Abstract
The nitidulid beetle Lobiopa insularis is an important pest of strawberry crops in the United States and Brazil. Both larvae and adults feed on ripe strawberries, causing 20–70% loss in production during serious infestations. Aiming at the development of efficient, clean, and highly specific pest management systems, semiochemicals, especially pheromones, are particularly useful. Analyses of the extracts of both males and females obtained from aeration of live beetles showed the presence of three male specific compounds, 2-nonanone, 2-undecanone, and 2-undecanol (in an enantiomeric ratio of S:R = 3.5:1). This is the first record of ketones and an alcohol as pheromone components in Nitidulidae. These compounds were emitted by males in amounts of 0.3:6:1.5 ng per insect within 24 h (1:30:3), respectively, during the scotophase, indicating nocturnal sexual activity. Field tests with pitfall traps containing different mixtures of compounds and ripe strawberries as a co-attractant summed up to five treatments with 25 replications. As a result, 59% males and 41% females (1:0.7) were caught, indicating the L. insularis pheromone to cause aggregation of both sexes. Results of the field tests showed that the attractivity of the binary mixture of ketones (T3) differed from the control (T5), from traps with 2-undecanone alone (T4), and from the mixture of 2-undecanone and racemic 2-undecanol (T2). Moreover, the activity of the ternary mixture of compounds (T1) was not different from that of T3, indicating that the racemic alcohol did not positively influence trap catches. In future applications, a mixture of synthetic strawberry-derived compounds that are attractive to L. insularis may substitute rapidly decaying fruit in the field, maintaining catches for longer periods. Because of its efficiency and low cost, a mixture of 2-undecanone and 2-nonanone is recommended to catch adult L. insularis in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various insect pests attack strawberry crops (Fragaria x ananassa Duchesne - Rosaceae) (Bernardi et al. 2015). During the fruiting period, the strawberry sap beetle, Lobiopa insularis (Castelnau, 1840) (Coleoptera: Nitidulidae) is the main pest, damaging ripe strawberries (Fornari et al. 2013; Guimãraes et al. 2009; Myers 2016; Potter et al. 2013; Rondon et al. 2014). In Brazil, losses in production range from 20% to 70% in severe outbreaks, especially during harvest of the crops (Fornazier et al. 1986; Salles and Williams 1986).
In the field, both larvae and adults of L. insularis feed on ripe strawberry fruits, damaging them for consumption and industrial use, because ripe fruits are contaminated by larvae and feces (Botton et al. 2014). The same damage is caused by the strawberry sap beetle, Stelidota geminata (Coleoptera: Nitidulidae) in the northeastern region of the US (Loughner et al. 2007). In Southern Brazil, sap beetles infest crops between August and February and overwinter in sheltering vegetation or similar protective places (Rondon et al. 2014). Results of a laboratory study indicated that the lifetime of males and females of L. insularis is of approximately 270 and 318 days, respectively, and that the oviposition period lasts 133 ± 27.5 days (Botton et al. 2014; Bortoli et al. 2014), indicating the potential damage that these insects may cause in the field.
Due to its location inside mature fruits and because the collection of fruits is performed daily by producers, pesticide applications could cause a serious residue problem. A study of the Brazilian Sanitary Agency (ANVISA), carried out between 2001 and 2010, revealed that approximately 80% of tested strawberry samples contained insecticide residues (e.g. organophosphorus compounds, triazoles, pyrethroids and carbamates) (Jardim and Caldas 2012). Despite the residue that these substances leave on the fruits, two pesticides, a pyrethroid-benzoylurea combination and an analogue of pyrazole, were approved for controlling L. insularis in strawberry crops (MAPA 2017). Moreover, an alternative monitoring system was described using plastic containers with squashed strawberries as attractants, water, and Malathion 1000® CE (dose of 4 mL/L), an organophosphorous compound (Botton et al. 2014; Guimãraes et al. 2009). However, already after seven days these traps, which then contain rotten strawberries, are no longer attractive (Botton et al. 2014).
In this scenario, aiming for a specific, cleaner, and effective control technique, semiochemicals stand out, particularly for organic farming, in which pesticides are not allowed. Consequently, the objective of this study was to identify the pheromone composition of L. insularis and to test a synthetic lure in the field.
