Abstract
Lodgepole pine (Pinus contorta) forests have experienced severe mortality from mountain pine beetle (MPB) (Dendroctonus ponderosae Hopkins) in western North America for the last several years. Although the mechanisms by which beetles kill host trees are unclear, they are likely linked to pine defense monoterpenes that are synthesized from carbohydrate reserves. However, how carbohydrates and monoterpenes interact in response to MPB colonization is unknown. Understanding this relationship could help to elucidate how pines succumb to bark beetle attack. We compared concentrations of individual and total monoterpenes and carbohydrates in the phloem of healthy pine trees with those naturally colonized by MPB. Trees attacked by MPB had nearly 300% more monoterpenes and 40% less carbohydrates. Total monoterpene concentrations were most strongly associated with the concentration of sugars in the phloem. These results suggest that bark beetle colonization likely depletes carbohydrate reserves by increasing the production of carbon-rich monoterpenes, and other carbon-based secondary compounds. Bark beetle attacks also reduce water transport causing the disruption of carbon transport between tree foliage and roots, which restricts carbon assimilation. Reduction in carbohydrate reserves likely contributes to tree mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The physiological and chemical mechanisms underlying tree death due to insect attacks and drought have received much attention from researchers during the last decade (de la Mata et al. 2017; Leuzinger et al. 2009; McDowell et al. 2008; Sala et al. 2010; van Mantgem et al. 2009). The mountain pine beetle (MPB) (Dendroctonus ponderosae Hopkins) (Coleoptera: Curculionidae) is the most important mortality agent in pine trees, particularly lodgepole pine (Pinus contorta) in Western North America (Raffa et al. 2008). Since 1990, MPB has killed millions of hectares of lodgepole pine in British Columbia (Canada) alone, costing the governments and forest industry billions of dollars (Safranyik et al. 2010). In addition, MPB has had serious ecological consequences such as reducing forest carbon sinks (Kurz et al. 2008). Meanwhile, it has expanded its host range into novel habitats (Erbilgin et al. 2014), which has had cascading impacts on bird and mammal populations in post-MPB stands (Saab et al. 2014). Studies on MPB-host tree interactions have mainly focused on host defense chemistry (see references in Erbilgin et al. 2017a, b). Yet, carbohydrate reserves used by trees to produce these defense chemicals have received less attention (Goodsman et al. 2013; Lahr and Krokene 2013; Page et al. 2012; Wiley et al. 2016). In particular, how tree defensive chemistry and carbohydrate reserves change in response to bark beetle attacks is less understood (Raffa et al. 2017).
Tree death is required by MPB for the depletion of host defenses, successful host colonization, and reproduction, and it involves close interactions between beetles and their symbiotic phytopathogenic fungi (Safranyik et al. 2010). Beetles directly girdle the phloem and inoculate their fungi into vascular tissues during host colonization. Their combined effects overcome tree defenses and disrupt nutrient and water transport between foliage and roots (Frank et al. 2014), causing tree death. Continuous resin production and allocation of carbon resources toward resin production drains carbon resources, especially considering that photosynthesis is hindered (Goodsman et al. 2013; Lahr and Krokene 2013; Wiley et al. 2016). Thus, the interaction between defense compounds and carbohydrate reserves during host aggregation should be investigated to help determine how lodgepole pine succumbs to MPB attack.
Pine trees produce a variety of chemicals in their resin to defend against insects and pathogens (Raffa et al. 2005). Some of these compounds are volatile organic chemicals, for example monoterpenes (Seybold et al. 2006). Monoterpenes are important because they are not only toxic to beetles, but can also affect symbiotic fungal pathogens transported in beetle mycangia (Erbilgin et al. 2017a, b). For example, within two weeks of the beetle’s entry into subcortical tissues, a localized response occurs, resulting in the increase of monoterpene concentrations to approximately 300 times that of the first few days of attack (Raffa et al. 2005). Some of these monoterpenes, such as 3-carene and limonene, and one phenylpropanoid, 4-allylanisol, can be anti-feedants as well as hinder beetle growth and reproduction. Others such as myrcene and terpinolene may amplify the effects of MPB pheromones (Erbilgin et al. 2017a). Pheromones are synthesized either by de novo or by modifying host monoterpene α-pinene. It is unknown, however, how such sudden increase in defense chemistry production affects the carbohydrate reserves in trees.
