Abstract
Research over the last decade has revealed extensive parsimony among pheromones within the large insect family Cerambycidae, with males of many species producing the same, or very similar aggregation pheromones. Among some species in the subfamily Cerambycinae, interspecific attraction is minimized by temporal segregation, and/or by minor pheromone components that synergize attraction of conspecifics or inhibit attraction of heterospecifics. Less is known about pheromone-based mechanisms of reproductive isolation among species in the largest subfamily, the Lamiinae. Here, we present evidence that the pheromone systems of two sympatric lamiine species consist of synergistic blends of enantiomers of (E)-6,10-dimethyl-5,9-undecadien-2-ol (fuscumol) and the structurally related (E)-6,10-dimethyl-5,9-undecadien-2-yl acetate (fuscumol acetate), as a mechanism by which species-specific blends of pheromone components can minimize interspecific attraction. Male Astylidius parvus (LeConte) were found to produce (R)- and (S)-fuscumol + (R)-fuscumol acetate + geranylacetone, whereas males of Lepturges angulatus (LeConte) produced (R)- and (S)-fuscumol acetate + geranylacetone. Field experiments confirmed that adult beetles were attracted only by their species-specific blend of the enantiomers of fuscumol and fuscumol acetate, respectively, and not to the individual enantiomers. Because other lamiine species are known to produce single enantiomers or blends of enantiomers of fuscumol and/or fuscumol acetate, synergism between enantiomers, or inhibition by enantiomers, may be a widespread mechanism for forming species-specific pheromone blends in this subfamily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent research on the chemical ecology of cerambycid beetles has revealed that the pheromone chemistry of related species often is highly conserved, with many sympatric species sharing pheromone components, or even having identical attractant pheromones (reviewed by Millar and Hanks 2017). Within the subfamilies Cerambycinae, Lamiinae, and Spondylidinae, volatile aggregation pheromones are produced by males, and attract both sexes. Among species in the large subfamily Cerambycinae, even species on different continents often share pheromone components such as 3-hydroxyalkan-2-ones and the related 2,3-alkanediols, despite the species having diverged many thousands of years ago. Thus, traps baited with single pheromone components may attract multiple species of cerambycines in different parts of the world (e.g., Hayes et al. 2016; Sweeney et al. 2014; Wickham et al. 2014). The results from such screening trials have provided valuable leads for the subsequent full identification of pheromones for various target species (e.g., Hanks et al. 2007; Mitchell et al. 2013, Mitchell et al. 2015; Narai et al. 2015; Zou et al. 2016).
Interspecific attraction to shared pheromone components may be averted, or at least minimized among sympatric cerambycines by seasonal segregation: adults of each species typically are active for brief and discrete periods of the season (3–6 wk), and species emerge in orderly progression from early spring through late fall (e.g., see Handley et al. 2015; Hanks and Millar 2013; Hanks et al. 2014). Furthermore, species that overlap seasonally may be segregated by diel phenology, because daily activity periods usually are limited to a few hours (Hanks and Wang 2017). Finally, those species which are fully synchronous, overlapping in both seasonal and diel phenology, may be segregated by the synergistic or antagonistic effects of the minor components of their pheromones (Mitchell et al. 2013; 2015).
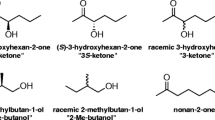
Less is known about the pheromone chemistry of cerambycid species in the largest subfamily, the Lamiinae (reviewed by Millar and Hanks 2017; for taxonomy, see Švácha and Lawrence 2014). Several species in the lamiine genus Monochamus are known to use 2-(undecyloxy) ethanol (termed monochamol) as an aggregation pheromone, and a structural analog, 4-(heptyloxy)butan-1-ol, is reported to be a pheromone component of the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky) (Zhang et al. 2002), and its congener, A. chinensis (Forster) (Hansen et al. 2015). In contrast, males of the South American lamiine Hedypathes betulinus (Klug) produce a blend of structurally related terpenoid derivatives, including (R)-(E)-6,10-dimethyl-5,9-undecadien-2-ol (fuscumol), (R)- and (S)-(E)-6,10-dimethyl-5,9-undecadien-2-yl acetate (fuscumol acetate), and (E)-6,10-dimethyl-5,9-undecadien-2-one (geranylacetone, Fig. 1; Fonseca et al. 2010; Vidal et al. 2010). Fuscumol also is produced by males of the South American species Steirastoma breve (Sulzer) (Liendo-Barandiaran et al. 2010).
Based on evidence that fuscumol and related compounds were pheromone components of lamiines, and the conserved nature of cerambycid pheromones in general, Mitchell et al. (2011) conducted a screening trial of racemic fuscumol and/or fuscumol acetate in three disparate areas of North America (east-central Illinois, northwestern Indiana, central Texas), attracting adults of several lamiine species. However, to date, a pheromone blend has been formally identified for only one of these species, Astyleiopus variegatus (Haldeman), consisting of (S)-fuscumol + (S)-fuscumol acetate (Hughes et al. 2013).
