Abstract
Jasmonic acid (JA) is a natural plant hormone ubiquitously distributed in plants and centrally involved in the induction of direct and indirect plant defenses. Defenses up-regulated by this hormone include trichomes—a direct, mechanical defense—and alkaloids—a direct chemical defense—as well as two indirect chemical defenses: volatile organic compounds (VOCs) and extrafloral nectar (EFN). Plant cyanogenesis—the release of toxic hydrogen cyanide (HCN) from preformed cyanogenic precursors in fruits, leaves, and seeds of many plants—is recognized as a direct, constitutive plant defensive trait, and is among the most widely distributed of all direct chemical plant defenses. The cyanogenic system in plants is composed of three parameters: The cyanogenic potential (HCNp; concentration of cyanogenic precursors), β-glucosidase activity, and cyanogenic capacity (HCNc; release of gaseous hydrogen cyanide). Here, we demonstrated that experimental application of aqueous solutions of JA ranging from 0.001 to 1.0 mmol L−1, as well as insect herbivory significantly enhanced HCNc via the induction of β-glucosidase activity in wild lima bean (Phaseolus lunatus L.). In choice feeding trials with JA induced and damaged leaves, adult Mexican bean beetles—natural herbivores of lima bean—rejected leaves with enhanced β-glucosidase activity and HCNc. Our findings suggest that jasmonic acid plays a critical role in regulating activity of β-glucosidases, which determines the rate of cyanogenesis, and thus mediates direct plant defense against herbivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jasmonic acid (JA) is a natural plant hormone synthesized from α-linolenic acid, a C18 poly-unsaturated fatty acid, via the octadecanoid pathway. It is involved in multiple plant processes ranging from the ability to act as a growth inhibitor and senescence-promoting substance (Meyer et al. 1984) to inducing vegetative storage proteins (Franceschi and Grimes 1991; Mason and Mullet 1990). It also plays a central role in regulating multiple defense-associated plant characteristics, including structural and chemical traits (Creelman and Mullet 1995; McConn et al. 1997; Wei 2010). For example, JA can regulate the expression of trichomes in Arabidopsis (Traw and Bergelson 2003), and it increases the number of chemical compounds in glandular trichomes in tomato (Boughton et al. 2005; Van Schie et al. 2007). Furthermore, JA plays a central role in regulating the biosynthesis of several toxic compounds, such as alkaloids, which act as a direct chemical defense against herbivores (Howe 2001). External application of JA, as well as its derivate methyl jasmonate, effectively induces production of these compounds in developing seedlings (Aerts et al. 1994), in cells (Gantet et al. 1998; Gundlach et al. 1992), in hairy root cultures (Rijhwani and Shanks 1998a, b), in detached leaves (El-Sayed and Verpoorte 2005, as well as in intact plants (Baldwin et al. 1996). Other defenses induced by JA include the phytoalexin-related enzymes chalcone synthase, PAL, and hydroxymethylglutaryl-COA reductase, as well as protease inhibitors and chitinases (CHI-B) (Hu et al. 2009).
In addition to inducing direct defenses, another well-studied function of JA is its central role in regulating the expression of induced indirect plant defenses. Indirect plant defenses involve a higher trophic level and usually attract carnivorous arthropods to plants under attack from herbivores (Kappers et al. 2011). In particular, the induced release of plant volatile organic compounds (VOCs) and the induction or priming of extrafloral nectar (EFN) have been studied in detail over the last decade (e.g., Ballhorn et al. 2008, 2013a; Heil and Silva Bueno 2007). Lima bean has been advanced as a model plant system for many studies on indirect plant defense (VOCs and EFN) both under laboratory (e.g., Ballhorn et al. 2008, 2011a; Choh et al. 2006; Kost et al. 2011; Radhika et al. 2008) and field conditions (Ballhorn et al. 2013b; Kost and Heil 2008).
In the present study, we analyzed the impact of JA treatment and insect herbivory (Mexican bean beetle; Cocinellidae: Epilachna varivestis Muls.) on plant cyanogenesis using wildtype lima bean plants (Fabaceae: Phaseolus lunatus L.). Cyanogenesis is one of the most widely distributed chemical plant defenses (Møller and Seigler 1999). About 10 % of vascular plant species produce and accumulate cyanogenic precursors, generally cyanogenic glucosides (Seigler and Brinker 1993; Webber and Miller 2008). Two types of enzymes accelerate the release of HCN from cyanogenic glucosides in response to cell damage: β-glucosidases and hydroxynitrile lyases (Selmar et al. 1987, 1989). In lima bean, white clover, and cassava, β-glucosidases critically determine the velocity of HCN release (Ballhorn et al. 2006; Swain et al. 1992; Till 1987), whereas in other plants (Euphorbiaceae: Hevea brasiliensis) hydroxy nitril lyases affect the speed of HCN production as well (Selmar et al. 1989). Even though the actual release of HCN takes place only in response to cell damage, cyanogenesis is considered a constitutive defense due to the damage-independent production of preformed cyanogenic precursors. Cyanogenesis acts as an efficient direct plant defense against a broad range of herbivores (Ballhorn et al. 2009, 2010) and is highly toxic because cyanide inhibits the mitochondrial respiration pathway by blocking the cytochrome a/a3 dependent oxidase, but other metal-containing enzymes also are affected (Solomonson 1981). In general, cyanogenesis is more efficient against chewing herbivores, since herbivores with a piercing or sucking mode of feeding avoid extensive cell damage, thus circumventing the damage-induced HCN release. Specialist herbivores often can tolerate cyanogenesis (Gleadow and Woodrow 2002a) but nevertheless can be negatively affected by extensive host plant cyanogenesis in the long run, likely due to the costs associated with detoxification mechanisms (Ballhorn et al. 2007).