Non-structural carbohydrates (NSCs) are thought to be the primary energy source supporting all physiological functions in trees (Barbaroux et al. 2003; Wiley et al. 2016), including the production of carbon-rich monoterpenes (Keeling and Bohlmann 2006). Starch and sugars constitute NSCs, which are found in stem, roots, branches, and needles (Wiley et al. 2016). The level of tree defense against bark beetles may be largely determined by NSC levels, because defense compounds such as monoterpenes are energetically costly and require carbohydrates. This suggests that an abundance of carbohydrate reserves is an important factor in pine defenses to beetles (Goodsman et al. 2013; Lahr and Krokene 2013; Miller and Berryman 1986; Wiley et al. 2016). Previous studies have investigated how NSCs are distributed throughout lodgepole pine after MPB attacks (Wiley et al. 2016) or fungal inoculations (Goodsman et al. 2013). However, these studies did not observe the effects of both MPB and their fungi on monoterpenes and NSC levels simultaneously. Our study is thus the first to investigate the relationship between monoterpene and NSC levels during the host colonization by MPB on pine trees.
Our objective was to determine monoterpene and NSC concentrations in the phloem shortly after MPB colonization of trees and compare them to those in non-attacked, healthy trees. Phloem is an ideal tissue to study these compounds because its content represents the first tier of defense against bark beetles and their fungi. The phloem also transports carbohydrates between the crown and roots (Vose and Ryan 2002). Therefore, studying phloem chemistry can be critical to determine the relationship between plant defenses and NSC concentrations in beetle-attacked trees. We hypothesized that lodgepole pine trees attacked by MPB will have elevated monoterpene concentrations combined with reduced NSCs in comparison to healthy trees, which would suggest that beetle colonization can deplete NSC reserves (Goodsman et al. 2013).
Methods and Materials
Site Location and Selection
A mixture of healthy and MPB-attacked lodgepole pine trees were selected at two sites 15 km apart in Jasper National Park, Alberta (Site 1: 52°41′50.3”N, 117°54′14.6”W; Site 2: 52°45′99.2”N, 118°01′53.7”W) in late August, 2016.
Tissue Sampling
Trees were visually inspected to determine if they were successfully colonized by MPB or healthy (no indications of bark beetle attacks or signs and symptoms of any major disease or insect attacks). Attacked trees were chosen based on how fresh the pitch tubes were, because hard and dry pitch tubes indicate a beetle attack from previous seasons. Successful MPB attacks were evident from the presence of early instar larval galleries and staining around oviposition galleries. All trees attacked by MPB died the following year (2017). Trees with the presence of disease or mechanical damage were not sampled. Once a tree was selected, bark was scraped off with a chisel, and a section of inner bark and phloem (5 × 2 cm) was extracted at 1.3 m height above the ground. Tissues from attacked trees were collected from healthy portions of phloem and were free of staining and beetle galleries. We estimated that MPB colonization on these trees likely began 6–8 weeks prior to sample collection based on observed larval development (a mixture of different life stages from eggs to 2nd larval instars). The 6–8 week timeframe also allows the tree to produce an adequate induced response (Erbilgin et al. 2017b). Samples from all attacked (N = 37) and healthy (N = 37) trees were collected on the same day and stored on dry ice in the field until they could be stored at −40 °C in the laboratory prior to chemical analysis.
Monoterpene Analysis
To prepare phloem samples for monoterpene extraction, the outer bark was removed to expose only the phloem. The samples were ground in liquid nitrogen using a cryogrinder (SPEX Sample Prep Freezer Mill 6770, NJ, USA) and then were stored at −40 °C.
