Abstract
Objective
This study aimed to assess the impact of a lung-protective ventilation strategy utilizing transpulmonary driving pressure titrated positive end-expiratory pressure (PEEP) on the prognosis [mechanical ventilation duration, hospital stay, 28-day mortality rate and incidence of ventilator-associated pneumonia (VAP), survival outcome] of patients with Acute Respiratory Distress Syndrome (ARDS).
Methods
A total of 105 ARDS patients were randomly assigned to either the control group (n = 51) or the study group (n = 53). The control group received PEEP titration based on tidal volume [A tidal volume of 6 mL/kg, flow rate of 30–60 L/min, frequency of 16–20 breaths/min, constant flow rate, inspiratory-to-expiratory ratio of 1:1 to 1:1.5, and a plateau pressure ≤ 30–35 cmH2O. PEEP was adjusted to maintain oxygen saturation (SaO2) at or above 90%, taking into account blood pressure], while the study group received PEEP titration based on transpulmonary driving pressure (Esophageal pressure was measured as a surrogate for pleural pressure using an esophageal pressure measurement catheter connected to the ventilator. Tidal volume and PEEP were adjusted based on the observed end-inspiratory and end-expiratory transpulmonary pressures, aiming to maintain a transpulmonary driving pressure below 15 cmH2O during mechanical ventilation. Adjustments were made 2–4 times per day). Statistical analysis and comparison were conducted on lung function indicators [oxygenation index (OI), arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2)] as well as other measures such as heart rate, mean arterial pressure, and central venous pressure in two groups of patients after 48 h of mechanical ventilation. The 28-day mortality rate, duration of mechanical ventilation, length of hospital stay, and ventilator-associated pneumonia (VAP) incidence were compared between the two groups. A 60-day follow-up was performed to record the survival status of the patients.
Results
In the control group, the mean age was (55.55 ± 10.51) years, with 33 females and 18 males. The pre-ICU hospital stay was (32.56 ± 9.89) hours. The mean Acute Physiology and Chronic Health Evaluation (APACHE) II score was (19.08 ± 4.67), and the mean Murray Acute Lung Injury score was (4.31 ± 0.94). In the study group, the mean age was (57.33 ± 12.21) years, with 29 females and 25 males. The pre-ICU hospital stay was (33.42 ± 10.75) hours. The mean APACHE II score was (20.23 ± 5.00), and the mean Murray Acute Lung Injury score was (4.45 ± 0.88). They presented a homogeneous profile (all P > 0.05). Following intervention, significant improvements were observed in PaO2 and OI compared to pre-intervention values. The study group exhibited significantly higher PaO2 and OI compared to the control group, with statistically significant differences (all P < 0.05). After intervention, the study group exhibited a significant increase in PaCO2 (43.69 ± 6.71 mmHg) compared to pre-intervention levels (34.19 ± 5.39 mmHg). The study group’s PaCO2 was higher than the control group (42.15 ± 7.25 mmHg), but the difference was not statistically significant (P > 0.05). There were no significant differences in hemodynamic indicators between the two groups post-intervention (all P > 0.05). The study group demonstrated significantly shorter mechanical ventilation duration and hospital stay, while 28-day mortality rate and incidence of ventilator-associated pneumonia (VAP) showed no significant differences. Kaplan-Meier survival analysis revealed a significantly better survival outcome in the study group at the 60-day follow-up (HR = 0.565, 95% CI: 0.320–0.999).
Conclusion
Lung-protective mechanical ventilation using transpulmonary driving pressure titrated PEEP effectively improves lung function, reduces mechanical ventilation duration and hospital stay, and enhances survival outcomes in patients with ARDS. However, further study is needed to facilitate the wider adoption of this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Acute Respiratory Distress Syndrome (ARDS) is a life-threatening condition characterized by severe hypoxemia and decreased lung compliance, posing a significant risk to patients’ lives [1, 2]. ARDS is associated with endothelial injury of the pulmonary capillaries and diffuse alveolar damage, often accompanied by varying degrees of pulmonary arterial vasoconstriction and the potential development of pulmonary hypertension [3]. Despite its high mortality rate, effective therapeutic strategies for alleviating this devastating disease remain limited [4].