Several abiotic factors affect the cyanogenic potential (HCNp; concentration of cyanogenic precursors in a given plant tissue) including temperature, carbon dioxide, water, light and nutrient availability, and soil salinity (Ballhorn et al. 2011b; Ballhorn and Elias 2014; Frehner et al. 1997; Gleadow and Woodrow 2002b). However, these examples represent more or less slow phenotypic adaptations of cyanogenic traits or even long-term changes of genotype frequencies of high and low cyanogenic plants in populations (Foulds and Grime 1972) rather than rapid plant responses to a distinct external stimulus. Activation of β-glucosidases in response to leaf damage has been shown in lima bean (Ballhorn et al. 2006) and rubber tree (Kadow et al. 2012; Voß 2001). In both plant systems, different mechanisms lead to an activation of this enzyme. Ballhorn and co-workers (2006) reported an induction of β-glucosidase activity in lima bean following mechanical and herbivore damage over the course of 72 hr, whereas Voß (2001) and Kadow et al. (2012) demonstrated a much faster induction of enzymatic activity (within seconds) following mechanical damage, which is attributed to post-translational mechanisms. In vitro assays with extracts prepared from leaf tissue removed at the site of damage have shown significantly enhanced kinetics in releasing HCN (Kadow et al. 2012). Remarkably, in these studies the plant response was local and only detectable in the mechanically damaged tissue itself (Kadow et al. 2012; Voß 2001) or in a limited area (< 2 cm) surrounding the site of mechanical or herbivore damage (Ballhorn et al. 2006) without systemically spreading to other, yet undamaged leaves.
Given the wide distribution of cyanogenesis in the plant kingdom, including many cultivated species (Jones 1998), a better understanding of this defensive trait is broadly important. At the same time, improving our understanding of the multiple functions of jasmonic acid is relevant for a variety of disciplines including both basic and applied plant sciences. In the present study, we provide evidence that plant cyanogenesis is inducible by a natural plant hormone.
Methods and Materials
Plants
Wildtype lima bean plants (Fabaceae: Phaseolus lunatus L.) used were grown from seeds collected in a natural population in Oaxaca, Mexico. Plants were cultivated under greenhouse conditions adjusted to resemble conditions at natural sites in Mexico as recorded in May 2012. Light in the chamber was provided by a combination (1:1) of HQI-BT 400 W (Osram) and RNP-T/LR 400 W (Radium) lamps with a light regime of 13:11 L:D under photon flux density of 450–500 μmol photons m−2 s−1 at table height. Temperature was 30 ° C in the light period and 23 ° C in the dark period, and relative air humidity was adjusted to 70–80 %. Plants were cultivated in containers of 10 × 10 × 11 cm (width, length, height; one plant per pot) in a 1:1 ratio of standard substrate (Fox Farms, Arcata, CA, USA) and sand (grain size 0.5–2.0 mm). All plants were fertilized with 50 ml of a 0.1 % aqueous solution of Shultz All Purpose Plant Food® [NPK(%); 15, 10, 15-Fertilizer] once a week and watered daily. Position of plants in the greenhouse chamber was changed every 3d to exclude any position effects. Jasmonic acid treatments, herbivore damage and subsequent chemical analyses of leaf material were conducted after a plant cultivation period of 7 weeks and when the plants had developed 6.2 ± 2.1 leaves (mean ± SD; N = 245 plants).
Jasmonic Acid and Herbivore Treatment of Plants
Foliage was sprayed with aqueous JA solutions of different concentration (0.001, 0.01, 0.1, and 1.0 mmol L−1) 90 min after the start of the light period (Ballhorn et al. 2013b). The solutions used caused no visible phytotoxic effects at any concentration. Plants were randomly selected for treatment with different solutions. Control plants were treated the same way but sprayed with water instead of JA solution. Leaves were sprayed with JA solutions until completely moistened and allowed to dry (10 min) before being sprayed a second time. Plants were allowed to dry again, and then were placed back on the table at a distance of at least 40 cm apart. According to recent studies on the low efficiency of long-distance signaling via herbivore or JA-induced volatiles, this distance is considered sufficient to avoid cross-induction (Heil and Adame-Álvarez 2010). Seven individual plants were used for every combination of JA treatment and incubation period resulting in an overall number of plants of 245 [resulting from 5 treatments (control, 0.001, 0.01, 0.1, and 1.0 mmol JA L−1) × 7 time points of sampling (0, 12, 24, 48, 72, 96, and 192 h) × 7 replicates = 245 JA-treated plants]. Each plant was sampled once (instead of multiple sampling of individual plants over the experimental period) to avoid induction of β-glucosidase activity due to damaging plants by leaf removal during the sampling process. Each seven plants used in experiments with JA treatment were analyzed directly after JA treatments (1 h) to test for potential rapid responses of β-glucosidase activity.
In addition to JA treatments, plants were exposed to damage by Mexican bean beetle larvae (30 first instars per plant, 7 plants per time point of sampling; N = 49 plants). Larvae were removed after a feeding period of 4 hr. At this developmental stage, larvae feed on the lower surface of leaves and consume leaf parenchyma without destroying the upper epidermis. This herbivore treatment resulted in light, homogenous damage of foliage. Herbivore treated leaves were used exclusively for chemical analyses of leaf traits (β-glucosidase activity, HCNp, HCNc, and soluble protein) and not for feeding trials to exclude potential direct effects of former presence of larvae (remaining feces or saliva) on choice behavior of adult beetles. Similar to JA treatments, seven plants were analyzed directly after the herbivore treatment to test for potential rapid responses of β-glucosidase activity. Leaf material damaged by Mexican bean beetle larvae was not used in the experiments.
Leaf Material Used for Chemical Analyses and Feeding Experiments
Individual leaves of a defined developmental stage (intermediate leaves; 6th node below the apex; 0.732 ± 0.124 g fw; N = 294) were used for the experiments to reduce variability of traits due to differences in leaf age (Ballhorn et al. 2011b). The trifoliate leaves were portioned for chemical analyses and feeding trails. For feeding trials, leaf discs were removed from one leaflet per plant. Leaf discs were kept on moist filter paper for 1 hr prior to feeding trials to ensure complete evaporation of gaseous cyanide from the cutting edge and to exclude any effects on the herbivores. The remaining two leaflets were used for biochemical analyses. One leaflet was used for analysis of the cyanogenic capacity (HCNc) according to Ballhorn et al. (2010) by using an airflow equipment for detection of gaseous hydrogen cyanide. The other leaflet was portioned to measure cyanogenic potential (HCNp) and soluble protein concentration. The position of leaflets used for chemical analyses and bioassays as well as the position (left or right the central vein) of leaf discs used in the different feeding trails was set at random. As the same individual leaves were used in bioassays and chemical analyses, leaf chemical parameters can be related directly to insect choice behavior. Leaf chemical characteristics are similar among all three leaflets of lima bean (Ballhorn et al. 2007, 2013b).