One hundred milligrams of each ground sample was extracted twice with 0.5 mL of dichloromethane plus 0.004% tridecane as an internal standard at room temperature as described in Goodsman et al. (2013). Samples were vortexed for 30 sec at 3000 rpm, sonicated for 10 min, then centrifuged for 15 min at 0 °C and 16,100 rpm at 2 °C for 15 min. Extracts were transferred to 2 ml glass vials and stored at −40 °C until analysis. Extracts (1 μl) with a split ratio of 10:1 were injected into a coupled Gas Chromatograph-Mass Spectrometer (GC-MS, 7890A/5975C, Agilent Tech., Santa Clara, CA, USA) equipped with a non-chiral column (HP-Innovax; ID 0.50 mm, length 30 m; Product ID: 9091IN233I; Agilent Tech.). Extracts were analyzed with helium as the carrier gas at a flow of 1.0 ml min−1 and with a temperature program of 55 °C for 1 min, then 40 °C min−1 to 65 °C (held for 1 min), then 40 °C min−1 to 75 °C (held for 0.5 min), then 12 °C min−1 to 130 °C (held for 0.08 min), then 25 °C min−1 to 220 °C (held for 0.08 min) and then 50 °C min−1 to 250 °C (held for 0.5 min). Peaks were identified using the following standards: α-pinene, β-pinene, 3-carene, myrcene, limonene, p-cymene, camphor, 4-allyanisole, borneol (Fluka, Sigma-Aldrich, Buchs, Switzerland; chem. Purity >90%), γ-terpinene, α-terpinene, pulegone, terpineol (Sigma-Aldrich, St. Louis, MO, USA; chem. Purity >90%), ocimene, terpinolene, bornyl acetate (chem. Purity >90%), camphene (SAFC Supply Solutions, St. Louis, MO, USA; chem. Purity >80%), and β-phellandrene (Erbilgin laboratory; chem. Purity >90%). Compounds were identified by comparing retention times and mass spectra to those of the authentic standard chemicals. The quantity of chemicals was calculated using calibrated curves generated from analyses of a serial of dilution of known quantities of standards (20 μg/ml, 2 μg/ml, 0.2 μg/ml), and calculated as μg of compound/mg of fresh tissue.
Non-structural Carbohydrate Analysis
Water-soluble sugar and starch concentration analysis followed the protocol listed in Chow and Landhäusser (2004). The first step began with three days of freeze-drying 150 mg of each ground sample to remove excess moisture. Then, 50 mg of each sample was oven dried at 60 °C for 24 hr to avoid the conversion of starches to sugars. Water-soluble sugar was extracted from the sample in hot ethanol (80%), combined with phenol-sulfuric acid, then measured using a spectrophotometer (Pharmacia LKB Ultrospec III, Sparta, NJ, USA) set at 490 nm. The starches residing in the pellet were digested and mixed with the resultant glucose hydrolyzate with peroxidase-glucose oxidase/o-dianisidine, a color reagent. Starch concentrations (glucose hydrolysate) were measured at a wavelength of 525 nm.
Data Analysis
Concentrations (μg/mg fresh weight of phloem) of seven most abundant monoterpenes (α-pinene, β-pinene, 3-carene, myrcene, limonene, terpinolene, β-phellandrene), total monoterpenes, one phenylpropanoid, 4-allylanisol, and three carbohydrates (sugar, starch, and total carbohydrates) were analyzed. Other monoterpenes were identified and included in calculating total monoterpenes, but were not statistically analyzed separately. Differences in each of these variables between attacked and healthy tree groups were evaluated for statistical significance using two-sample t-tests. The relationship between total monoterpenes and potential explanatory variables, that are sugar, starch, total carbohydrates, tree group (attacked or attacked), and interactions between the carbohydrate variables and tree groups was investigated using a GLM in order to identify which of the individual carbohydrate variables was most strongly associated with overall monoterpene production. Differences in monoterpene-carbohydrate profiles between tree groups were tested using a permutational multivariate analysis of variance (perMANOVA), the results of which were visualized in a non-metric multidimensional scaling (NMDS) ordination plot. Variables were log-transformed in order to satisfy statistical assumptions, as needed. All analyses were conducted in the R software environment version 3.4.0 (R Core Team 2017). Multivariate analyses performed with functions provided in R package “vegan” version 2.4–3 (Oksanen et al. 2017).
Results
Overall monoterpene-carbohydrate profiles differed between attacked and healthy trees by MPB (perMANOVA F1,71 = 34.80, p < 0.001). NMDS indicated clear separation between these groups of trees (Fig. 1). Furthermore, NMDS showed attacked trees were associated with the monoterpene levels, whereas healthy trees were associated with carbohydrate levels (Fig. 1). These associations support the significant differences between means of attacked and healthy trees mentioned below and shown in Fig. 2.