Methods and Materials
Collection and Maintenance of Insects in the Laboratory
Adults and immatures of L. insularis were collected manually in an ordinary strawberry field (cv. Albion), located at São José dos Pinhais, Paraná, Brazil (Latitude: −25.613302, Longitude: −49.082794), between August and December 2015, and taken to the Laboratório de Semioquímicos, Chemistry Department of the Universidade Federal do Paraná (UFPR). Species assignment followed Parson (1943), and sex determination was based on Guimãraes et al. (2009).
Insects were kept in rearing chambers under controlled conditions of temperature, humidity, and photoperiod (25 ± 1 °C, 70 ± 10%, 12:12 h L/D) in polystyrene boxes (11 cm × 11 cm × 3.5 cm) containing autoclaved soil to avoid contamination by fungi and spider mites. Boxes also contained pieces of ripe strawberry, banana or a strawberry based artificial diet (Bortoli et al. 2014), which was changed whenever necessary.
Volatile Collection
Collections of headspace volatiles were performed inside glass chambers (35 cm × 4.5 cm) placed in an air-conditioned room (24 ± 2 °C and 12:12 h L/D). Samples were collected by using a humidified and charcoal-filtered airflow at 1 L/min/chamber. Volatiles were trapped on glass tubes containing 20 mg of HayeSep Q 80–100 mesh (Althech, Lokeren, Belgium) and eluted with 400 μL of doubly distilled HPLC grade hexane (Zarbin et al. 1999). Extracts were produced every 24 h for comparison of volatiles of males and females and every 12 h to determine the profile of pheromone production. Control experiments were run under the same conditions, but without insects. Extractions were performed at the same time every day until the end of the experiments. All extracts obtained were stored at −20 °C until analysis.
Analytical Procedures
Extracts were analyzed by GC, GC/MS, and GC/FT-IR (all equipped with a GC-QP 2010 Plus (Shimadzu) and with a RTX-5 column (30 m × 0.25 mm i.d. and 0.25 mm film thickness; Restek, Bellefonte, Pennsylvania, USA). Injections of 1 μL of extract were performed using splitless injection at 250 °C. The oven temperature started at 50 °C (1 min hold) and was ramped to 250 °C at a rate of 7 °C/min. Helium was used as the carrier gas at a column head pressure of 170 kPa. The same parameters were used for all analyses.
Infrared spectra were recorded using a DiscovIR-GC Spectra Analysis gas chromatograph coupled to a Shimadzu gas chromatograph (GC) (model 2010). The GC was operated in splitless mode and was equipped with a DB-5 (0.25 μm, 0.25 m × 30 m) (J&W Scientific, Folsom, California, EUA) capillary column. Helium served as the carrier gas. The temperature program was the same as that used in the GC/MS analysis. A liquid-nitrogen-cooled photoconductive mercury-cadmium-telluride (MCT) detector was used with a FT-IR resolution of 8 cm−1.
Identification and Quantification of Volatile Compounds
Volatiles released by L. insularis were identified on the basis of their mass spectra, Kovats indices (KI), and co-injections with commercial standards (Aldrich Chemical Company - Milwaukee, Wisconsin, USA). Pure enantiomers of 2-undecanol were synthesized in our lab.
The quantification of pheromone components released by L. insularis was calculated in nanograms (ng) based on peak areas of GC/MS chromatograms and on calibration curves produced with different concentrations (0.05, 0.1, 1, and 5 ng) of synthetic standards of the identified natural products.
Kinetic Enzymatic Resolution of 2-Undecanol
A solution of racemic 2-undecanol (41.5 mg, 0.24 mmol), CAL-B (65 mg, Novozym 435 ®), and vinyl acetate (0.1 mL) in tetrahydrofuran (2.5 mL) was stirred on a shaker at 32 °C for 2 h at 200 rpm. Subsequently, the solution was filtered and the solvent removed under vacuum. The acetate formed and the unreacted alcohol were separated by column chromatography with silica gel using hexane as the eluent. As a result, 19 mg of (R)-2-undecyl acetate with [α]D = −1.81 (C = 1 in CHCl3) were obtained and 17 mg of (S)-2-undecanol with [α]D = +6.72 (C = 1 in CHCl3). Using enantioselective GC analysis (β-cyclodextrin stationary phase column), the enantiomeric excess was shown to be higher than 99% for both compounds. To determine the enantiomeric composition of natural 2-undecanol it was necessary to acetylate the sample as the racemate was not well resolved under the described conditions.