It appears unlikely that interspecific attraction among the 12 lamiine species caught by Mitchell et al. (2011) would be averted by seasonal segregation because they all overlap broadly in seasonal phenology (during late June to early August; Handley et al. 2015; Hanks and Millar 2013; Hanks et al. 2014). Moreover, many of these species overlap in diel phenology as well, being crepuscular to nocturnal (unpub. data), a common trait among lamiines (Linsley 1959; Švácha and Lawrence 2014). Thus, it seemed likely that pheromone chemistry plays a critical role in averting interspecific attraction among these species, as suggested by differences among them as to whether the adults are attracted by racemic fuscumol or fuscumol acetate, or only by the blend of the two (Mitchell et al. 2011).
Here, we describe further screening trials of fuscumol and fuscumol acetate in east-central Illinois that were intended to target lamiine species for pheromone identification. Traps were baited with both racemic and chiral chemicals to provide more detailed insight into the nuances of their pheromone chemistry. The two species captured in greatest numbers were Astylidius parvus (LeConte) and Lepturges angulatus (LeConte). Mitchell et al. (2011) had already reported that adult A. parvus were attracted to racemic fuscumol, and adult L. angulatus to racemic fuscumol acetate, suggesting that the species differed in their pheromone chemistry. Larvae of both species are polyphagous, feeding within woody tissues of hardwood trees, shrubs, and vines of many families (Linsley and Chemsak 1995). Adults of both species are active from late June to early August (Hanks and Millar 2013; Hanks et al. 2014). Overlap in diel phenology was confirmed during the present study using capture data from traps that had been fitted with a mechanism that changed collecting jars at programmable time intervals (see Methods and Materials).
We tested the hypothesis that cross attraction between A. parvus and L. angulatus is averted by differences in the chemistry of their respective pheromones. Thus, we identified the compounds produced by males, and confirmed that adults of each species were attracted only to their species-specific blends. Remarkably, both species were found to exhibit the rare phenomenon of synergism between the enantiomers of their pheromone components (Mori 2007), with beetles being attracted only to the appropriate blends of the enantiomers, and not to the individual enantiomers.
Methods and Materials
Sources of Chemicals
(Fig. 1) Racemic (E)-fuscumol, (E)-fuscumol acetate, and (E)-geranylacetone were purchased from Bedoukian Research (Danbury, CT, USA), and (E/Z)-geranylacetone from Sigma-Aldrich (St. Louis, MO, USA). (R)-Fuscumol (96.6 % enantiomeric excess, ee), (S)-fuscumol (98 % ee), (R)-fuscumol acetate (96.6 % ee), and (S)-fuscumol acetate (98 % ee) were synthesized by enzymatic kinetic resolution of the racemic compounds as described in Hughes et al. (2013).
Study Sites
Field work to identify pheromones of cerambycid species and to characterize diel phenology and attraction to synthesized pheromones, was conducted at five study sites in east-central Illinois (Table 1), all of which were wooded with mature second-growth or successional hardwoods and dominated by oaks (Quercus species), hickories (Carya species), maples (Acer species), and ash (Fraxinus species).
Diel Phenology of Adults
Diel flight phenology of adult A. parvus and L. angulatus was characterized by capturing beetles with cross-vane panel traps (black corrugated plastic, Alpha Scents, Portland, OR, USA) coated with the fluoropolymer dispersion Fluon® (10 % aqueous dilution; Northern Products, Woonsocket, RI, USA) to improve capture efficiency (for details on trapping methods, see Graham et al. 2010). Traps were hung from inverted L-shaped frames of polyvinylchloride pipe with trap bottoms ~0.5 m above the ground. Trap lures consisted of polyethylene sachets (5.1 × 7.6 cm, Bagettes® model 14770, Cousin Corp., Largo, FL, USA) that were loaded with 50 mg of synthesized pheromone dissolved in 1 ml isopropanol (a neutral solvent for cerambycids; Hanks et al. 2012), with separate lures for racemic fuscumol and fuscumol acetate. Traps also were baited with ethanol, because it synergizes attraction to pheromones for some lamiine species (Hanks et al. 2012). The ethanol lures consisted of polyethylene sachets (10 × 15 cm, 0.05 mm thick, Bagettes® model 14,772, Cousin Corp., Largo, FL, USA) loaded with 100 ml of ethanol. The supplied collection basins of traps were replaced with a mechanism that changed eight trap jars at programmable intervals (henceforth “timer traps”; model #2850, BioQuip Products, Rancho Dominguez, CA, USA). Individual traps were deployed at the Allerton Park and Brownfield Woods study sites (Table 1) during 16 June to 5 September 2013, except during periods of inclement weather. Timer traps were programmed to rotate jars at seven 1-h intervals, beginning at 19:00 h and ending at 2:00 h, so as to encompass the expected crepuscular to nocturnal activity periods of lamiines. The eighth jar was not rotated for 17 h (2:00–19:00), so as to confirm that beetles were not active during the remainder of each 24 h cycle. The hour that beetles were caught was estimated as the median time that their trap jar was positioned under the trap. Overlap between the two species in diel flight period was assessed by calculating, for each species, the percentage of adults which were captured during the flight period of the other species.
Identification of Pheromones
Beetles for collection of headspace odors were captured alive with panel traps as described above, but with trap basins replaced with 2-L plastic jars with their bottoms replaced with aluminum screen to allow rainwater to drain. Trap lures consisted of polyethylene sachets as described above, but loaded with the blend of racemic fuscumol and fuscumol acetate (50 mg each) dissolved in 1 ml isopropanol. Single traps were deployed during May to August 2013 and 2014 at all five study sites (Table 1). Traps were serviced and beetles were collected every 1 or 2 d. Trap lures were replaced as needed, usually after 10–14 d.