NMDS ordination showing the separation of mountain pine beetle (Dendroctonus ponderosae)-attacked (open circle) or non-attacked, healthy (closed circle) lodgepole pine (Pinus contorta) trees on the basis of phloem monoterpene and carbohydrate variables (concentrations, μg/mg fresh weight). Abbreviations: Total mono = Total monoterpenes; Total carb. = Total carbohydrates
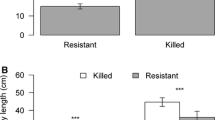
Means (±S.E.) of phloem monoterpenes (a) and carbohydrates (b) (concentrations, μg/mg fresh weight) in mountain pine beetle (Dendroctonus ponderosae)-attacked (open circle) or non-attacked, healthy (closed circle) lodgepole pine (Pinus contorta) trees. Results of t-tests comparing the mean concentrations of each compound between these groups of trees are indicated with “***” when p < 0.001 or “**” when p = 0.01–0.001
Trees attacked by MPB had higher concentrations of monoterpenes (both individual and total) and lower concentrations of NSCs compared to healthy trees (Fig. 2a, b). The mean total monoterpene concentration in attacked trees was 278% that of healthy trees (Fig. 2a). Similarly, concentrations of each individual monoterpene significantly differed between attacked and healthy trees (Fig. 2a). Each of the carbohydrates significantly differed between tree groups. Mean total carbohydrates, sugar, and starch were all less concentrated in phloem of attacked trees (Fig. 2b).
The total monoterpene concentration in lodgepole pine phloem showed a strong negative association with phloem sugar concentration (Table 1; Fig. 3). This relationship was indicated by the general linear model, which further supported the above results that total monoterpene concentrations differed between tree groups (Table 1; Fig. 2a). However, neither starch or total carbohydrate concentrations nor any of the carbohydrate-tree group interactions were significantly associated with total monoterpene concentrations (Table 1).
Discussion
Attacked and healthy lodgepole pine trees differed in their monoterpene and NSC concentrations. The former trees had nearly 300% more monoterpenes and had nearly 40% less NSCs. Similar reductions of carbohydrate concentrations in the stems of MPB-attacked trees have been previously reported (Page et al. 2012; Wiley et al. 2016). Although our approach does neither account for resistance/tolerance levels of trees sampled nor for the variation in environmental conditions in the expression of chemical defenses and NSC levels, our results suggest that MPB attacks increased monoterpene production while simultaneously decreasing carbohydrate reserves.
Our study provides evidence that bark beetles and their fungi can affect tree carbohydrate reserves in three possible ways. First, beetle attacks increase the production of carbon-rich terpenes (Raffa and Berryman 1983a). Monoterpenes are one of the primary defense compounds against MPB (Boone et al. 2011; Erbilgin et al. 2017a, b; Raffa and Berryman 1983a). The increased production of these compounds can occur as quickly as 3–7 days after beetle attacks (Cale et al. 2017; Raffa and Berryman 1983b). Such a rapid and large increase (ranging from 300% to 500%) in the production of monoterpenes requires a proportional increase in the metabolism of NSC reserves and allocation of carbon, ultimately depleting carbohydrate reserves (Goodsman et al. 2013).
Second, bark beetles and their associated fungi can restrict and disrupt carbon transport between needles and roots by killing the tree’s phloem, the primary tissue involved in translocating photosynthate such as sugars (Paine et al. 1997; Wiley et al. 2016). Thus, through the consumption of phloem, bark beetle feeding reduces not only local NSC availability for defense metabolism, but also inhibits the host’s ability to replenish carbohydrate reserves.
Finally, bark beetles and their fungi can reduce carbon assimilated during photosynthesis by disrupting water transport (Lahr and Krokene 2013; Miller and Berryman 1986; Regier et al. 2010; Wiley et al. 2016). Since phloem is not the major structure for water transport in trees, the activities of bark beetles themselves likely do not directly affect water transport. However, their associated phytopathogenic fungi can infect, proliferate in, and consequently occlude the xylem, thus disrupting water flow (Miller and Berryman 1986; Regier et al. 2010). Furthermore, reduced vascular water transport and availability likely leads to stomatal closure, causing reduced stomatal conductance and photosynthetic rates (Arango-Velez et al. 2016). Indeed, a loss of water conductance can be a primary cause of pine death following MPB colonization (Wiley et al. 2016). In fact, all trees attacked by MPB in our experiment died the following year (2017), whereas all control trees remained alive. We conclude that MPB and their associated fungi together may not only affect the production of carbohydrates, but also alter the allocation carbon to the production of defense compounds (Goodsman et al. 2013), further hastening the death of MPB-attacked trees (Wiley et al. 2016).