Mechanical ventilation is the mainstay treatment for patients with acute respiratory distress syndrome, with Lung Protective Ventilation (LPV) serving as a fundamental approach in ARDS management [5]. The core principles of LPV for ARDS involve the use of low tidal volume (VT), application of relatively high positive end-expiratory pressure (PEEP), and restriction of plateau pressure to mitigate the occurrence of ventilator-induced lung injury (VILI) and improve survival outcomes [6, 7]. Moreover, PEEP plays a crucial role in alveolar recruitment, preventing the collapse of recruitable alveoli, thereby improving oxygenation and minimizing lung injury [8,9,10]. The European Society of Intensive Care Medicine (ESICM) guidelines on the treatment of acute respiratory distress syndrome (ARDS) [11] provide multiple recommendations regarding definitions, phenotype analysis, and respiratory support strategies. These recommendations cover various aspects, including high-flow nasal oxygen (HFNO), non-invasive ventilation (NIV), tidal volume settings, positive end-expiratory pressure (PEEP) and lung recruitment maneuvers (RM), prone positioning, neuromuscular blockade, and extracorporeal life support (ECLS). The aim of these guidelines is to update the ESICM’s 2017 Clinical Practice Guidelines (CPG) and has been developed in collaboration with an international panel of clinical experts, methodologists, and patient representatives representing ESICM.
Nevertheless, the optimal weighting of lung protective parameters in predicting ARDS prognosis remains inadequately understood [12]. While low tidal volume and high PEEP serve as the cornerstones of LPV, it is worth noting that tidal volumes below 6 ml/kg may lead to partial atelectasis, and a cyclic pattern of alveolar opening and closing occurs at the interface between normally ventilated and atelectatic lung regions, as well as between atelectatic and consolidated regions [13]. Therefore, the exploration of effective treatment methods for ARDS is still ongoing.
Furthermore, when increasing PEEP fails to recruit functional lung units and improve lung compliance, it results in excessive lung distension and energy transfer to the lung parenchyma, thereby increasing the risk of lung injury, dead space formation, pneumothorax, and adverse hemodynamic consequences [14, 15]. Only low tidal volume combined with a decrease in transpulmonary driving pressure and an increase in PEEP can effectively confer lung protection, while an elevation in plateau pressure associated with an increase in transpulmonary driving pressure leads to lung injury [16]. Consequently, transpulmonary driving pressure-guided lung-protective ventilation has emerged as a promising approach in the management of ARDS.
Therefore, the integration of the ESICM guidelines on ARDS treatment provides crucial guidance for clinical practice, our aim is to investigate the impact of a tailored lung-protective ventilation strategy based on transpulmonary driving pressure on patient outcomes in ARDS. We hypothesize that optimizing tidal volume and PEEP according to transpulmonary driving pressure measurements will result in improved survival rates in ARDS patients.
2 Materials and methods
2.1 Study population
This prospective study was conducted at our hospital’s intensive care unit (ICU) from January 2020 to December 2023. The study included patients diagnosed with moderate to severe acute respiratory distress syndrome (ARDS) who required mechanical ventilation with lung-protective strategies. Random assignment of patients into either the control group or the study group was performed using a random number table. The control group received positive end-expiratory pressure (PEEP) titration based on tidal volume, while the study group underwent PEEP titration based on transpulmonary driving pressure. The study design adhered to the ethical principles outlined in the Helsinki Declaration for clinical research and received approval from the ethics committee of our hospital.
2.2 Inclusion and exclusion criteria
Inclusion criteria:
-
Age between 18 and 80 years, and both genders.
-
Diagnosis of ARDS based on the 2012 Berlin definition.
-
Initiation of invasive mechanical ventilation within 48 h of inclusion, and an anticipated need for mechanical ventilation for more than 72 h.
Exclusion criteria:
-
Pre-ICU mechanical ventilation duration exceeding 48 h.
-
Arterial blood gas analysis showing a PaO2/FiO2 ratio greater than 150 mmHg before enrollment.
-
Duration of ARDS exceeding 72 h.
-
Presence of conditions such as pneumothorax, mediastinal emphysema, large pleural effusion, diaphragmatic hernia, chest wall deformities, or significant lung bullae.
-
Patients with severe respiratory central depression, neuromuscular conduction block diseases, or severe central nervous system lesions.