Extraction and Purification of β-Glucosidase
Fully expanded trifoliate leaves were selected for the experiments (N = 1 leaf per plant). Leaves of similar positions at the stem were selected to reduce uncontrolled variability (see above) due to differences in leaf age and development. Leaves were cut off with a razor blade and were processed immediately. The petiole was excluded from the analyses. Leaves used for determination of β-glucosidase activity were weighed and homogenized in a fourfold volume of 67 mmol L−1 phosphate buffer (4 ml buffer per g leaf fresh weight) adjusted to pH 6.4. The extract was filtered through cotton fabric and centrifuged at 20,000 g and 4 ° C. The protein-containing supernatant was concentrated by ammonium sulfate fractionation (231 mg ammonium sulfate per ml of protein solution) and filtered through membrane caps with a pore size <10,000 kD (Schleicher and Schuell BioScience GmbH, Dassel, Germany).
β-Glucosidase Activity
Quantification of β-glucosidase activity in the extracts was conducted according to Ballhorn et al. (2006). The assay is based on the detection of p-nitrophenol released by hydrolysis of the chromogenic artificial substrate p-NP-glucoside by β-glucosidases (Merck, Darmstadt, Germany) (Hösel and Nahrstedt 1975; Selmar et al. 1987). The incubation mixture for analysis of the enzymatic activity contained 1 ml substrate solution (2 mmol L−1) in citrate buffer adjusted to pH 5.6. The incubation mixture consisted of 4.9 ml citrate buffer (pH 5.6) and 0.1 ml extract. After 10 min incubation at 30 °C, the reaction was stopped by adding 1 ml ice-cold sodium carbonate solution (1 mol L−1), and the released p-nitrophenol was quantified spectrophotometrically at 400 nm (Pharmacia Biotech, Ultraspec 3000). β-glucosidase activity was calculated per gram leaf dry weight as katal (kat). An enzyme activity of 1 kat is defined as a substrate conversion rate of 1 mol substrate per second under standard temperature and pressure. The calculation of the enzyme activity was carried out using a molar extinction coefficient for p-nitrophenol of ε400 nm = 16,159 l/mol cm (Voß 2001).
Cyanogenic Potential (HCNp)
The cyanogenic potential (HCNp) of leaves was measured by extracting cyanogenic precursors from plant material according to Ballhorn et al. (2005). For analysis, leaves were removed with a razor blade, weighed to the nearest 0.001 g and ground with liquid nitrogen in a cooled mortar and pestle at 4 ° C under addition of 2 ml ice-cold Na2HPO4 buffer (67 mmol L−1). Samples were quantitatively analyzed for their HCNp by complete enzymatic hydrolysis of cyanogenic precursors with specific β-glucosidase isolated from rubber tree (Hevea brasiliensis). We used gas-tight glass vessels (Thunberg vessels) for incubation of leaf extracts together with enzyme solution adjusted to an activity of 20 nkat per 100 μl (Ballhorn et al. 2005). Quantitative detection of the released HCN was carried out spectrophotometrically at 585 nm using the Spectroquant® cyanide test (Merck, Darmstadt, Germany; method described in detail in Ballhorn et al. 2005).
Cyanogenic Capacity (HCNc)
The analysis of gaseous HCN release from experimentally treated lima bean leaves was carried out using an airflow system according to Ballhorn et al. (2005). This vessel system allowed a constant airflow adjusted to 7 L h−1. Leaflets were treated with chloroform (250 μl per leaflet) to achieve complete tissue disintegration at the cellular level, and consequently, the release of gaseous hydrogen cyanide from the accumulated cyanogenic precursors. At the discharge opening of the equipment, the HCN-containing air was led into a test tube containing 0.1 mol L−1 NaOH solution. In this solution, cyanide was bonded as NaCN and then, as with HCNp quantification, was spectrophotometrically quantified at 585 nm as a polymethine dye formed using the Spectroquant® cyanide test. The release of cyanide was analyzed quantitatively over a time period of 20 min after solvent-induced cell damage. Under the adjusted experimental conditions (7 L air per hr, 250 μl chloroform per leaflet at room temperature), typically a HCN release over a time period of about 60 min can be observed (Ballhorn et al. 2005, 2006). However, the initial release over 20 min represents a good estimate for the overall cyanogenic capacity (Ballhorn et al. 2010).
Soluble Protein Concentration
Concentration of soluble proteins in leaves and plants was quantified according to Bradford (1976) with modifications following Ballhorn et al. (2007). Bradford reagent (Biorad Laboratories, Hercules, CA, USA) was diluted 1:5 with ddH2O, and 20 μl of each homogenized plant sample were combined with 1 ml of diluted Bradford solution. Bovine serum albumin (BSA; Fluka ChemieAG, Buchs, Switzerland) at different concentrations served as a standard. After 5 min incubation, concentration of protein was spectrophotometrically measured at 595 nm.
Feeding Trials
We used two different feeding experiments to determine the effects of JA treatment and incubation period on feeding choice of adult Mexican bean beetles. In Experiment 1, for each JA treatment (0.001, 0.01, 0.1, and 1.0 mmol L−1), as well as for non-induced controls, we used leaf discs that were removed at different times after JA application (0, 12, 24, 48, 72, 96, and 192 hr respectively) simultaneously in a choice arena. In Experiment 2, to obtain further insights into the impact of JA concentration, we offered leaf discs removed from plants induced at the same time (0, 12, 24, 48, 72, 96 and 192 hr prior to the experiment, respectively) but which were treated with JA solutions of different concentrations. In these choice experiments, we excluded discs from control plants, i.e., non-induced plants, to detect potential quantitative differences in herbivore choice behavior depending on different levels of induction (which otherwise would be overlaid by the disproportionate preference for the controls). In both experiments, we used leaf discs (13 mm diam, cut with a cork-borer) instead of intact leaflets to avoid potential effects of leaf size on beetle choice behavior. For the choice arena, we used Petri dishes (14 cm diam) supplemented with slightly moist filter paper. Leaf discs were placed upside down (beetles preferred to feed on the lower surface of leaves) and in equal distance to each other on the filter paper; the position of leaflets was random. Beetles were food-deprived for 2 hr before the experiment, and then a single beetle was placed in the middle of the Petri dish. Beetles were allowed to feed for 2 hr at the same ambient conditions as adjusted for plants. Natural sex ratios of beetles were used. Leaf area consumption was quantified by digitally photographing leaflets and leaf discs on a scale (Canon, EOS 5D Mark III; 22.3 MP) and computer-based determination of missing leaf area using analySIS software (Olympus, Hamburg, Germany).