In conclusion, we found that trees colonized by MPB had substantially higher monoterpene levels than healthy trees. Lower levels of NSCs in the former group of trees suggest that the production of monoterpenes likely consumed NSCs and altered the carbon balance in trees (Goodsman et al. 2013). Furthermore, bark beetles and their associated fungi possibly affected the translocation of carbon and photosynthate. Together this may have led to the death of MPB-colonized trees that were no longer capable of producing defense chemicals or failed to allocate sufficient resources toward tolerating MPB colonization (Raffa and Berryman 1983a). Because the decrease in NSCs only partially explained the enhanced production of monoterpenes in the current study, other physiological processes such as hydraulic failure (McDowell et al. 2008; Wiley et al. 2016) or the differential allocation of resources along tree stem (Goodsman et al. 2013) likely contributed to tree death as well. Further studies should quantify other carbon-based secondary compunds, such as diterpene acids and phenolics, as well as primary compounds, such as lipids and proteins, in relation to NSC in pines.
References
Arango-Velez A, El Kayal W, Copeland CCJ, Zaharia LI, Lusebrink I, Cooke JEK (2016) Differences in defense responses of Pinus contorta and Pinus banksiana to the mountain pine beetle fungal associate, Grosmannia clavigera are affected by water deficit. Plant Cell Environ 39:726–744
Barbaroux C, Bréda N, Dufrêne E (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157:605–615
Boone CK, Aukema BH, Bohlmann J, Carroll AL, Raffa KF (2011) Efficacy of tree defense physiology varies with bark beetle population density: a basis for positive feedback in eruptive species. Can J For Res 41:1174–1188
Cale JA, Muskens M, Najar A, Ishangulyyeva G, Hussain A, Kanekar SS, Klutsch JG, Taft S, Erbilgin N (2017) Infection by a mycangial fungus causes differential feedbacks in the susceptibility of historical and novel host pines to mountain pine beetle. Tree Physiol 37:1597–1610
Chow PS, Landhäusser SM (2004) A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol 24:1129–1136
de la Mata R, Hood S, Sala A (2017) Insect outbreak shifts the direction of selection from past to slow growth rates in the long-lived conifer Pinus ponderosa. Proc Natl Acad Sci U S A 114:7391–7396
Erbilgin N, Ma C, Whitehouse C, Shan B, Najar A, Evenden M (2014) Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naive host ecosystem. New Phytol 201:940–950
Erbilgin N, Cale JA, Hussain A, Ishangulyyeva G, Klutsch JG, Najar A, Zhao S (2017a) Weathering the storm: how lodgepole pine trees survive mountain pine beetle outbreaks. Oecologia 184:469–478
Erbilgin N, Cale JA, Lusebrink I, Najar A, Klutsh JG, Sherwood P, Bonello E, Evenden ML (2017b) Water-deficit and fungal infection can differentially affect the production of different classes of defense compounds in two host pines of mountain pine beetle. Tree Physiol 37:338–350
Frank JM, Massman WJ, Ewers BE, Huckaby LS, Negrón JF (2014) Ecosystem CO2/H2O fluxes are explained by hydraulically limited gas exchange during tree mortality from spruce bark beetles. J Geophys Res Biogeosci 119:1195–1215
Goodsman DW, Lusebrink I, Landhäusser SM, Erbilgin N, Lieffers VJ (2013) Variation in carbon availability, defense chemistry and susceptibility to fungal invasion along the stems of mature trees. New Phytol 197:586–594
Keeling CI, Bohlmann J (2006) Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence on conifers against insects and pathogens. New Phytol 170:657–675
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Abata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–990
Lahr EC, Krokene P (2013) Conifer stored resources and resistance to a fungus associated with the spruce bark beetle Ips typographus. PLoS One 8(8):e72405. https://doi.org/10.1371/journal.pone.0072405
Leuzinger S, Bigler C, Wolf A, Körner C (2009) Poor methodology for predicting large-scale tree die-off. Proc Natl Acad Sci U S A 106:E106