2.3 Intervention methods
Both groups received invasive mechanical ventilation, lung recruitment maneuvers, and sedation to control spontaneous breathing and prevent its impact on measured parameters. Muscle relaxants were administered when necessary.
The control group underwent PEEP titration based on tidal volume. A volume-controlled mode was used, with a tidal volume of 6 mL/kg, flow rate of 30–60 L/min, frequency of 16–20 breaths/min, constant flow rate, inspiratory-to-expiratory ratio of 1:1 to 1:1.5, and a plateau pressure ≤ 30–35 cmH2O. PEEP was adjusted to maintain oxygen saturation (SaO2) at or above 90%, taking into account blood pressure.
Selection of PEEP [11]:
-
1.
Initial setting: The initial PEEP is typically set at a lower level, such as 3–5 cmH2O, and subsequently adjusted gradually based on the patient’s response and physiological indicators.
-
2.
Goals and adjustments: The goal is to maintain alveolar recruitment at end-expiration, reducing the risk of alveolar collapse and atelectasis. Adjustments to PEEP should be based on parameters such as PaO2, SaO2, lung compliance, and chest wall compliance.
-
3.
Monitoring and feedback: Close monitoring of gas exchange, respiratory mechanics, hemodynamics, oxygenation, and ventilation status is necessary during PEEP adjustments. This feedback information assists physicians in determining the optimal PEEP level.
Selection of tidal volume [11]:
-
1.
Protective lung ventilation principles: Implementing a protective lung ventilation strategy is crucial for ARDS patients, aiming to use lower tidal volumes to avoid alveolar overdistension and barotrauma.
-
2.
Individualized settings: Tidal volume selection should be based on factors such as patient weight, lung compliance, and chest wall compliance. Generally, tidal volume is set at 6–8 ml/kg of predicted body weight.
-
3.
Monitoring and adjustments: Close monitoring of respiratory mechanics and gas exchange changes is necessary during mechanical ventilation. Setting tidal volume too low may result in inadequate ventilation and acidosis, while setting it too high may increase the risk of barotrauma. Therefore, timely adjustments to tidal volume should be made based on the patient’s response and physiological indicators.
The study group underwent PEEP titration based on transpulmonary driving pressure. Esophageal pressure was measured as a surrogate for pleural pressure using an esophageal pressure measurement catheter connected to the ventilator. Inspiratory and expiratory esophageal pressures were continuously monitored. The ventilator provided measurements of tidal volume, flow rate, and airway peak pressure. Inspiratory transpulmonary pressure was calculated as the difference between inspiratory airway plateau pressure and inspiratory esophageal pressure, while expiratory transpulmonary pressure was calculated as the difference between expiratory airway plateau pressure and expiratory esophageal pressure. Transpulmonary driving pressure was determined by subtracting the end-expiratory transpulmonary pressure from the end-inspiratory transpulmonary pressure. Tidal volume and PEEP were adjusted based on the observed end-inspiratory and end-expiratory transpulmonary pressures, aiming to maintain a transpulmonary driving pressure below 15 cmH2O during mechanical ventilation. Adjustments were made 2–4 times per day. Transpulmonary driving pressure serves as a more precise indicator of the pressure gradient experienced by the alveoli between the end-inspiration and end-expiration phases during the attainment of target tidal volume. The selection of end-inspiratory transpulmonary pressure as the foundation for calculating transpulmonary driving pressure is primarily grounded in its capacity to reflect the lung’s expansion status throughout the inspiratory phase. As inspired gas infiltrates the lung, a gradual expansion takes place, accompanied by a progressive rise in alveolar pressure. Consequently, end-inspiratory transpulmonary pressure effectively captures the pressure milieu when the lung attains its maximum degree of expansion [11].
2.4 Outcome measures
2.4.1 Baseline characteristics
Baseline characteristics included gender, age, body mass index, pre-ICU hospital stay, etiology of ARDS, Acute Physiology and Chronic Health Evaluation (APACHE) II score, and Murray Acute Lung Injury score. These parameters were collected at the beginning of the study.