Statistics
Differences in β-glucosidase activity, cyanogenic potential (HCNp), cyanogenic capacity (HCNc), and protein among plants and treatments were statistically analyzed using post-hoc analyses (Tukey’s HSD) after one-way ANOVA. The interaction of the respective plant traits (β-glucosidase activity, HCNp, and HCNc) with concentration of applied JA solutions and incubation period were analyzed using a generalized linear model (GLM). We used a Pearson’s correlation to test for significant associations between β-glucosidase activity and HCNc, as well as between plant traits and leaf consumption by beetles. All statistical analyses were carried out with Statistical Package for Social Sciences (SPSS) 16.0 (SPSS for Windows, SPSS, Chicago, IL, USA).
Results
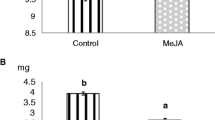
Treatment of lima bean plants with jasmonic acid (JA) at concentrations ranging from 0.001 to 1.0 mmol L−1 significantly induced β-glucosidase activity (Fig. 1a, Table S1). Compared to control plants, the increase of β-glucosidase activity became significant 24 hr after induction in all JA treatment groups. We observed a non significant trend of further increase between 24 and 48 hr for most treatments. β-Glucosidase activity remained at significantly elevated levels until the end of the experiment (192 hr after induction) (Table S2). After reaching a peak of enzymatic activity, between hours 48 and 72, lima bean plants showed only a marginal, non-significant decrease of β-glucosidase activity over the remaining experimental period. The enzymatic activity showed a higher but also non significant trend in plants sprayed with higher concentrations of JA. Spraying JA solution at the lowest concentration of 0.001 mmol L−1 resulted in an enzymatic activity which at its peak was by a factor of 7.5 higher than the controls. Treatments with JA solutions of 0.01, 0.1, and 1.0 mmol L−1 increased β-glucosidase activity by factors of 7.9, 8.4, and 9.2. However, differences in β-glucosidase activity among the JA-treatment groups were not significant at any time of sampling (Fig. 1a). Control plants not treated with JA showed no enhanced levels of β-glucosidase activity. Herbivore damage resulted in similar patterns of enzyme activation as compared to JA treatment. The activity of β-glucosidases showed a distinct increase within the first 48 hr after induction, and remained largely unchanged until the end of the experimental period. Herbivore damage resulted in a peak of enzymatic activity (at 48 hr) which was higher by a factor of 8.0 than in control plants and, thus, was comparable to the effects of JA treatment at a concentration of 0.01 mmol L−1 (Fig. 1a).
Effect of jasmonic acid concentration, herbivore damage, and incubation period on leaf traits of lima bean (Phaseolus lunatus) Foliage of lima bean was treated with different concentrations of aqueous jasmonic acid (JA) solution or exposed to insect herbivory and β-glucosidase activity (a), cyanogenic potential (HCNp) (b), cyanogenic capacity (HCNc) (c), and soluble protein content (d) were quantified at different time points (0–192 hr) after the treatment. Values shown are means (+SD); N = 7 plants per treatment and period of incubation (N = 294 plants in total). Different non-capitalized letters at the columns represent significant differences among plant treated with different JA concentration at a given time point after induction. Different capital letters below the columns refer to significant differences among plant treated with the same JA concentration but at different time points after induction [according to post hoc test (Tukey’s; P < 0.05) after one-way ANOVA]
The wildtype lima bean plants used showed natural variation of cyanogenic potential (HCNp) in leaves. The HCNp of the selected developmental stage ranged between 45.7 and 57.2 μmol HCN g−1 fw. Neither JA treatments nor insect herbivory significantly affected HCNp (Fig. 1b; Table S1, S2).
Corresponding to the observed changes in β-glucosidase activity (Fig. 1a), the cyanogenic capacity (HCNc) was significantly enhanced in response to JA treatments as well as to herbivore damage (Fig. 1c; Table S1). Changes of HCNc in response to JA treatments were correlated to the observed patterns of JA-induced β-glucosidase activity (according to Pearson correlation: r = 0.994; P < 0.001). Jasmonic acid treatments resulted in a maximum increase of HCN release by the factors 2.9, 3.1, 3.1, and 3.3 in plants sprayed with 0.001, 0.01, 0.1, and 1.0 mmol L−1 compared to controls. Damage by Mexican bean beetle larvae resulted in elevated HCNc by a factor of 2.8. In plants induced with 0.1 and 1.0 mmol L−1 JA, the increase in HCNc became significant after 12 hr (Fig. 1c, Table S2) and it showed a further significant increase over the next 12 hr. Treatment with lower JA concentration (0.001 and 0.01 mmol L−1 as well as herbivore damage) resulted in increased HCNc levels 12 hr after treatment. However, differences became significant after 24 hr. After 24 hr, a significantly increased HCNc was observed among all JA treatments and herbivore damaged plants. While 24 hr after induction, plants treated with 1.0 mmol L−1 JA showed a higher HCNc than plants induced by herbivory, at all other time points HCNc was not significantly different among JA and herbivore induced plants. For all treatments, cyanogenic capacity remained at significantly enhanced levels until the end of the experiment after 192 hr (Fig. 1c; Table S2).
Soluble protein concentration in leaves increased in response to JA treatments and herbivory (Fig. 1d). While the overall quantitative increase was low and ranged by factors of 1.05–1.12 compared to controls, it became significant after 12 hr (0.1 and 1.0 mmol L−1 JA) or 24 hr (all treatments) (Fig. 1d; Table S2). The increase in protein concentration was positively correlated to both β-glucosidase activity (r = 0.074; P < 0.001) and HCNc (r = 0.976; P < 0.001; according to Pearson correlation). Control plants did not show any change in leaf protein concentration over the experimental period.
In cafeteria-style feeding trials with adult Mexican bean beetles, we tested leaf material derived from plants that had been treated with identical concentration of aqueous JA solutions (0.0, 0.001, 0.01, 0.1, and 1.0 mmol L−1, respectively) but that were incubated for different time periods after the treatment. In these trials, incubation period of plants after JA treatment significantly affected beetle feeding choice (Fig. 2; Table S3). The attractiveness of JA induced plants significantly decreased 12 hr after induction, and decreased further over the following 12 h. Among all feeding trials leaf material derived from plants that were treated with JA 24–192 hr prior the bioassay was equally (un-) attractive to the herbivores. Leaf area consumption by beetles and β-glucosidase activity (r = −0.974; P < 0.001), HCNc (r = −0.961; P < 0.001) as well as soluble protein content (r = −0.932; P = 0.002) were negatively correlated.