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought. New Phytol 178:719–739
Miller RH, Berryman AA (1986) Carbohydrate allocation and mountain pine beetle attack in girdled lodgepole pines. Can J For Res 16:1036–1040
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt P, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P et al (2017) Vegan: community ecology package. R package version 2.4–3. https://CRAN.R-project.org/package=vegan
Page WG, Jenkins MJ, Runyon JB (2012) Mountain pine beetle attack alters the chemistry and flammability of lodgepole pine foliage. Can J For Res 42:1631–1647
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:179–206
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. URL https://www.R-project.org/
Raffa KF, Berryman AA (1983a) The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: Scolytidae). Ecol Monogr 53:27–49
Raffa KF, Berryman AA (1983b) Physiological aspects of lodgepole pine wound responses to a fungal symbiont of the mountain pine beetle. Can Entomol 115:723–734
Raffa KF, Aukema BH, Erbilgin N, Klepzig KD, Wallin KF (2005) Interactions among conifer terpenoids and bark beetles across multiple levels of scale: an attempt to understand links between population patterns and physiological processes. Recent Adv Phytochem 39:79–118
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517
Raffa KF, Mason CJ, Bonello P, Cook S, Erbilgin N, Keefover-Ring K, Klutsch JG, Villari C, Townsend PA (2017) Defense sydromes in lodgepole-whitebark pine ecosystems related to degree of historical exposure to mountain pine beetles. Plant Cell Environ 40:1791–1806
Regier N, Streb S, Zeeman SC, Frey B (2010) Seasonal changes in starch and sugar content of poplar (Populus deltoides x nigra cv. Dorskamp) and the impact of stem girdling on carbohydrate allocation to roots. Tree Physiol 30:979–987
Saab VA, Latif QS, Rowland MM, Johnson TN, Chalfoun AD, Buskirk SW, Heyward JE, Dresser MA (2014) Ecological consequences of mountain pine beetle outbreaks for wildfire in western north American forests. For Sci 60:539–559
Safranyik LL, Carroll AL, Régnière J, Langor DW, Riel WG, Shore TL, Peter B, Cooke BJ, Nealis VG, Taylor SW (2010) Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can Entomol 142:415–442
Sala A, Piper F, Hoch G (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol 186:274–281
Seybold SJ, Huber DP, Lee JC, Graves AD, Bohlmann J (2006) Pine monoterpenes and pine bark beetles: a marriage of convenience for defense and chemical communication. Phytochem Rev 5:143–178
van Mantgem PJ, Stephenson NL, Byrne JC, Daniels LD, Franklin JF, Fulé PZ, Harmon ME, Larson AJ, Smith JM, Taylor AH et al (2009) Widespread increase of tree mortality rates in the western United States. Science 323:521–524
Vose JM, Ryan MG (2002) Seasonal respiration of foliage, fine roots, and woody tissues in relation to growth, tissue N, and photosynthesis. Glob Chang Biol 8:182–193
Wiley R, Rogers BJ, Hodgkinson R, Landhäusser SM (2016) Nonstructural carbohydrate dynamics of lodgepole pine dying from mountain pine beetle attack. New Phytol 209:550–562
Acknowledgements
The project received funding from the NSERC-Discovery to NE, The University of Alberta – Undergraduate Research Initiative for MR. Carbohydrate analysis was conducted by Maksat Igdyrov in Dr. Simon Landhäusser’s lab (Univ. Alberta). We also acknowledge that all research presented in the manuscript was conducted in accordance with all applicable laws and rules set forth by provincial (Alberta) and federal governments and the University of Alberta and all necessary permits were in hand when the research was conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roth, M., Hussain, A., Cale, J.A. et al. Successful Colonization of Lodgepole Pine Trees by Mountain Pine Beetle Increased Monoterpene Production and Exhausted Carbohydrate Reserves. J Chem Ecol 44, 209–214 (2018). https://doi.org/10.1007/s10886-017-0922-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0922-0