Field Tests
From September to December 2016, field tests were performed at the same location as the collection of insects the year before. Pitfall traps containing a rubber septum impregnated with 150 μL of five different mixtures (T1-T5) and half a ripe strawberry were used. Baits were formulated by combining the strawberry with the three male specific compounds identified in the extracts of L. insularis according to the proportions and quantities emitted by the insects (1:30:3 or 1.3, 4 and 40 ng/150 μL of 2-undecanol, 2-nonanone and 2-undecanone, respectively). Lures were T1: 2-undecanone +2-undecanol +2-nonanone + strawberry; T2: 2-undecanone +2-undecanol + diet; T3: 2-undecanone +2-nonanone + strawberry; T4: 2-undecanone + strawberry; T5: control - hexane + strawberry; racemic 2-undecanol was used in the field.
Pitfall traps were modifications of that described by Kelts (2005). They were made from 500 mL plastic cups (diameter 7 cm, height 12 cm) covered with a yellow lid. Five holes were made circularly on the lids, and two rows of four aligned holes (2 cm in diameter) were made around the cups to allow insects to enter. A solution of water and detergent was placed at the bottom of the trap (± 100 mL). To support the diet, a 30 mL cup (bottom 4 cm, height 3 cm) was pierced and tied with a wire on the upper part of the trap. Rubber septa were also pierced and tied internally with a wire to the lid of the trap.
Traps were marked with yellow strings to facilitate their re-collection and maintenance and put in a shallow hole of approximately 5 cm in the soil, among strawberry plants. In a greenhouse (120 m of extension), pitfall traps were set up approximately 24 m apart from each other and set up 10 m away from the entrance and exit of the greenhouse. Baits T1 to T5 were placed randomly in each greenhouse. Five greenhouses were used in total. After 14 days, traps were collected and insects sexed and counted. Five repetitions were carried out.
Statistical Analysis
Field tests analyses were performed in the statistical program BioEstat 5.0 (Ayres 2007). A square-root transformation was done followed by an ANOVA and a t test (5%) (LSD – Least Significant Difference), which compared the mean number of insects collected per treatment. The outliers were discarded from the analyses.
Results
Analysis of the Extracts of Lobiopa insularis
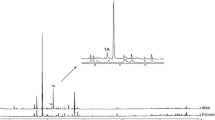
Comparisons of volatiles collected from both sexes showed the presence of three male specific compounds (Fig. 1).
The Kovats Indices (KIs) of the three components on the RTX-5 column were: 1: KI = 1087; 2: KI = 1296; and 3: KI = 1303. GC/MS-analysis suggested the compounds to be 2-nonanone (1), 2-undecanone (2) and 2-undecanol (3), respectively (Fig. 2).
The minor compound 1 showed the mass spectrum depicted in Fig. 2, 1a. Its infrared spectrum (Fig. 2, 1b) with a strong C = O stretching absorption band at 1712 cm−1, which is characteristic for ketones (Silverstein et al. 1991), a molecular ion of m/z 142, and a search in the NIST database suggested the target compound to be 2-nonanone. Co-injection of an authentic sample of 2-nonanone and the natural extract proved that compound 1 was indeed 2-nonanone.
Similar to 1, the mass spectrometric fragmentation pattern of 2 (Fig. 2, 2a) and its infrared spectrum (Fig. 2, 2b) with again a strong C = O stretching absorption band at 1712 cm−1 (Silverstein et al. 1991) as well as a molecular ion of m/z 170 suggested the compound to be 2-undecanone. Comparison of the analytical data of the natural product with those of an authentic sample of 2-undecanone and GC-coinjection confirmed our structure assignment.
The mass spectrum of compound 3 (Fig. 2, 3a) showed a diagnostic base peak at m/z 45, revealing the sub-structure of a methyl carbinol, and the NIST database suggested compound 3 to be 2-undecanol. This was supported by the infrared spectrum, which showed a stretching band at 3285 cm−1 (Fig. 2, 3b), indicating the presence of a hydroxyl group (Silverstein et al. 1991). The identification of 2-undecanol was confirmed by co- injection of an authentic sample and the natural extract.
To investigate the stereochemical composition of 2-undecanol, reference samples of (R)- and (S)-2-undecanol were obtained through kinetic enzymatic resolution of racemic 2-undecanol, using the enzyme CAL-B (Novozym 435 ®) (Vidal et al. 2010). The reaction showed a good selectivity (E > 200), and only one stereoisomer was acetylated. The produced acetate and the unreacted part of the alcohol were separated by flash chromatography. The optical rotation of the two samples was measured to check which isomer had been esterified. The alcohol and the acetate showed an [α]D = +6.72 and an [α]D = −1.81, respectively (both samples C = 1 in CHCl3). According to the literature (S)-2-undecanol has an [α]D = +6,60 (C = 0.95 in CHCl3) (Hillbur et al. 2005; Ohtaki et al. 2005), which proves that in the present kinetic resolution the (R)-enantiomer had been acetylated, following the Kazlauskas rule (Kazlauskas et al. 1991).