Effect of jasmonic acid concentration and incubation period on leaf area consumption by Mexican bean beetles (Epilachna varivestis). Leaf material from lima bean plants treated with different concentrations of jasmonic acid (JA) (0.0–1.0 mmol L−1) and at different times prior the feeding trial (0–192 hr) were simultaneously offered to individual Mexican bean beetles. Consumed leaf area was quantified after 6 hr. In feeding trial (a) beetles were offered untreated leaf materials exclusively, whereas in the other feeding trials (b-e) leaf material from JA treated plants was offered together with untreated leaves (white bars). Values shown are means (+SD); N = 12 feeding trials per JA treatment). Different non-capitalized letters at the columns represent significant differences in leaf consumption [according to post hoc test (Tukey’s; P < 0.05) after one-way ANOVA]
To test the effect of induction with different concentrations of JA on herbivore choice behavior, we conducted another cafeteria feeding trial offering plant material that has been treated with different JA concentrations but at the same time prior the bioassay (Fig. 3). While incubation period after JA application had a strong effect on leaf consumption by beetles (Fig. 2), no significant differences in leaf area consumption among plants treated with 0.001, 0.01, 0.1, and 1.0 mmol L−1 JA solutions were observed when comparing plant material that had been treated at the same time prior to the choice experiments but with different concentrations of JA (Fig. 3; Table S4). This feeding trial showed further that not only the choice behavior of insects was significantly affected by the incubation time after JA treatment (Fig. 2) but also the total consumption of leaf material in the different feeding trials (F 6,77 = 7.673, P < 0.001) (Fig. 3). Total leaf area consumption was significantly decreased in plants treated with JA 24 h prior to the assay compared to plants sampled directly after treatment, and remained at significantly reduced levels until the end of the experiment (Fig. 3).
Effect of jasmonic acid concentration on herbivore choice behavior and total leaf consumption . Leaf material induced with different concentrations of jasmonic acid (JA) but incubated for identical periods (0, 12, 24, 48, 72, 96, and192 hr, respectively) was offered simultaneously to individual Mexican bean beetles (each of the stacked bars represents a different feeding trial setup) and leaf area consumption was quantified (N = 12 trials per incubation period). Capital letters to the right columns refer to significant differences in leaf consumption [according to post hoc test (Tukey’s; P < 0.05) after one-way ANOVA]. In addition, total leaf consumption was compared among the different feeding trials. Different non-capitalized letters on top of columns represent significant differences in total leaf consumption among the feeding trials [according to post hoc test (Tukey’s; P < 0.05) after one-way ANOVA]
Discussion
Plants have evolved a plethora of defenses to defend themselves against multiple enemies (Walling 2000). Jasmonic acid (JA) is one of the key hormones involved in the expression of many induced defenses against herbivores, including both mechanical and chemical traits (Stintzi et al. 2001). In the present study, we report a JA-mediated induction of β-glucosidase activity in the course of several hours, and subsequently, enhanced cyanogenic capacity (HCNc) in wild lima bean plants. Induction of these traits resulted in an improved plant resistance to a natural insect herbivore of lima bean, the Mexican bean beetle. Thus, we showed for the first time that JA controls the expression of plant cyanogenesis (i.e., the release of cyanide from preformed cyanogenic precursors), which suggests that cyanogenesis can be interpreted as an inducible rather than constitutive plant defense. Although we did not observe any significant changes in HCNp throughout the experimental period, we cannot exclude variation in HCNp before our first measurement about 1 hr after JA application. Rapid turnover of cyanogenic glucosides has been repeatedly reported indicating plasticity of cyanogenesis also with respect to the HCNp (Ballhorn and Elias 2014; Gleadow and Møller 2014). Nevertheless, in our present study, JA treatments resulted in a significantly enhanced release of HCN—the cyanogenic capacity (HCNc)— which is a key parameter determining plant resistance to herbivores (Alonso-Amelot and Oliveros-Bastidas 2005; Ballhorn et al. 2006, 2010).
In our study, the induction of resistance due to elevated β-glucosidase activity and HCNc became significant after 24 hr and remained at a high level of activity, approximately eight-fold compared to the controls for at least 192 hr. This time-lag of induction possibly suggests the involvement of mRNA synthesis in the course of JA-induced gene activation (Dammann et al. 1997). The increase of β-glucosidase activity and HCNc in lima bean over the course of several hours is in contrast to the findings on cyanogenic rubber tree (Euphorbiaceae: Hevea brasiliensis) reported by Kadow et al. (2012). Kadow and co-workers observed a rapid increase of enzymatic activity (3.9–8.7 fold increase) within seconds after mechanically damaging leaves resulting in an enhanced release of hydrogen cyanide from cyanogenic precursors under in vitro conditions, i.e., in extracts prepared from previously damaged leaf tissue. The rate of activation of β-glucosidase in H. brasiliensis leaves indicates that the effect is probably induced by post-translational enzyme modification. These findings are in line with results from Nagano et al. (2005), who reported a rapid formation of catalytically active β-glucosidase polymers in root tissue of Arabidopsis. In Arabidopsis roots, the β-glucosidase PYK10 is stored in ER (endoplasmatic reticulum) bodies as an inactive monomer. When PYK10 comes into contact with cytoplasmic PBP1 (PYK Binding Protein 1) upon homogenization, cross-linking of the monomers results in polymerization of the protein. The PYK10 (PBP1) polymers are insoluble but are catalytically active (Nagano et al. 2005). A similar mechanism might occur in H. brasiliensis and lead to the activation of β-glucosidase, as this enzyme tends to form oligomers of variable size (2–26 identical subunits) (Selmar et al. 1987). A further example of β-glucosidase activation due to polymer formation has been described for common wheat (Triticum aestivum). β-Glucosidase isolated from wheat leaf tissue forms hexamers and lower order oligomers, while only the hexamers are catalytically active (Sue et al. 2006). The kinetics of β-glucosidase activation we describe here are not in line with the observations on rapid induction in other plant species, and are likely not due to post-translational effects. The increase in soluble protein concentration, which was significantly correlated to variation in β-glucosidase activity and HCNc among the different treatments, rather suggests synthesis of defense-associated proteins—including β-glucosidase. However, as we have not purified the specific β-glucosidase in the present study, we cannot rule out the possibility that this fraction contained other proteins than β-glucosidase potentially affecting the herbivores.