Captured beetles were sexed by the morphology of the fifth abdominal sternite (Linsley and Chemsak 1995), or by pairing beetles in the laboratory and observing their behavior (i.e., males will attempt to mate by mounting females). Beetles were caged, separately by species and sex, under ambient laboratory conditions (~12:12 h L:D, ~20 °C). Adults of both study species feed on bark of oak branchlets (pers. obs.), and so caged beetles were provided sections of branches (2–5 cm diam, ~8 cm long) freshly cut from oak trees (Quercus alba L. and Q. rubra L.) at the same field sites where beetles were trapped. Beetles also were provided sugar water as a source of moisture (10 % aqueous sucrose solution in a glass vial with cotton wick). Beetles were allowed to acclimatize for at least 24 h before being aerated, and between aerations.
Volatiles produced by beetles were collected by aerating them in glass Mason-style canning jars placed adjacent to closed exterior windows (natural photoperiod, ~14:10 h L:D, ~20 °C). Clean air (1 L/min) was pulled through the jars by vacuum for 24 h. Headspace volatiles were collected with glass tube cartridges that contained a layer of the adsorbent polymer HayeSep® Q (150 mg; Sigma-Aldrich) between plugs of glass wool, attached to the chamber outlet. Beetles usually were aerated individually, but in some cases two or three beetles of the same sex were aerated together in case the presence of conspecifics enhanced pheromone release. Once it was confirmed that females did not produce volatile chemicals in detectable quantities (see Results), the sexes were sometimes aerated together, in case the presence of females stimulated males to call. Beetles usually were aerated with fresh twigs of oak (provided as food), and aerations of jars without beetles (including host material when appropriate) were run simultaneously as controls for system contaminants. Additional controls included jars containing twigs that had been damaged mechanically, by scraping or gouging the bark, to simulate feeding damage by beetles that may result in release of plant volatiles. Numbers of males and females that were aerated were 33 and 20 for A. parvus, and 16 and 8 for L. angulatus.
Insect-produced chemicals were recovered from adsorbent cartridges by extraction with 1.5 ml of dichloromethane. Extracts were analyzed with a gas chromatograph (GC) interfaced to a mass selective detector (Models 6890 and 5973, Hewlett-Packard, Palo Alto, CA, USA) fitted with a AT-5 ms column (30 m × 0.25 mm i.d., 0.25 μm film; Alltech Associates Inc., Deerfield, IL, USA). The GC oven was programmed from 35 °C/1 min, 10 °C/min to 210 °C, hold 3 min. Injections were made in splitless mode, with an injector temperature of 250 °C, and helium carrier gas. Sex-specific compounds were identified by comparing spectra and retention times to those of authentic standards.
The enantiomeric ratio and absolute configuration of insect-produced fuscumol acetate and the double bond configuration of geranylacetone were determined by analyzing aliquots of extracts with an HP 5890 GC fitted with a chiral stationary phase Cyclodex B column (30 m × 0.25 mm i.d., 0.25 μm film; Agilent Technologies, Inc., Santa Clara, CA, USA). The oven temperature was programmed from 50 °C/1 min, 2.5 °C/min to 200 °C, hold 5 min, with an injector temperature of 210 °C. Structures were confirmed by coinjection of an aliquot of aeration extract with the mixture of synthetic stereoisomers (Millar et al. 2009); (R)- and (S)-fuscumol acetate (retention times 51.33 and 51.72 min, respectively) and (Z)- and (E)-geranylacetone (retention times 14.53 and 14.74 min, respectively) were resolved to baseline.
Because the enantiomers of fuscumol did not resolve on the chiral column, fuscumol in extracts was esterified with (S)-O-acetyl lactic acid chloride (Slessor et al. 1985), with the method modified as described by Hughes et al. (2013). The resulting diastereomeric derivatives were separated to baseline on an achiral DB-5 GC column (30 m × 0.25 mm i.d., 0.25 μ film, J&W Scientific, Folsom, CA, USA). The oven temperature was programmed from 40 °C/1 min, 10 °C/min to 250 °C, hold 5 min, with an injector temperature of 250 °C, and helium carrier gas. The retention times of the diastereomeric esters of (R)- and (S)-fuscumol were 18.94 min and 19.03 min, respectively. Samples of racemic and (R)-fuscumol were esterified under the same conditions to distinguish between the diastereomers, and to verify that the derivatizing reagent was enantiomerically pure. Overall, chirality determinations were made with four samples from male A. parvus and five samples from L. angulatus.
Field Bioassays of Pheromones
Attraction of beetles to synthesized pheromones was tested with four independent field bioassays because the large number of possible combinations of compounds would have resulted in excessively long trap lines if they had all been tested at once. Beetles were caught with panel traps as described above, but with the trap basins filled with ~300 ml of saturated aqueous NaCl solution to kill and preserve captured beetles. Traps were positioned 10 m apart in linear transects, with one treatment per transect randomly assigned to one trap on the first day. Traps were serviced every 1–3 d, at which time treatments were rotated one position along transects to control for positional effects.