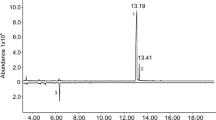
Enantioselective gas chromatography employing a modified β-cyclodextrin as the stationary phase failed to satisfactorily separate racemic 2-undecanol. However, a good resolution was achieved with 2-undecyl acetate, as shown in Fig. 3. (S)-2-Undecanol, obtained in the resolution reaction, was acetylated and injected for comparison with the racemic sample. The synthetic (R)-2-undecyl acetate was also analyzed. This enantioselective analysis proved that both alcohol and acetate obtained in the kinetic resolution showed an enantiomeric excess higher than 99%. Subsequently, a micro-derivatization was performed with extracts of males to obtain the acetate of the natural product. Comparison of retention times showed that L. insularis produced 2-undecanol in an enantiomeric ratio of (S):(R) = 3.5:1 (Fig. 3). This was confirmed by GC/MS-analysis, which showed that both peaks present in the acetylated extract were definitely 2-undecyl acetates and not impurities with the same retention time.
Quantification and Emission of Pheromone Components
During 24 h one insect emitted a mean amount of 0.30 ± 0.15 ng (1–2-nonanone), 6.03 ± 5.15 ng (2–2-undecanone), and 1.5 ± 1.37 ng (3–2-undecanol) (Fig. 4), in proportions of 1:30:3, respectively. Furthermore, a close look at the circadian rhythm of the release of volatiles showed that the compounds are only emitted during the scotophase.
Field Tests
ANOVA indicated differences among treatments (F: 4.380; P = 0.008), being T3 (2-undecanone +2-nonanone + strawberry) different from T2 and T4 (P = 0.01), and T5 (P = 0.001) (Fig. 5). The following comparisons were not different from each other (P > 0.05): T1 x T2, T1 x T3, T2 x T4, T2 x T5, and T4 x T5. Moreover, all traps except the control (N = 1 male) captured both sexes (N = 44 insects), with a mean proportion of approximately 60% of males to 40% of females or 1:0.7 respectively.
Discussion
Pheromone Components of Lobiopa insularis
Three male specific volatiles, 2-nonanone, 2-undecanone, and 2-undecanol were identified from the sap beetle L. insularis. This is the first time that ketones were identified as pheromone components in the Nitidulidae family and the first time that 2-undecanol was identified as a pheromone component in Coleoptera (see El-Sayed 2016).
In the field, pitfall traps containing different mixtures of the three male-specific compounds captured both sexes, indicating that the pheromone has the function of an aggregation pheromone. Furthermore, this pheromone could be an “aggregation-sex pheromone”, according to Cardé (2014), which means that it is produced by one sex, attracting both sexes, and the primary driving force is mate procurement.
Aggregation pheromones are commonly found in nitidulid beetles, especially in Carpophilus, which is the most intensively studied genus of this family. The pheromones described for this genus are usually methyl- and ethyl-branched polyenes. For example, the pheromone of Carpophilus mutilatus Erichson is composed of (E,E,E)-5-ethyl-7-methyl-3,5,7-undecatriene and (E,E,E)-6-ethyl-4-methyl-3,5,7-decatriene (Bartelt et al. 1993), and the pheromone of Carpophilus hemipterus (L.) (Coleoptera: Nitidulidae) is composed by even four polyenes: (E,E,E,E)-3,5,7-trimethyl-2,4,6,8-decatetraene, (E,E,E,E)-3,5,7-trimethyl-2,4,6,8-undecatetraene, (E,E,E,E)-7-ethyl-3,5-dimethyl-2,4,6,8-decatetraene, and (E,E,E,E)-7-ethyl-3,5-dimethyl-2,4,6,8-undecatetraene (Bartelt et al. 1992).
The pheromone components of L. insularis have also been found as plant volatiles. For example, 2-nonanone is naturally occurring as a component of fruit aroma that has antimicrobial activity, and it was effective in preventing postharvest deterioration in the packaging of strawberries (Almenar et al. 2009). In addition, 2-nonanone is released by fermented pollen, which attracted the sap beetle Aethina tumida Murray in laboratory bioassays (Torto et al. 2007).