In our study, differences in concentrations of applied JA solutions (0.001, 0.01, 0.1, and 1.0 mmol L−1) did not result in significant differences of induction of β-glucosidase activity and HCNc (Fig. 1), which suggests that even low levels of JA are sufficient to induce a strong defense response. Aqueous JA solutions of 1.0 mmol L−1 frequently are applied in experimental studies to induce or prime plant defenses such as induced volatile organic compounds or extrafloral nectar (e.g., Ballhorn et al. 2008, 2013a; Kost and Heil 2008). The experiments on the induction of β-glucosidase activity and HCNc we report here demonstrate that much lower concentrations are effective for induction of plant defense. High concentrations of 1.0 mmol L−1, however, did not result in any visible detrimental effects on the plants such as enhanced senescence (He et al. 2002), and apparently are suitable to experimentally induce plant defense responses.
The similar response of lima bean β-glucosidase activity towards JA treatments of different concentrations is in contrast with the distinct quantitative effects of mechanical damage on β-glucosidase activity reported for rubber tree. In this plant species, Kadow et al. (2012) related the enhanced enzyme activity to the severity or type of tissue damage. In their study, extensive damage caused higher activation. This discrepancy can be explained by the almost maximum induction of lima bean β-glucosidase activity even with the low concentrations of experimentally applied JA. A gradient of even lower JA concentrations may have resulted in more quantitative effects.
The induction of β-glucosidase activity in lima bean resulted in enhanced HCNc and enhanced plant defense against an insect herbivore. Thus, these findings show that the activation of β-glucosidase activity in lima bean is affecting cyanogenesis and is directly defense related. However, it should be considered that β-glucosidases have been reported to have a multifunctional character. In plants, β-glucosidases play important roles in diverse aspects of plant physiology, e.g., (1) formation of intermediates in cell wall lignification (Dharmawardhana et al. 1995; Escamilla-Treviño et al. 2006), (2) cell wall degradation in endosperm during germination (Leah et al. 1995), and (3) activation of phytohormones (Kristoffersen et al. 2000; Lee et al. 2006). In addition to these regulatory functions in intact plants, β-glucosidases are involved in a broad range of defense-associated reactions (reviewed by Morant et al. 2008) including activation of chemical defense compounds such as saponins (Nisius 1988), glucosinolates (Halkier and Gershenzon 2006), and, in soybean Glycine max, the release of free isoflavones from their conjugates by an isoflavone conjugate-hydrolyzing β-glucosidase (Suzuki et al. 2006). Isoflavones repeatedly have been reported to be involved in plant defense against pathogens (Barz and Welle 1992; Dakora and Phillips 1996; Graham and Graham 1996, 2000; Rivera-Vargas et al. 1993).
In particular, the putative role of β-glucosidases for systemic acquired resistance against pathogens (SAR) may be of high ecological relevance (Selmar et al. 1987). SAR is primarily induced by pathogens, but tissue damage by piercing and sucking insects and mites induce similar responses (Bostock 1999; Grinberg et al. 2005; Russo et al. 1997; Walling 2000). Two important aspects of SAR are the accumulation of phytoalexins and enhanced local lignification of cell walls (Hammerschmidt 1999a, b; De Bruxelles and Roberts 2001), and β-glucosidases are involved in both processes. Furthermore, β-glucosidases have a critical function in the elicitation of herbivore-induced volatiles (Mattiacci et al. 1995; Wang et al. 2008 and references therein), thus, an overlapping function with jasmonic acid, which also regulates the release of these VOCs (Ballhorn et al. 2011a). The induction of β-glucosidases is likely to have functions beyond the increase in HCN release.
Induced plant defenses against herbivores and pathogens are proposed as a sustainable plant protection strategy in agricultural ecosystems. There is controversy regarding applied approaches that target indirect plant defenses, as there generally is a broad range of biotic and abiotic factors that determine their efficiency. However, the induction of direct plant defenses by natural plant hormones, as we report here, has potential as an efficient plant protection mechanism, as no pathogens or herbivores are required as inducing agents. Furthermore, the results show that the induction of β-glucosidases does not affect the concentration of cyanogenic compounds—which at enhanced levels might also reduce food plant quality for humans or livestock—but rather increases the velocity of HCN release as the toxic agent from an existing pool of cyanide-containing precursors. With regard to potential application of jasmonic acid in agriculture, the very low concentration of JA that we show was sufficient to induce plant responses, also promotes potential economical approaches. As many food plants are cyanogenic and other important crops such as Brassica sp. rely on similar defense mechanisms, i.e., the release of toxic compounds (isothiocyanates) from inactive precursors via the activity of β-glucosidases, the observed effects might be broadly applicable. In the present study, we did not find any treatment-associated costs due to enhanced synthesis of β-glucosidases, such as reduced growth of induced plants. This makes the induction of cyanogenesis by JA treatment highly interesting for potential application in sustainable plant protection.