2.4.2 Pulmonary function parameters
Pulmonary function parameters, including the oxygenation index (OI), arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), pulmonary shunt fraction (Qs/Qt), and dead space in the respiratory tract (Vd/Vt) were measured 48 h after initiation of mechanical ventilation. The OI, calculated as the ratio of PaO2 to FiO2, normally ranges from 400 to 500 mmHg. An OI below 300 mmHg indicates lung dysfunction. PaO2 and PaCO2 represent the levels of oxygen and carbon dioxide dissolved in the blood, respectively.
2.4.3 Hemodynamic parameters
Heart rate, mean arterial pressure, and central venous pressure were measured 48 h after initiation of mechanical ventilation.
2.4.4 Prognostic measures
Prognostic measures included 28-day mortality, duration of mechanical ventilation, length of hospital stay, and incidence of ventilator-associated pneumonia (VAP). All patients were followed up for 60 days after discharge to record their survival status.
2.5 Statistical methods
Data analysis was performed using IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as mean ± standard deviation (SD) or as median (interquartile range) depending on their distribution. Categorical variables were reported as frequencies and percentages. Baseline characteristics and demographic data were compared between the control group and the study group using independent t-tests for continuous variables and chi-square tests for categorical variables. Independent t-tests were also employed to compare the primary outcomes, including pulmonary function parameters and hemodynamic parameters, between the two groups. The secondary outcomes, such as 28-day mortality, duration of mechanical ventilation, length of hospital stay, and incidence of ventilator-associated pneumonia (VAP), were compared using chi-square tests or Fisher’s exact tests, as appropriate. Survival analysis was conducted using the Kaplan-Meier method, and the log-rank test was utilized to compare the survival curves between the control group and the study group. Cox regression analysis was performed to identify independent predictors of mortality. Statistical significance was set at a p-value less than 0.05. In handling missing data, this study takes into account the proportion of missing data and the characteristics of the data. Considering this factor during sample size calculation, the study can employ the method of complete case analysis, also known as simple deletion method.
3 Results
3.1 Sample size
Based on the preliminary clinical findings, this study considers the effect size (Δ) of the new intervention compared to the existing treatment to be 10%. Assuming a probability (P0) of adverse events occurring in patients under the existing treatment is 25%, which can be estimated from historical data. The significance level (α) is set at 0.05 (i.e., a 5% probability). A desired level of statistical power (1-β) is set at 80% or above to ensure the reliability of the study results.
Based on these parameters, the required sample size can be calculated using the formula: n=[(Zα/2 + Zβ)²×2×P0 × (1-P0)]/(Δ²), where n represents the required sample size. This formula takes into account the expected effect size difference, event occurrence probability, significance level, and statistical power, providing a reasonably accurate estimation of the required sample size.
However, in practical implementation, it may be necessary to make appropriate adjustments to the sample size. For example, considering potential loss to follow-up, data incompleteness, or non-compliance with analysis criteria, it may be necessary to increase the sample size. Additionally, to balance the sample sizes between the experimental and control groups, slight adjustments to the calculated sample size can be made. Obtaining a sample size of 100–120 patients would be appropriate.
3.2 Baseline characteristics
The general characteristics of the two patient groups were collected and compared, as shown in Table 1. In the control group, the mean age was (55.55 ± 10.51) years, with 33 females and 18 males. The average body mass index (BMI) was (22.50 ± 2.88) kg/m2, and the pre-ICU hospital stay was (32.56 ± 9.89) hours. Among the patients in the control group, 30 had pulmonary-related causes of ARDS, while 21 had non-pulmonary causes. The mean APACHE II score was (19.08 ± 4.67), and the mean Murray Acute Lung Injury score was (4.31 ± 0.94). In the study group, the mean age was (57.33 ± 12.21) years, with 29 females and 25 males. The average BMI was (21.84 ± 2.83) kg/m2, and the pre-ICU hospital stay was (33.42 ± 10.75) hours. Among the patients in the study group, 38 had pulmonary-related causes of ARDS, while 16 had non-pulmonary causes. The mean APACHE II score was (20.23 ± 5.00), and the mean Murray Acute Lung Injury score was (4.45 ± 0.88). They presented a homogeneous profile (all P > 0.05).
