Z J Crop Hortic Sci:1–11. https://doi.org/10.1080/01140671.2017.1365732
Struble DL, Arn H (1984) Combined gas chromatography and Electroantennogram recording of insect olfactory responses. In: Hummel HE, Miller TA (eds) Techniques in pheromone research. Springer New York, New York, pp 161–178
Tatsuka K, Suekane S, Sakai Y, Sumitani H (1990) Volatile constituents of kiwifruit flowers: simultaneous distillation and extraction versus headspace sampling. J Agric Food Chem 38:2176–2180. https://doi.org/10.1021/jf00102a015
Thiery D, Bluet JM, Pham-Delegue MH, Etievant P, Masson C (1990) Sunflower aroma detection by the honeybee. Study by coupling gas chromatography and electroantennography. J Chem Ecol 16:701–711. https://doi.org/10.1007/bf01016481
Twidle AM, Mas F, Harper AR, Horner RM, Welsh TJ, Suckling DM (2015) Kiwifruit flower odor perception and recognition by honey bees, Apis mellifera. J Agric Food Chem 63:5597–5602. https://doi.org/10.1021/acs.jafc.5b01165
Twidle AM, Suckling DM, Seal AG, Fedrizzi B, Pilkington LI, Barker D (2017) Identification of in situ flower volatiles from kiwifruit (Actinidia chinensis Var. deliciosa) cultivars and their male pollenisers in a New Zealand orchard. Phytochemistry 141:61–69. https://doi.org/10.1016/j.phytochem.2017.05.011
von Frisch K (1974) Decoding the language of the bee. Science (New York, NY) 185:663–668. https://doi.org/10.1126/science.185.4152.663
Acknowledgements
Funding for this project was provided by the New Zealand Ministry of Business, Innovation and Employment (Contract # C11X1309). We thank Flore Mas and Kye Chung Park for useful discussions and Ruth Butler for advice on statistical analyses. We are grateful to Heywood Orchards Ltd. for access to kiwifruit vines, and thank Shona Seymour for identifying vine cultivars.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Twidle, A.M., Barker, D., Seal, A.G. et al. Identification of Floral Volatiles and Pollinator Responses in Kiwifruit Cultivars, Actinidia chinensis var. chinensis. J Chem Ecol 44, 406–415 (2018). https://doi.org/10.1007/s10886-018-0936-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-018-0936-2