All four experiments shared two treatments for comparative purposes (Table 2): racemic fuscumol and, separately, racemic fuscumol acetate (which simulated the pheromone of L. angulatus, see Results). Experiment 1 was designed as a follow-up to the screening trial of Mitchell et al. (2011), but with treatments added to test the influence of geranylacetone (see Results), and to test for attraction to geranylacetone alone (Table 2). In this experiment, synthesized pheromones were dispensed from sachets made from heat-sealed, lay-flat polyethylene tubing (Associated Bag, Milwaukee, WI, USA), which were loaded with 1 ml of neat chemical. Lure release rates were standardized to ~15 mg/d by using sachets with different wall thicknesses and adding a cotton roll (1 × 4 cm dental wick, Patterson Dental Supply, Inc., St. Paul, MN, USA) to some lures, as follows: fuscumol (1.5 mil [38 μm] wall thickness, with cotton roll); fuscumol acetate (3 mil [76 μm] wall thickness, with cotton roll), geranylacetone (3 mil wall thickness, without cotton roll). An empty 3 mil wall thickness sachet served as a blank control. The experiment was conducted during three years: 5 August to 5 September 2013 at Vermilion River Observatory (Table 1), 22 June to 6 August 2014 at Allerton Park and Brownfield Woods (one transect at each site), and 15 July to 16 September 2015 at Forest Glen Preserve. Lures were replaced every 14 d.
For the remaining three experiments, trap lures consisted of the Bagettes® model 14770 polyethylene sachets loaded with dilute synthetic pheromones. The experiments were conducted at Brownfield Woods and Nettie Hart Woods (Table 1). Experiment 2 (25 July to 3 September 2014 and 15 June to 17 September 2015) tested attraction to various blends of racemic and chiral fuscumol and fuscumol acetate, including simulations of the pheromone blends of both species (Table 2; see Results). Experiment 3 (15 June to 17 September 2015) was similar to the second experiment, but included different combinations of chiral compounds (Table 2). Experiment 4 (15 June to 17 September 2015) tested a third combination of treatments so as to assess attraction to individual chiral compounds vs. individual racemic blends (Table 2).
For each experiment, differences between treatment means, blocked by site and date, were tested separately for each species using the nonparametric Friedman’s Test (PROC FREQ, option CMH; SAS Institute 2011) because data violated homoscedasticity assumptions of ANOVA (Sokal and Rohlf 1995). Thus, replicates were defined by study site and collection date. Assuming a significant overall Friedman’s test, pairs of treatment means were compared with the nonparametric Dunn-Nemenyi multiple comparison test (Elliot and Hynan 2011; Zar 2010). Replicates that contained no specimens of the species in question were dropped from analyses.
Taxonomy of captured beetles follows Monné and Hovore (2005). Specimens of species that were represented in the data set are available from the laboratory collection of LMH, and voucher specimens have been deposited with the collection of the Illinois Natural History Survey, Champaign, IL.
Results
Diel Phenology of Adults
Timer traps captured 26 adult A. parvus and 45 adult L. angulatus, with most adults of both species caught between 29 June and 28 August 2013. Both species showed a strongly skewed frequency distribution (Fig. 2), with most beetles caught within the first hour, which was between 1950 and 2050 h for A. parvus, and 2050 and 2150 h for L. angulatus. Thus, adults became active with the onset of complete darkness (solar radiation fell to zero between 1800 and 2000 h during the trapping period; Water and Atmospheric Resources Monitoring Program, Illinois Climate Network 2014, Illinois State Water Survey, Champaign, IL: http://dx.doi.org/10.13012/J8MW2F2Q). The two species overlapped broadly in diel phenology, with ~61 % of A. parvus caught during the activity period of L. angulatus, and 100 % of L. angulatus caught during the activity period of A. parvus.
Identification of Pheromones
Extracts of volatiles emitted by males of both species contained fuscumol and/or fuscumol acetate, and (E)-geranylacetone, with detectable quantities in 13 of 33 extracts from male A. parvus and 11 of 16 extracts from male L. angulatus. These compounds were not detected in any aeration extracts from females, nor in any system controls. Male A. parvus produced (R)- + (S)-fuscumol, (R)-fuscumol acetate, and (E)-geranylacetone in a ratio of ~3:1:1 (R:S-fuscumol ranging from 1:10 to 10:1; N = 4), and male L. angulatus produced (R)- + (S)-fuscumol acetate and (E)-geranylacetone in a ratio of ~3:1 (R:S-fuscumol acetate ranging from 2:1 to 8:3; N = 5).
Field Bioassays of Pheromones
Exactly 1800 cerambycid beetles of 46 species were trapped during the four field experiments (Table 3), including representatives of the subfamilies Cerambycinae, Lamiinae, Lepturinae, Parandrinae, Prioninae, and one species in the closely related family Disteniidae. Most of the trapped beetles (~91 %) were lamiines, with the species caught in largest numbers being the two targeted species, A. parvus and L. angulatus, followed by three species in the same tribe (Acanthocinini), Graphisurus fasciatus (Degeer), its congener G. despectus (LeConte), and Astylopsis macula (Say), as well as A. variegatus in the tribe Acanthoderini (Table 3). Both sexes of A. parvus and L. angulatus were caught, for example with sex ratios of 43 and 50 % female in Experiment 1.