2-Undecanone has been isolated from the trichomes of wild tomatoes (Scott 2002) and proved to be a powerful repellent of mosquitos, ticks, and cockroaches (Roe 2004). In contrast, 2-undecanol is a flower emitted volatile, used by various species of Hymenoptera as a pheromone component (e.g. Apidae, Formicidae) (see El-Sayed 2016).
Both, 2-undecanone and 2-nonanone, have been identified as components of the volatile bouquet of ripe strawberries, attracting adults of L. insulars in the field (Kelts 2005). Therefore, both ketones may be sequestered by L. insularis through feeding. Furthermore, the structural similarity among the three pheromonal compounds indicates a widespread, common biosynthesis. Starting from myristic or palmitic acid, β-oxidation (chain shortening) would furnish the less stable 3-oxododecanoic acid, which would yield 2-undecanone upon decarboxylation. Subsequent reduction would produce 2-undecanol. Similarly, the immediate precursor of 2-nonanone would be decanoic acid (generated from dodecanoic acid by another chain shortening step). Consequently, the compounds can also be insect-produced through de novo biosynthesis. Besides acting as pheromones, both 2-nonanone and 2-undecanone might also act as antimicrobial agents (Almenar et al. 2009), and as repellents against parasitoids and predators (Roe 2004).
Field Tests
Results indicated that the activity of the binary mixture of the ketones (T3) did not differ from that of the ternary mixture (T1), and both treatments differed from the control, indicating that the ketones could be used in the field to monitor and control L. insularis populations. It also indicates that 2-undecanol did not improve the efficiency of catch, and more studies are necessary to understand the biological activity of this compound.
Field tests were performed with a co-attractant (ripe strawberry), and even though treatments containing pheromone components without the co-attractant were not performed in this study, fruit plus 2-undecanone and 2-nonanone (T3) was clearly more effective in captures compared to fruit alone (T5). With regards to the positive effect of co-attractants in the field, effective captures of sap beetles were shown with traps containing the pheromone of C. hemipterus combined with host-related co-attractants (e.g. fermenting whole wheat bread dough or fermenting fig juice), which caught more than 1000 insects per trap, whereas the pheromone alone caught only about 20 individuals (Bartelt et al. 1992). Similar effects have been frequently reported from other species.
References
Almenar E, Catala R, Hernandez-Muños P, Gavara R (2009) Optimization of an active package for wild strawberries based on the release of 2-nonanone. Food Sci Technol-LEB 42:587–593
Ayres M, Ayres JM, Ayres DL, Santos AS (2007) Bioestat 5.0 - Aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém, PA, pp 364.
Bartelt RJ, Dowd PF, Vetter RS, Shorey HH, Baker TC (1992) Responses of Carpophilus hemipterus (Coleoptera: Nitidulidae) and other sap beetles to the pheromone of C. hemipterus and host-related coattractants in California field tests. Environ Entomol 21:1143–1153
Bartelt RJ, Carlson DG, Vetter RS, Baker TC (1993) Male-produced aggregation pheromone of Carpophilus mutilatus (Coleoptera: Nitidulidae). J Chem Ecol 19:107–118
Bernardi D, Botton M, Nava DE, Zawadneak MAC (2015) Guia para a identificação e monitoramento de pragas e seus inimigos naturais em morangueiro, 1st edn. Embrapa Clima Temperado, p 46
Bortoli LC, Machota Júnior R, Botton M (2014) Biologia e tabela de vida de fertilidade da broca-do-morangueiro criada em dieta artificial. Pesq Agropec Bras 49:144–147
Botton M, Bernardi D, Fornari R, Machota Júnior R, Bortoli LC (2014) Bioecologia, monitoramento e controle de Lobiopa insularis (Castelnau, 1840) (Coleoptera: Nitidulidae) na cultura do morangueiro no Rio Grande do Sul. Embrapa, Circular Técnica n. 113, pp 8
Cardé RT (2014) Defining attraction and aggregation pheromones: teleological versus functional perspectives. J Chem Ecol 40:519–520
El-Sayed AM (2016) The Pherobase: Database of Pheromones and Semiochemicals