3.3 Comparison of pulmonary function parameters
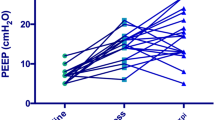
The PEEP and Vd/Vt of the two groups were significantly lower than those of the control group, and the difference was statistically significant (all P < 0.05). (Table 2)
Comparison of pulmonary function parameters before and 48 h after intervention in the two patient groups revealed no significant differences in PaO2, PaCO2, and OI before intervention (all P > 0.05). However, after intervention, both groups showed a significant increase in PaO2, PaCO2, and OI compared to before intervention, with the study group demonstrating significantly higher PaO2 and OI than the control group, with statistically significant differences (all P < 0.05). (Table 3)
3.4 Hemodynamic parameters
Comparison of hemodynamic parameters after 48 h of intervention in the two patient groups showed that the mean arterial pressure (MAP) in the control group was (82.36 ± 14.02) mmHg, central venous pressure (CVP) was (10.36 ± 1.68) mmHg, and heart rate was (102.03 ± 18.29) beats/min. In the study group, the MAP was (80.14 ± 12.39) mmHg, CVP was (11.21 ± 1.93) mmHg, and heart rate was (97.26 ± 20.36) beats/min. There were no significant differences in hemodynamic parameters between the two groups after intervention (all P > 0.05). (Table 4)
3.5 Prognostic analysis
Comparison of 28-day mortality, duration of mechanical ventilation, length of hospital stay, and incidence of VAP between the two patient groups revealed that in the control group, 23 patients died within 28 days, with a total of 16 cases of VAP. The duration of mechanical ventilation was (15.36 ± 3.39) days, and the length of hospital stay was (29.26 ± 5.92) days. In the study group, 15 patients died within 28 days, with a total of 14 cases of VAP. The duration of mechanical ventilation was (11.23 ± 3.17) days, and the length of hospital stay was (24.73 ± 4.85) days. The study group showed a significantly shorter duration of mechanical ventilation and hospital stay compared to the control group, but there were no significant differences in 28-day mortality and VAP incidence. (Table 5)
To further analyze the differences in prognosis between the two intervention methods, all patients were followed up after discharge, with a follow-up period of 60 days from enrollment, and their survival status was recorded. Kaplan-Meier curve analysis showed that the study group had a significantly better survival outcome than the control group (HR = 0.565, 95% CI: 0.320–0.999). The results are shown in Fig. 1.
Among the 20 patients who died in the study group, 6 died of respiratory failure, 10 died of multi-organ failure, and 4 died of sepsis. Of the 28 patients who died in the control group, 9 died of respiratory failure, 11 died of multi-organ failure, 8 died of sepsis.
4 Discussion
The findings of this study demonstrate that a lung-protective ventilation strategy guided by transpulmonary driving pressure significantly improves respiratory function, reduces the duration of mechanical ventilation, and shortens hospital stay for ARDS patients. The selected time point for measurement in this study is 48 h after the initiation of mechanical ventilation, as this period is characterized by significant changes in pulmonary function parameters:
-
1.
Improved oxygenation: Positive pressure mechanical ventilation assists in regulating the fraction of inspired oxygen and positive end-expiratory pressure, which helps reduce pulmonary edema, increase lung volumes, and thereby enhance oxygenation. This can lead to an increase in PaO2 and a potential decrease in PaCO2, reflecting improved gas exchange efficiency [17].
-
2.
Reduced oxygen consumption and respiratory work: Mechanical ventilation can improve alveolar ventilation, increase functional residual capacity, and consequently decrease oxygen consumption and respiratory workload. This helps alleviate respiratory muscle fatigue, reduce respiratory rate and depth, and enable more stable and effective breathing.
-
3.
Cardiac function impact: Mechanical ventilation may lead to a reduction in venous return, subsequently decreasing cardiac output. This effect can be more pronounced in patients with congestive heart failure, as mechanical ventilation can alleviate the cardiac burden by reducing pulmonary circulation and right ventricular afterload.
-
4.
Risks of lung injury and barotrauma: Mechanical ventilation also carries inherent risks, such as the potential for lung injury and barotrauma. These injuries can impact pulmonary function parameters, such as decreased lung compliance and impaired oxygenation capacity [18].