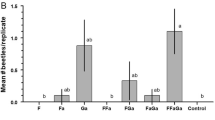
In all four experiments, adults of both A. parvus and L. angulatus were significantly influenced by the experimental treatments, and generally in accordance with their pheromone chemistry. In Experiment 1, the greatest number of adult A. parvus were captured by traps baited with racemic fuscumol, with or without fuscumol acetate and/or geranylacetone (Fig. 3a; treatments F, F + GA, F + FA, and F + GA + FA), consistent with males producing both enantiomers of fuscumol. These findings suggest that both enantiomers of fuscumol are necessary and sufficient for attraction, that the (R)-fuscumol acetate component is not essential, and that geranylacetone does not influence attraction and is not attractive alone. This experiment also indicated that (S)-fuscumol acetate, which is not produced by males of A. parvus, was not antagonistic. The remaining experiments supported these conclusions (Figs. 4a, 5a, 6a), confirming that both enantiomers of fuscumol are necessary, and that neither enantiomer of fuscumol acetate influences attraction.
Mean (± SE) number of adult Astylidius parvus (b) and Lepturges angulatus (b) that were caught per replicate during Experiment 1. Chemical abbreviations: F = racemic fuscumol, FA = racemic fuscumol acetate, GA = geranylacetone. Means significantly different: Friedman’s Q 7,297 = 118.2, P < 0.001, and Q 7,249 = 106.9, P < 0.001, respectively. Means with different letters are significantly different (Dunn-Nemenyi multiple comparison test, P < 0.05)
Mean (± SE) number of adult Astylidius parvus (a) and Lepturges angulatus (b) that were caught per replicate during Experiment 2. Chemical abbreviations: F = racemic fuscumol, FA = racemic fuscumol acetate, with enantiomers indicated by R and S. Means significantly different: Friedman’s Q 7,96 = 29.7, P < 0.001, and Q 7,120 = 49.6, P < 0.001, respectively. Means with different letters are significantly different (Dunn-Nemenyi multiple comparison test, P < 0.05)
Mean (± SE) number of adult Astylidius parvus (a) and Lepturges angulatus (b) that were caught per replicate during Experiment 3. Chemical abbreviations: F = racemic fuscumol, FA = racemic fuscumol acetate, with enantiomers indicated by R and S. Means significantly different: Friedman’s Q 7,240 = 56.0, P < 0.001, and Q 7,264 = 68.6, P < 0.001, respectively. Means with different letters are significantly different (Dunn-Nemenyi multiple comparison test, P < 0.05)
Mean (± SE) number of adult Astylidius parvus (a) and Lepturges angulatus (b) that were caught per replicate during Experiment 4. Chemical abbreviations: F = racemic fuscumol, FA = racemic fuscumol acetate, with enantiomers indicated by R and S. Means significantly different: Friedman’s Q 6,91 = 52.1, P < 0.001, and Q 6,35 = 33.9, P < 0.001, respectively. Means with different letters are significantly different (Dunn-Nemenyi multiple comparison test, P < 0.05)
In contrast, Experiment 1 revealed that adult L. angulatus were significantly attracted to racemic fuscumol acetate alone (Fig. 3b), consistent with males producing both enantiomers, but attraction was significantly enhanced by geranylacetone. Fuscumol apparently antagonized attraction to both fuscumol acetate (in treatment F + FA, Fig. 3b), and the blend of fuscumol acetate and geranylacetone (in treatment F + FA + GA). There was some support for this antagonism in Experiment 2 (Fig. 4b), with the mean for the blend of racemic fuscumol and fuscumol acetate being not significantly different from the mean for the control, although the individual enantiomers of fuscumol apparently did not influence attraction. Nevertheless, there was no evidence that racemic fuscumol antagonized attraction of L. angulatus to fuscumol acetate in Experiment 3 (Fig. 5b). Finally, Experiment 4 reconfirmed that both enantiomers of fuscumol acetate were necessary for attraction of L. angulatus (Fig. 6b).
Species in subfamilies other than Lamiinae generally were represented by few specimens that probably were captured by random encounters with traps. The fact that 41 adults of the cerambycine Xylotrechus colonus (F.) were caught might suggest significant attraction, but that species is among the most common and abundant cerambycids in eastern North America, and even low levels of attraction would have resulted in capture of much greater numbers of beetles (e.g., see Hanks et al. 2014). Other non-lamiine species that were fairly numerous are among those that typically are captured by panel traps regardless of how they are baited, such as the cerambycine Elaphidion mucronatum (Say), the parandrine Neandra brunnea (F.), and the prionine Orthosoma brunneum (Forster) (e.g., see Hanks and Millar 2013).
Discussion
Astylidius parvus and L. angulatus overlap broadly in the phenology of their adults, as revealed in earlier studies of their seasonal phenology (Hanks et al. 2014), and in the study of diel phenology reported here. Identification of the possible pheromone components produced by the males and field bioassays of racemic and chiral pheromone components supported the hypothesis that interspecies attraction is minimized by differences in the chemistry of their pheromones. Thus, adult A. parvus required both enantiomers of fuscumol for attraction, neither of which are produced by L. angulatus, and adult L. angulatus required both enantiomers of fuscumol acetate for attraction, whereas male A. parvus produce only the (R)-enantiomer. Therefore, the mechanisms that prevent interspecific attraction are complementary, effectively preventing attraction in either direction, as has been reported among some sympatric cerambycine species (Mitchell et al. 2015).