Fornari RA, Machot R Jr, Bernardi D, Botton M, Pastori PL (2013) Evaluation of damage, food attractants and population dynamics of strawberry sap beetle. Hort Bras 31:380–385
Fornazier MJ, Carmo CAS, Teixeira CP, Pereira EB (1986) Finding of the strawberry borer Lobiopa insularis in the state of Espírito Santo. EMCAPA. Comunicado Técnico n. 44, pp 3
Guimãraes AJ, Michereff FM, Ribeiro MGPM, Liz RS, Guedes IMR (2009) Ocorrência e Manejo da Broca-do-Morango no Distrito Federal. Embrapa, Comunicado Técnico n. 74, pp 5
Hillbur Y, Celander M, Baur R, Rauscher S, Haftmann J, Franke S, Francke W (2005) Identification of the sex pheromone of the swede midge, Contarinia nasturii. J Chem Ecol 31:1807–1828
Jardim ANO, Caldas ED (2012) Brazilian monitoring programs for pesticide residues in food – Results from 2001 to 2010. Food Control 25:607–616
Kazlauskas RJ, Weissfloch ANE, Rappaport AT, Cuccia LA (1991) A rule to predict which enantiomer of a secondary alcohol reacts faster in reactions catalyzed by cholesterol estarase, lipase from Pseudomonas cepacia, and lipase from Candida rugosa. J Org Chem 56:2656–2665
Kelts CA (2005) Evaluation of attractants and monitoring for sap beetle control in strawberries. Thesis for Master in Sciences - University of Florida, Florida, p 88 http://ufdcimages.uflib.ufl.edu/UF/E0/01/20/62/00001/kelts_c.pdf
Loughner RL, Loeb GM, Demchak K, Schloemann S (2007) Evaluation of strawberry sap beetle (Coleoptera: Nitidulidae) use of habitats surrounding strawberry plantings as food resources and overwintering sites. Environ Entomol 36:1059–1065
MAPA - Ministério da Agricultura, Pecuária e Abastecimento (2017). AGROFIT: sistema de agrotóxicos fitossanitários http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons
Myers L (2016) Sap beetles of Florida. University of Florida. http://entnemdept.ufl.edu/creatures/field/corn/sap_beetles.htm
Ohtaki T, Akasaka K, Kabuto C, Ohrui H (2005) Chiral discrimination of secondary alcohols by both 1H-NMR and HPLC after labeling with a chiral derivatization reagent, 2-(2,3-Anthracenedicarnoximide)cyclohexane carboxylic acid. Chirality 17:S171–S176
Potter MA, Price JF, Habeck DH, Schuster DJ, McCord E Jr (2013) A survey of sap beetles (Coleoptera: Nitidulidae) in strawberry fields in west central Florida. Fla Entomol 96:1188–1189
Roe RM (2004) Method of repellency insects. US patent 6:437,001
Rondon SI, Price JF, Cantliffe DJ (2014) Sap beetle (Coleoptera: Nitidulidae) Management in Strawberries. University of Florida, Horticultural Sciences Department, n. HS993, 2. rev
Salles LAB, Williams RN (1986) Broca-do-morango (Lobiopa insularis). Embrapa-UEPAE. Documentos n. 17, pp 10
Scott A (2002) Insect repellent designed from tomatoes. Chem Week 164:30
Silverstein RM, Bassler GC, Morril TC (1991) Spectrometric identification of organic compounds, 5th edn. Wiley, New York, p 512
Torto B, Arbogast RT, Alborn H, Alonso S, van Englesdorp D, Boucias D, Tumlinson JH, Teal PEA (2007) Composition of volatiles from fermenting pollen dough and attractiveness to the small hive beetle Aethina tumida, a parasite of the honeybee Apis mellifera. Apidologie 38:380–389
Vidal DM, Fonseca MG, Zarbin PHG (2010) Enantioselective synthesis and absolute configuration of the sex pheromone of Hedypathes betulinus (Coleoptera: Cerambycidae). Tetrahedron Lett 51:6704–6706
Zarbin PHG, Ferreira JTB, Leal WS (1999) Metodologias gerais empregadas no isolamento e identificação estrutural de feromônios de insetos. Quim Nova 1999:263–268
Acknowledgements
The authors would like to thank Marcelo Leschnhak for allowing the field tests and collections of L. insularis in his property, Dr. Maurício Osvaldo Moura for the statistical analyses, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Semioquímicos na Agricultura (INCT), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Fundação Araucária (FA) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moliterno, A.A.C., Martins, C.B.C., Szczerbowski, D. et al. The Male Produced Aggregation Pheromone of a Strawberry Sap Beetle, Lobiopa insularis (Coleoptera: Nitidulidae). J Chem Ecol 43, 550–556 (2017). https://doi.org/10.1007/s10886-017-0851-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0851-y