Although there was no significant improvement in the 28-day mortality rate, there was a notable enhancement in survival at 60 days. Transpulmonary driving pressure represents the ratio between tidal volume and respiratory system compliance, which is closely related to ventilated lung volume [19]. It has been shown that transpulmonary driving pressure is a more informative predictor of clinical outcomes associated with lung-protective mechanical ventilation than tidal volume alone. Large-scale registry studies involving non-cardiac thoracic surgical patients under general anesthesia and mechanical ventilation have demonstrated a continuous and dose-dependent relationship between transpulmonary driving pressure and major postoperative pulmonary complications, including pneumonia, pulmonary edema, reintubation, and ARDS [20].
Mechanical ventilation is the primary intervention for patients with ARDS, but its application is limited due to the risk of lung injury [17]. Recent literature has highlighted the importance of lung-protective strategies in mechanical ventilation research, with a focus on tidal volume and PEEP settings. Evidences are continuously emerging in the realm of acute respiratory distress syndrome (ARDS) and surgical patients, shedding light on the pivotal role played by transpulmonary driving pressure in determining the associated benefits [18]. Transpulmonary driving pressure-titrated positive end-expiratory pressure (PEEP) is a more precise therapeutic approach. By titrating the PEEP level, clinicians can individualize the optimal PEEP setting based on the patient’s specific condition and clinical needs [21]. This helps avoid the pitfalls of setting PEEP too low, which may result in incomplete alveolar recruitment and persistent hypoxia, as well as the potential complications of excessive PEEP, such as alveolar overdistension, increased intrathoracic pressure, impaired venous return, and decreased cardiac output.
Clinical studies have demonstrated that using transpulmonary pressure as a guide to set the optimal PEEP during mechanical ventilation for ARDS patients can improve respiratory dynamic parameters and enhance treatment outcomes [22]. This provides a robust clinical basis for the use of transpulmonary driving pressure-titrated PEEP in the management of ARDS.
Selecting transpulmonary driving pressure-titrated PEEP as the treatment approach for ARDS patients is well-grounded in physiological principles and supported by both clinical practice and research evidence, making it an effective and safe therapeutic modality. However, in real-world application, individualized settings and adjustments by experienced clinicians or respiratory therapists are still necessary, based on the patient’s specific circumstances and clinical requirements.
Moreover, a comprehensive meta-analysis encompassing multiple randomized controlled trials examining protective ventilation strategies during general anesthesia has revealed a distinctive finding: transpulmonary driving pressure emerges as the sole ventilatory parameter significantly correlated with a heightened risk of postoperative pulmonary complications [21]. By quantifying the mechanical load imposed on the lungs during ventilation, transpulmonary driving pressure provides a tangible measure of the stress endured by the lung parenchyma, thereby offering valuable insights into the pathophysiological mechanisms underlying pulmonary complications. Consequently, it provides a more accurate assessment of lung stress and the risk of lung injury. In ARDS patients, optimizing PEEP settings based on end-expiratory transpulmonary pressure ensures positive pressure is maintained at the end of expiration to prevent alveolar collapse [21]. By eliminating the influence of extrapulmonary factors, transpulmonary driving pressure reflects changes in lung pressure more precisely. It represents the distending force applied solely to the lungs and serves as a reliable indicator of lung injury during protective ventilation [14]. A study conducted by Dianti et al. revealed that PEEP titration based on transpulmonary driving pressure effectively protects the lungs and diaphragm in the majority of patients with acute hypoxemic respiratory failure [22]. Likewise, in a recent prospective, randomized, crossover physiological study, transpulmonary driving pressure was assessed in recovering ARDS patients under different levels of inspiratory effort, establishing a significant correlation between inspiratory effort and transpulmonary driving pressure [23].
The primary function of PEEP is to improve the patient’s residual volume, mitigate lung injury, and reduce the risk of pulmonary edema. However, increasing PEEP can also lead to adverse effects, such as reduced cardiac output and decreased venous return. Therefore, in experimental settings, it is crucial to carefully monitor and record the impact of PEEP elevation on intrathoracic pressures and cardiovascular function.
Lung elasticity and compliance are key factors that influence the efficacy of PEEP. Lung elasticity determines the degree of resistance to external forces, while compliance reflects the lung tissue’s ability to expand and contract in response to pressure changes. When PEEP is increased, if lung elasticity and compliance remain unchanged, the rise in intrathoracic pressure will be more pronounced. This may result in alveolar overdistension, increasing the risk of barotrauma and potentially leading to alveolar rupture and hemorrhage [24].