Although the two enantiomers of fuscumol were essential for attraction of adult A. parvus, neither the (R)-fuscumol acetate nor the geranylacetone found in the male-produced volatiles appeared to play a role in attraction of conspecifics. However, geranylacetone did enhance attraction of L. angulatus to fuscumol acetate, indicating that for this species, it is indeed a pheromone component. Geranylacetone also is a component of the volatile blend produced by males of the South American H. betulinus, possibly being sequestered directly from host plants of the adults (Fonseca et al. 2010) as a biosynthetic precursor of fuscumol and fuscumol acetate (Zarbin et al. 2013).
Synergism between enantiomers of pheromone components, such as that shown by A. parvus and L. angulatus, is uncommon in insects (Mori 2007), but most of the known examples are from the Coleoptera. In the first example reported, females of the ambrosia beetle Gnathotrichus sulcatus LeConte (Curculionidae: Scolytinae) produced a 35:65 blend of (R)- and (S)-sulcatol, and field bioassays showed that beetles were attracted only to blends of the enantiomers, and not to either pure enantiomer (Borden et al. 1976; Bordon et al. 1980; Byrne et al. 1974). Bordon et al. (1980) also found that the sympatric congener Gnathotrichus retusus (LeConte) produced and responded only to (S)-sulcatol, demonstrating how differences in the enantiomeric composition created species-specific pheromone channels. Even more intriguing, Miller et al. (1996) found that in the pine engraver, Ips pini (Say) (Scolytinae), the enantiomeric ratio of ipsdienol varied among geographic populations, possibly resulting from character displacement where I. pini was sympatric with other species that also used ipsdienol as a pheromone component. In another beetle family (Laemophloeidae), the stored products pest Cryptolestes turcicus (Grouvelle) uses non-racemic mixtures of the enantiomers of macrocyclic lactones in its pheromone blend (Millar et al. 1985; Oehlschlager et al. 1987).
These precedents for enantiomeric synergism raise the possibility that the manipulation of enantiomeric ratios to create unique aggregation pheromone channels may be common among sympatric cerambycid species that produce fuscumol and fuscumol acetate as pheromone components. For example, in addition to the examples described in this paper, males of H. betulinus produce an 82:18 blend of (R)- and (S)-fuscumol, but pure (R)-fuscumol acetate (Vidal et al. 2010). However, field bioassays of the enantiomers, alone or in blends, have not yet been reported for H. betulinus. In contrast, males of the North American A. variegatus produce pure (S)-fuscumol and (S)-fuscumol acetate (Hughes et al. 2013, 2016), although bioassays comparing the racemic and enantiomeric forms of the two compounds have not yet been reported. Moreover, racemic fuscumol and fuscumol acetate, individually or in combination, have been shown to attract a number of other sympatric lamiine species, plus a species in the Cerambycinae, Obrium maculatum (Olivier) (e.g., Hanks and Millar 2013; Hanks et al. 2012; Mitchell et al. 2011). As the pheromones of these lamiine species are identified, other examples of enantiomeric synergism may be found, as well as examples of inhibition by enantiomers.
Similarly, among European species in the subfamily Spondylidinae, males of Tetropium fuscum (F.) produce (S)-fuscumol (Silk et al. 2007), and adults of the congener T. castaneum (L.) are attracted by the same compound when released along with host plant volatiles (Sweeney et al. 2010). These two species overlap in their geographical distribution in Europe (Bense 1995), suggesting that mechanisms other than pheromone chemistry serve to limit interspecific attraction.
Previous research already had shown that adult A. parvus were attracted by fuscumol and not fuscumol acetate, and vice versa for L. angulatus (Mitchell et al. 2011), suggesting that the composition of their pheromones could have been predicted beforehand. However, trap catches in field bioassays of blends of synthetic pheromones may sometimes be misleading, because even weak attraction to incorrect ratios, or blends that lack synergistic components, may nevertheless result in statistical significance relative to unbaited controls. For example, Mitchell et al. (2011) found that adult A. variegatus were significantly attracted to racemic fuscumol acetate in comparison to controls, despite the absence of the (S)-fuscumol component of its pheromone. However, those authors subsequently discovered that attraction to racemic fuscumol fell to insignificant levels in the presence of lures containing a blend of racemic fuscumol + fuscumol acetate (Mitchell et al. 2011). It still remains to be seen what effect the “non-natural” (R)-enantiomers of fuscumol and fuscumol acetate have on attraction of A. variegatus, and to what degree attraction is influenced by the ratios of the various components.
Identification of the pheromones of A. parvus, L. angulatus, and previously of A. variegatus (Hughes et al. 2013, 2016), and earlier reports that adults of other species in the tribes Acanthocinini and Acanthoderini are attracted by fuscumol and/or fuscumol acetate (Mitchell et al. 2011), indicate that these compounds comprise another conserved pheromone motif within the Cerambycidae. However, there are nuances at play, because the fuscumol/fuscumol acetate pheromone channel appears to be quite finely partitioned among these species. If indeed species specificity in pheromone composition could be afforded only by the chirality of fuscumol and fuscumol acetate, and assuming that pheromones could be composed of one to four components (i.e., a single enantiomer of either fuscumol and fuscumol acetate vs. both enantiomers of each), there are 14 unique combinations available as species-specific pheromone blends, even without considering differences in blend ratios. Moreover, the role of geranylacetone in enhancing attraction of L. angulatus to the other pheromone components suggests that it may further extend the number of unique pheromone blends that are available. Thus, different subsets and ratios of the five compounds can accommodate a substantial number of blends, more than enough to minimize cross attraction within individual communities of sympatric and synchronic lamiine species that use fuscumol, fuscumol acetate, and/or geranylacetone as pheromone components.