References
Aerts RJ, Gisi D, Carolis E, Luca V, Baumann TW (1994) Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J 5:635–643
Alonso-Amelot ME, Oliveros-Bastidas A (2005) Kinetics of the natural evolution of hydrogen cyanide in plants in neotropical Pteridium arachnoideum and its ecological significance. J Chem Ecol 31:315–331
Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (1996) Volatile signaling in plant-plant interactions: “Talking Trees” in the genomics era. Science 311:812–815
Ballhorn DJ, Elias JD (2014) Salinity-mediated cyanogenesis in white clover (Trifolium repens) affects trophic interactions. Ann Bot 114:357–366
Ballhorn DJ, Lieberei R, Ganzhorn JU (2005) Plant cyanogenesis of Phaseolus lunatus and its relevance for herbivore-plant interaction: The importance of quantitative data. J Chem Ecol 31:1445–1473
Ballhorn DJ, Heil M, Lieberei R (2006) Phenotypic plasticity of cyanogenesis in lima bean Phaseolus lunatus - Activity and activation of beta-glucosidase. J Chem Ecol 32:261–275
Ballhorn DJ, Heil M, Pietrowski A, Lieberei R (2007) Quantitative effects of cyanogenesis on an adapted herbivore. J Chem Ecol 33:2195–2208
Ballhorn DJ, Kautz S, Lion U, Heil M (2008) Trade-offs between direct and indirect of lima bean (Phaseolus lunatus). J Ecol 96:743–745
Ballhorn DJ, Kautz S, Heil M, Hegeman AD (2009) Cyanogenesis of wild lima bean (Phaseolus lunatus L.) is an efficient direct defense in nature. PLoS ONE 4:e5450
Ballhorn DJ, Kautz S, Lieberei R (2010) Comparing responses of generalist and specialist herbivores to various cyanogenic plant features. Entomol Exp Appl 134:245–259
Ballhorn DJ, Kautz S, Jensen M, Schmitt I, Heil M, Hegeman AD (2011a) Genetic and environmental interactions determine plant defences against herbivores. J Ecol 99:313–326
Ballhorn DJ, Reisdorff C, Pfanz H (2011b) Quantitative effects of enhanced CO2 on jasmonic acid induced plant volatiles of lima bean (Phaseolus lunatus L.). J Appl Bot Food Qual 84:65–71
Ballhorn DJ, Kautz S, Heil M (2013a) Distance and sex determine host plant choice by herbivorous beetles. PLoS ONE 8:e55602
Ballhorn DJ, Kautz S, Schädler M (2013b) Induced plant defense via volatile production is dependent on rhizobial symbiosis. Oecologia 172:833–846
Barz W, Welle R (1992) Biosynthesis and metabolism of isoflavones and pterocarpan phytoalexins in chickpea, soybean and phytopathogenic fungi. In: Stafford HA, Ibrahim RK (eds) Phenolic metabolism in plants. Plenum Press, New York, pp 139–164
Bostock RM (1999) Signal conflicts and synergies in induced resistance to multiple attackers. Physiol Mol Plant Pathol 55:99–109
Boughton AJ, Hoover K, Felton GW (2005) Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol 31:2211–2216
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Choh Y, Kugimiya S, Takabayashi J (2006) Induced production of extrafloral nectar in intact lima bean plants in response to volatiles from spider mite-infested conspecific plants as a possible indirect defense against spider mites. Oecologia 147:455–460
Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci U S A 92:4114–4119
Dakora FD, Phillips DA (1996) Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Mol Plant Pathol 49:1–20
Dammann C, Rojo E, Sánchez-Serrano JJ (1997) Abscisic acid and jasmonic acid activate wound-inducible genes in potato through separate, organ-specific signal transduction pathways. Plant J 11:773–782
De Bruxelles GL, Roberts MR (2001) Signals regulating multiple responses to wounding and herbivores. Crit Rev Plant Sci 20:487–521
Dharmawardhana DP, Ellis BE, Carlson JE (1995) A beta-Glucosidase from lodgepole pine xylem specific for the lignin precursor coniferin. Plant Physiol 107:331–339
El-Sayed M, Verpoorte R (2005) Methyljasmonate accelerates catabolism of monoterpenoid indole alkaloids in Catharanthus roseus during leaf processing. Fitoterapia 76:83–90
Escamilla-Treviño LL, Chen W, Card ML, Shih MC, Cheng CL, Poulton JE (2006) Arabidopsis thaliana β-Glucosidases BGLU45 and BGLU46bv hydrolyse monolignol glucosides. Phytochemistry 67:1651–1660
Foulds W, Grime JP (1972) Influence of soil-moisture of frequency of cyanogenic plants in populations of Trifolium-repens and Lotus-corniculatus. Heredity 28:143–146
Franceschi VR, Grimes HD (1991) Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A 88:6745–6749
Frehner M, Lüscher A, Hebeisen T, Zanetti S, Schubiger F, Scalet M (1997) Effects of elevated partial pressure of carbon dioxide and season of the year on forage quality and cyanide concentration of Trifolium repens L. from a FACE experiment. Acta Oecol 18:297–304
Gantet P, Imbault N, Thiersault M, Doireau P (1998) Necessity of a functional octadecanoic pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant Cell Physiol 39:220–225
Gleadow RM, Møller BL (2014) Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annu Rev Plant Biol 65:155–185
Gleadow RM, Woodrow IE (2002a) Constraints on effectiveness of cyanogenic glycosides in herbivore defense. J Chem Ecol 28:1301–1313
Gleadow RM, Woodrow IE (2002b) Defense chemistry of cyanogenic Eucalyptus cladocalyx seedlings is affected by water supply. Tree Physiol 22:939–945
Graham TL, Graham MY (1996) Signaling in soybean phenylpropanoid responses (dissection of primary, secondary, and conditioning effects of light, wounding, and elicitor treatments). Plant Physiol 110:1123–1133
Graham TL, Graham MY (2000) Defense potentiation and competency: redox conditioning effects of salicylic acid and genistein. Mol Plant-Microbe Inter 4:60–68
Grinberg M, Perl-Treves R, Palevsky E, Shomer I, Soroker V (2005) Interaction between cucumber plants and the broad mite, Polyphagotarsonemus latus: from damage to defense gene expression. Entomol Exp Appl 115:135–144
Gundlach H, Müller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A 89:2389–2393
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 57:303–333
Hammerschmidt R (1999a) Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol 55:77–84
Hammerschmidt R (1999b) Phytoalexins: what have we learned after 60 years? Annu Rev Phytopathol 37:285–306
He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128:876–884
Heil M, Adame-Álvarez RM (2010) Short signalling distances make plant communication a soliloquy. Biol Lett 6:843–845
Heil M, Silva Bueno JC (2007) Herbivore-Induced Volatiles as Rapid Signals in Systemic Plant Responses: How to Quickly Move the Information? Plant Signal Behav 2:191–193
Hösel W, Nahrstedt A (1975) Glucosidases specific for the cyanogenic glucoside triglochinin. Purification and characterization of beta-glucosidases from Alocasia macrorrhiza Schott. Hoppe Seylers Z Physiol Chem 356:1265–1275
Howe GA (2001) Cyclopentenone signals for plant defense: remodeling the jasmonic acid response. Proc Natl Acad Sci U S A 98:12317–12319