To control and account for this factor, researchers can implement the following measures: Prior to the study, assess the research subjects’ lung function and compliance to ensure they are within the normal range. For patients with impaired lung function or reduced compliance, PEEP levels should be adjusted with caution to avoid pressure-induced lung injury. During the study, use precise monitoring equipment to continuously track changes in intrathoracic pressures and cardiovascular function [23]. This helps to promptly identify any abnormal responses and implement appropriate interventions. Set the appropriate PEEP level and adjust it in a timely manner based on the subjects’ reactions [25], which can be achieved by gradually increasing PEEP and observing the changes in intrathoracic pressures and cardiovascular function.
When studying and applying PEEP, it is crucial to thoroughly consider the impact of lung elasticity and compliance on pressure dynamics and take appropriate measures to control this factor. This helps to ensure the accuracy of experimental results and mitigate potential risks and complications.
However, it is worth noting that the measurement of transpulmonary driving pressure using esophageal catheters is time-consuming and presents challenges for routine application in anesthesia practice.
Limitations however should be acknowledged.
-
1.
Sample size and study design: The study had a relatively small sample size. A larger sample size would provide more robust results and enhance the generalizability of the findings. Additionally, the study design was randomized, but it would be beneficial to consider a multicenter, prospective study design to further validate the results.
-
2.
Generalizability: This study was conducted in a specific ICU setting, and the results may not be directly applicable to other clinical settings or patient populations. The research results did not stratify the analysis based on the severity of ARDS. Patients with different stages of ARDS severity may have varying needs and responses to treatment measures such as PEEP. The lack of stratification analysis could mean that the research findings may not accurately reflect the actual conditions of ARDS patients with different degrees of severity. When formulating treatment strategies, it is crucial to tailor them to best meet the individual patient’s needs. Going forward, future studies should incorporate more rigorous stratification analysis of ARDS patients based on the severity of their condition.
-
3.
Outcome measures: The study primarily focused on short-term outcomes such as PaO2, PaCO2, OI, mechanical ventilation time, and length of hospital stay. Future studies should consider evaluating long-term outcomes, such as quality of life, functional status, and mortality rates beyond the 28-day follow-up period, to provide a more comprehensive assessment of the intervention’s effects.
Further investigation is needed to elucidate the underlying mechanisms by which transpulmonary driving pressure titrated PEEP exerts its lung-protective effects. This can involve exploring molecular and cellular pathways, as well as conducting animal studies or in vitro experiments. In addition, future research should focus on evaluating the feasibility and practicality of implementing transpulmonary driving pressure titrated PEEP in different clinical settings. This can involve assessing the training requirements for healthcare providers, evaluating cost-effectiveness, and considering potential barriers to widespread adoption of the intervention.
5 Conclusion
Lung-protective mechanical ventilation guided by PEEP titration based on transpulmonary driving pressure yields significant improvements in lung function, reduces the duration of mechanical ventilation, shortens hospital stay, and contributes to enhanced survival outcomes. Nevertheless, further efforts are required to promote its widespread adoption and implementation.
Data availability
No datasets were generated or analysed during the current study.
References
Yıldırım F, Karaman İ, Kaya A. Current situation in ARDS in the light of recent studies: classification, epidemiology and pharmacotherapeutics. Tuberk Toraks. 2021;69:535–46.
Radermacher P, Maggiore SM, Mercat A. Fifty years of Research in ARDS. Gas Exchange in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2017;196:964–84.
Yadav H, Thompson BT, Gajic O. Fifty years of Research in ARDS. Is Acute Respiratory Distress Syndrome a Preventable Disease? Am J Respir Crit Care Med. 2017;195:725–36.
Gorman EA, O’Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet. 2022;400:1157–70.
Karageorgos V, Proklou A, Vaporidi K. Lung and diaphragm protective ventilation: a synthesis of recent data. Expert Rev Respir Med. 2022;16:375–90.
Baedorf Kassis EN, Bastos AB, Schaefer MS, Capers K, Hoenig B, Banner-Goodspeed V, Talmor D. Adaptive support ventilation and Lung-Protective Ventilation in ARDS. Respir Care. 2022;67:1542–50.
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8.
Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013; 2013: Cd003844.