References
Bense U (1995) Longhorn beetles: illustrated key to the Cerambycidae and Vesperidae of Europe. Margraf, Weikersheim, Germany
Borden JH, Chong L, McLean JA, Slessor KN, Mori K (1976) Gnathotrichus sulcatus: synergistic response to enantiomers of the aggregation pheromone sulcatol. Science 192:894–896
Bordon JH, Handley JR, McLean JA, Silverstein RM, Chong L, Slessor KN, Johnston BD, Schuler HR (1980) Enantiomer-based specificity in pheromone communication by two sympatric Gnathotrichus species (Coleoptera: Scolytidae). J Chem Ecol 6:445–456
Byrne KJ, Swigar AA, Silverstein RM, Borden JH, Stokkink E (1974) Sulcatol: population aggregation pheromone in the scolytid beetle, Gnathotrichus sulcatus. J Insect Physiol 20:1895–1900
Elliot AC, Hynan LS (2011) A SAS® macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Comput Methods Prog Biomed 102:75–80
Fonseca MG, Vidal DM, Zarbin PHG (2010) Male-produced sex pheromone of the cerambycid beetle Hedypathes betulinus: chemical identification and biological activity. J Chem Ecol 36:1132–1139
Graham EE, Mitchell RF, Reagel PF, Barbour JD, Millar JG, Hanks LM (2010) Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J Econ Entomol 103:641–647
Handley K, Hough-Goldstein J, Hanks LM, Millar JG, D'Amico V (2015) Diversity and phenology of cerambycid beetles in urban forest fragments of northern Delaware. Ann Entomol Soc Am 108:251–262
Hanks LM, Millar JG (2013) Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 23:21–44
Hanks LM, Wang Q (2017) Reproductive biology of cerambycid beetles. In: Wang Q (ed) Cerambycidae of the world: biology and management. CRC Press/Taylor & Francis, Boca Raton (in press)
Hanks LM, Millar JG, Moreira JA, Barbour JD, Lacey ES, McElfresh JS, Reuter FR, Ray AM (2007) Using generic pheromone lures to expedite identification of aggregation pheromones for the cerambycid beetles Xylotrechus nauticus, Phymatodes lecontei, and Neoclytus modestus modestus. J Chem Ecol 33:889–907
Hanks LM, Millar JG, Mongold-Diers JA, Wong JCH, Meier LR, Reagel PF, Mitchell RF (2012) Using blends of cerambycid beetle pheromones and host plant volatiles to simultaneously attract a diversity of cerambycid species. Can J For Res 42:1050–1059
Hanks LM, Reagel PF, Mitchell RF, Wong JCH, Meier LR, Silliman CA, Graham EE, Striman BL, Robinson KP, Mongold-Diers JA, Millar JG (2014) Seasonal phenology of the cerambycid beetles of east-Central Illinois. Ann Entomol Soc Am 107:211–226
Hansen L, Tian X, Wickham J, Hanks LM, Millar JG, Teale SA (2015) Identification of a male-produced pheromone component of the citrus longhorned beetle, Anoplophora chinensis. PLoS One 10:e0134358
Hayes R, Griffiths MW, Nahrung HF, Arnold PA, Hanks LM, Millar JG (2016) Optimizing generic cerambycid pheromone lures for Australian biosecurity and biodiversity monitoring. J Econ Entomol. doi:10.1093/jee/tow100
Hughes GP, Zou Y, Millar JG, Ginzel MD (2013) (S)-Fuscumol and (S)-fuscumol acetate produced by a male Astyleiopus variegatus (Coleoptera: Cerambycidae). Can Entomol 145:1–6
Hughes GP, Meier LR, Zou Y, Millar JG, Hanks LM, Ginzel MD (2016) Stereochemistry of fuscumol and fuscumol acetate influences attraction of longhorned beetles of the subfamily Lamiinae. Environ Entomol 45:1271–1275
Liendo-Barandiaran CV, Herrara B, Morillo F, Sánchez P, Hernández JV (2010) Identification of male sexual pheromone in Steirastoma breve (Coleoptera: Cerambycidae). Abstract, First Latin-American Meeting of Chemical Ecology, Colonia del Sacramento, Uruguay, 17–20 Oct 2010, p O-7
Linsley EG (1959) The ecology of the Cerambycidae. Annu Rev Entomol 4:99–138
Linsley EG, Chemsak JA (1995) The Cerambycidae of North America, part VII, no. 2: taxonomy and classification of the subfamily Lamiinae, tribes Acanthocinini through Hemilophini. Univ Calif Publ Entomol 114:1–292
Millar JG, Hanks LM (2017) Chemical ecology of cerambycid beetles. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC Press/Taylor & Francis, Boca Raton (in press)
Millar JG, Pierce HD Jr, Pierce AM, Oehlschlager AC, Borden JH (1985) Aggregation pheromones of the grain beetle, Cryptolestes turcicus (Coleoptera: Cucujidae). J Chem Ecol 11:1071–1081
Millar JG, Hanks LM, Moreira JA, Barbour JD, Lacey ES (2009) Pheromone chemistry of cerambycid beetles. In: Nakamuta K, Millar JG (eds) Chemical ecology of wood-boring insects. Forestry and Forest Products Research Institute, Ibaraki, Japan, pp. 52–79
Miller DR, Borden JH, Slessor KN (1996) Enantiospecific pheromone production and response profiles for populations of pine engraver, Ips pini (say) (Coleoptera: Scolytidae), in British Columbia. J Chem Ecol 22:2157–2172