Hu X, Li W, Chen Q, Yang Y (2009) Early signal transduction linking the synthesis of jasmonic acid in plant. Plant Signal Behav 4:696–697
Jones DA (1998) Why are so many food plants cyanogenic? Phytochemistry 47:155–162
Kadow D, Voß K, Selmar D, Lieberei R (2012) The cyanogenic syndrome in rubber tree Hevea brasiliensis: tissue-damage-dependent activation of linamarase and hydroxynitrile lyase accelerates hydrogen cyanide release. Ann Bot 109:1253–1262
Kappers IF, Hoogerbrugge H, Bouwmeester HJ, Dicke M (2011) Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. J Chem Ecol 37:150–160
Kost C, Heil M (2008) The defensive role of volatile emission and extrafloral nectar secretion for lima bean in nature. J Chem Ecol 34:2–13
Kost C, Tremmel M, Wirth R (2011) Do leaf cutting ants cut undetected? Testing the effect of ant-induced plant defences on foraging decisions in Atta colombica. PLoS ONE 6:e22340
Kristoffersen P, Brzobohaty B, Höhfeld I, Bako L, Melkonian M, Palme K (2000) Developmental regulation of the maize Zm-p60. 1 gene encoding a β-glucosidase located to plastids. Planta 210:407–415
Leah R, Kigel J, Svendsen I, Mundy J (1995) Biochemical and molecular characterization of a barley seed-glucosidase. J Bio Chem 270:15789–15797
Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I (2006) Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126:1109–1120
Mason HS, Mullet JE (1990) Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell Physiol 2:569–579
Mattiacci L, Dicke M, Posthumus MA (1995) Beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci U S A 92:2036–2040
McConn M, Creelman RA, Bell E, Mullet JE (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci U S A 94:5473–5477
Meyer A, Miersch O, Büttner C, Dathe W, Sembdner G (1984) Occurrence of the plant growth regulator jasmonic acid in plants. J Plant Growth Regul 3:1–8
Møller BL, Seigler DS (1999) Biosynthesis of cyanogenic glycosides, cyanolipids, and related compounds. In: Singh BK (ed) Plant amino acids. Biochemistry and biotechnology. Marcel Dekker, New York, pp 563–609
Morant AV, Jørgensen K, Jørgensen C, Michelle Paquette S, Sánchez-Pérez R, Møller BL, Bak S (2008) β-Glucosidases as detonators of plant chemical defense. Phytochemistry 69:1795–1813
Nagano AJ, Matsushima R, Hara-Nishimura I (2005) Activation of an ER-body-localized β-glucosidase via a cytosolic binding partner in damaged tissues of Arabidopsis thaliana. Plant Cell Physiol 46:1140–1148
Nisius A (1988) The stromacenter in avena plastids – an aggregation of betaglucosidase responsible for the activation of oat leaf saponins. Planta 173:474–481
Radhika V, Kost C, Bartram S, Heil M, Boland W (2008) Testing the optimal defence hypothesis for two indirect defences: extrafloral nectar and volatile organic compounds. Planta 228:449–457
Rijhwani SK, Shanks JV (1998a) Effect of subculture cycle on growth and indole alkaloid production by Catharanthus roseus hairy root cultures. Enzyme Microb Technol 22:606–611
Rijhwani SK, Shanks JV (1998b) Effect of elicitor dosage and exposure time on biosynthesis of indole alkaloids by Catharanthus roseus hairy root cultures. Biotechnol Prog 14:442–449
Rivera-Vargas LI, Schmitthenner AF, Graham TL (1993) Soybean flavonoid effects on and metabolism by Phytophthora sojae. Phytochemistry 32:851–857
Russo VM, Russo BM, Peters M, Perkins-Veazie P, Cartwright B (1997) Interaction of Colletotrichum orbiculare with thrips and aphid feeding on watermelon seedlings. Crop Prot 16:581–584
Seigler DS, Brinker AM (1993) Characterisation of cyanogenic glycosides, cyanolipids, nitroglycosides, organic nitro compounds and nitrile glucosides from plants. In: Waterman PG (ed) Methods in plant biochemistry vol. 8: Alkaloids and sulphur compounds. Academic, London, pp 51–131
Selmar D, Lieberei R, Biehl B, Voigt J (1987) Hevea linamarase—a nonspecific β-glycosidase. Plant Physiol 83:557–563
Selmar D, Lieberei R, Conn EE, Biehl B (1989) Alpha-hydroxynitrile lyase in Hevea brasiliensis and its significance for rapid cyanogenesis. J Plant Physiol 75:97–101
Solomonson L (1981) Cyanide as a metabolic inhibitor. In: Vennesland B, Conn EE, Knowles CJ, Westby J, Wissing F (eds) Cyanide in biology. Academic, London, pp 11–28
Stintzi A, Weber H, Reymond P, Farmer EE (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci U S A 98:12837–12842
Sue M, Yamazaki K, Yajima S, Nomura T, Matsukawa T, Iwamura H, Miyamoto T (2006) Molecular and structural characterization of hexameric β-D-glucosidases in wheat and rye. Plant Physiol 141:237–1247
Suzuki H, Takahashi S, Watanabe R, Fukushima Y, Fujita N, Noguchi A, Yokoyama R, Nishitani K, Nishino T, Nakayama T (2006) An isoflavone conjugate-hydrolyzing beta-glucosidase from the roots of soybean (Glycine max) seedlings – purification, gene cloning, phylogenetics, and cellular localization. J Biol Chem 281:30251–30259
Swain E, Li CP, Poulton JE (1992) Tissue and subcellular localization of enzymes catabolizing (R)-amygdalin in mature Prunus serotina seeds. Plant Physiol 100:291–300
Till I (1987) Variability of expression of cyanogenesis in white clover (Trifolium repens L.). Heredity 59:265–271
Traw MB, Bergelson J (2003) Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol 133:1367–1375
Van Schie CC, Haring MA, Schuurink RC (2007) Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol 64:251–263
Voß K (2001) Biologische Bedeutung und Aktivierbarkeit der β-D-Glycosidase in Blättern von Hevea brasiliensis (Willd.) Muell. Arg. (1865). PhD thesis, University of Hamburg, Germany.
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Wang X, Zhou G, Xiang C, Du M, Cheng J, Liu S, Lou Y (2008) β-Glucosidase treatment and infestation by the rice brown planthopper Nilaparvata lugens elicit similar signaling pathways in rice plants. Chin Sci Bull 53:53–57
Webber BL, Miller RE (2008) Gynocardin from Baileyoxylon lanceolatum and a revision of cyanogenic glycosides in Achariaceae. Biochem Syst Ecol 36:545–553
Wei S (2010) Methyl jasmonic acid induced expression pattern of terpenoid indole alkaloid pathway genes in Catharanthus roseus seedlings. Plant Growth Regul 61:243–251
Acknowledgments
We thank Adrienne Godschalx for helpful comments and discussion. This work was financially supported with start-up funds provided by Portland State University. SK acknowledges support by the German Academy of Sciences Leopoldina (grant LPDS 2009–29). The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kautz, S., Trisel, J.A. & Ballhorn, D.J. Jasmonic Acid Enhances Plant Cyanogenesis and Resistance to Herbivory in Lima Bean. J Chem Ecol 40, 1186–1196 (2014). https://doi.org/10.1007/s10886-014-0524-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0524-z