Mitchell RF, Graham EE, Wong JCH, Reagel PF, Striman BL, Hughes GP, Paschen MA, Ginzel MD, Millar JG, Hanks LM (2011) Fuscumol and fuscumol acetate are general attractants for many species of cerambycid beetles in the subfamily Lamiinae. Entomol Exp Appl 141:71–77
Mitchell RF, Millar JG, Hanks LM (2013) Blends of (R)-3-hydroxyhexan-2-one and alkan-2-ones identified as potential pheromones produced by three species of cerambycid beetles. Chemoecology 23:121–127
Mitchell RF, Reagel PF, Wong JCH, Meier LR, Silva WD, Mongold-Diers J, Millar JG, Hanks LM (2015) Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J Chem Ecol 41:431–440
Monné MA, Hovore FT (2005) Checklist of the Cerambycidae (Coleoptera) of the western hemisphere. BioQuip, Rancho Dominguez
Mori K (2007) Significance of chirality in pheromone science. Bioorg Med Chem 15:7505–7523
Narai Y, Zou Y, Nakamuta K, Mongold-Diers JA, Hanks LM, Millar JG (2015) Candidate aggregation pheromones of two potentially invasive Asian cerambycid species in the genus Xylotrechus. J Econ Entomol 108:1444–1446
Oehlschlager AC, King GGS, Pierce HD Jr, Pierce AM, Slessor KN, Millar JG, Borden JH (1987) Chirality of macrolide pheromones of grain beetles in the genera Oryzaephilus and Cryptolestes and its implications for species specificity. J Chem Ecol 13:1543–1554
SAS Institute (2011) SAS/STAT 9.3 user's guide. SAS Institute Inc., Cary NC, USA
Silk PJ, Sweeney JD, Wu J, Price J, Gutowski JM, Kettela EG (2007) Evidence for a male-produced pheromone in Tetropium fuscum (F.) and Tetropium cinnamopterum (Kirby) (Coleoptera: Cerambycidae). Naturwissenschaften 94:697–701
Slessor KN, King GGS, Miller DR, Winston ML, Cutforth TL (1985) Determination of chirality of alcohol or latent alcohol semiochemicals in individual insects. J Chem Ecol 11:1659–1667
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. WH Freeman and Co, New York
Švácha P, Lawrence JF (2014) Chapter 2.4 Cerambycidae Latreille, 1802, pp. 77–177. In: Leschen RAB, Beutel RG (eds) Handbook of zoology: Arthropoda: Insecta: Coleoptera, beetles. Vol. 3: Morphology and systematics (Phytophaga), Walter de Gruyter, Berlin/Boston
Sweeney JD, Silk PJ, Gutowski JM, Wu J, Lemay MA, Mayo PD, Magee DI (2010) Effect of chirality, release rate, and host volatiles on response of Tetropium fuscum (F.), Tetropium cinnamopterum Kirby, and Tetropium castaneum (L.) to the aggregation pheromone, fuscumol. J Chem Ecol 36:1309–1321
Sweeney JD, Silk PJ, Grebennikov V (2014) Efficacy of semiochemical-baited traps for detection of longhorn beetles (Coleoptera: Cerambycidae) in the Russian far east. Eur J Entomol 111:397–406
Vidal DM, Fonseca MG, Zarbin PH (2010) Enantioselective synthesis and absolute configuration of the sex pheromone of Hedypathes betulinus (Coleoptera: Cerambycidae). Tetrahedron Lett 51:6704–6706
Wickham JD, Harrison RD, Lu W, Guo Z, Millar JG, Hanks LM, Chen Y (2014) Generic lures attract cerambycid beetles in a tropical montane rain forest in southern China. J Econ Entomol 107:259–267
Zar J (2010) Biostatistical analysis, 5th edn. Pearson Prentice-Hall, Upper Saddle River
Zarbin PHG, Fonseca MG, Szczerbowski D, Oliveira ARM (2013) Biosynthesis and site of production of sex pheromone components of the cerambycid beetle, Hedypathes betulinus. J Chem Ecol 39:358–363
Zhang A, Oliver JE, Aldrich JR, Wang B, Mastro VC (2002) Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky). Z Naturforsch 57c:553–558
Zou Y, Rutledge CE, Nakamuta K, Maier CT, Hanks LM, Richards AB, Lacey ES, Millar JG (2016) Identification of a pheromone component and a critical synergist for the invasive beetle Callidiellum rufipenne (Coleoptera: Cerambycidae). Environ Entomol 45:216–222
Acknowledgments
We thank S. buck and the University of Illinois Committee on Natural Area for access to field sites. We appreciate funding support from The Alphawood Foundation of Chicago (to LMH), and the USDA National Institute of Food and Agriculture (grant number 2012-67013-19303, to JGM and LMH).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10886-016-0796-6.
Rights and permissions
About this article
Cite this article
Meier, L.R., Zou, Y., Millar, J.G. et al. Synergism between Enantiomers Creates Species-Specific Pheromone Blends and Minimizes Cross-Attraction for Two Species of Cerambycid Beetles. J Chem Ecol 42, 1181–1192 (2016). https://doi.org/10.1007/s10886-016-0782-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0782-z