Dianti J, Matelski J, Tisminetzky M, Walkey AJ, Munshi L, Del Sorbo L, Fan E, Costa EL, Hodgson CL, Brochard L, Goligher EC. Comparing the effects of Tidal volume, driving pressure, and Mechanical Power on Mortality in trials of lung-protective mechanical ventilation. Respir Care. 2021;66:221–7.
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–73.
Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49:727–59.
Grissom CK, Lanspa MJ, Groat D, Jacobs JR, Carpenter L, Kuttler KG, Leither L, Peltan ID, Brown SM, Srivastava R. Implementation of Lung-Protective Ventilation in patients with Acute Respiratory failure. Crit Care Med. 2023;51:797–807.
Rezoagli E, Laffey JG, Bellani G. Monitoring lung Injury Severity and Ventilation Intensity during mechanical ventilation. Semin Respir Crit Care Med. 2022;43:346–68.
Placenti A, Fratebianchi F. Interpretation and use of intraoperative protective ventilation parameters: a scoping review. Anaesthesiol Intensive Ther. 2022;54:320–33.
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–55.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33.
Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of Lung Injury in Acute Respiratory failure. Am J Respir Crit Care Med. 2017;195:438–42.
Paudel R, Trinkle CA, Waters CM, Robinson LE, Cassity E, Sturgill JL, Broaddus R, Morris PE. Mechanical power: a New Concept in Mechanical Ventilation. Am J Med Sci. 2021;362:537–45.
Pérez J, Dorado JH, Accoce M, Plotnikow GA. Airway and Transpulmonary driving pressure by end-inspiratory holds during pressure support ventilation. Respir Care. 2023;68:1483–92.
Williams EC, Motta-Ribeiro GC, Vidal Melo MF. Driving pressure and Transpulmonary pressure: how do we Guide Safe Mechanical. Ventilation? Anesthesiology. 2019;131:155–63.
Dianti J, Fard S, Wong J, Chan TCY, Del Sorbo L, Fan E, et al. Strategies for lung- and diaphragm-protective ventilation in acute hypoxemic respiratory failure: a physiological trial. Crit Care. 2022;26:259.
Liu L, Li HL, Lu C, Patel P, Wang D, Beck J, Sinderby C. Estimation of transpulmonary driving pressure during synchronized mechanical ventilation using a single lower assist maneuver (LAM) in rabbits: a comparison to measurements made with an esophageal balloon. Crit Care. 2023;27:325.
Bertoni M, Telias I, Urner M, Long M, Del Sorbo L, Fan E, Sinderby C, Beck J, Liu L, Qiu H, Wong J, Slutsky AS, Ferguson ND, Brochard LJ, Goligher EC. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit Care. 2019;23:346.
Dianti J, Fard S, Wong J, Chan TCY, Del Sorbo L, Fan E, Amato MBP, Granton J, Burry L, Reid WD, Zhang B, Ratano D, Keshavjee S, Slutsky AS, Brochard LJ, Ferguson ND, Goligher EC. Strategies for lung- and diaphragm-protective ventilation in acute hypoxemic respiratory failure: a physiological trial. Crit Care. 2022;26:259.
Wang WZ, Ying LJ, Liu WD, Zhang P, Li SF. Findings of ventilator-measured P0.1 in assessing respiratory drive in patients with severe ARDS. Technol Health Care. 2024;32:719–26.
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
No external funding received to conduct this study.
Author information
Authors and Affiliations
Contributions
Jian Sun, Wei-xi Zhong, Qing Xu, Qi-ming Feng, and Jing Gao conceived the idea and conceptualised the study. Jian Sun, Yan-ping Yang, Min-jie Zhou, Gang Zhao, and Guan-dong Huang, collected the data. Jing Gao, Lei Geng, Min-jie Zhou, and Xiao-guang Zhu analysed the data. Jian Sun, Guan-dong Huang, Lei Geng, Qi-ming Feng, Gang Zhao, and Xiao-guang Zhu drafted the manuscript, then Jing Gao, Wei-xi Zhong, Qing Xu, Qi-ming Feng, and Yan-ping Yang reviewed the manuscript. All authors read and approved the final draft.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted with approval from the Ethics Committee of Shanghai Sixth People’s Hospital. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants or their guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jian Sun and Jing Gao contributed equally to this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, J., Gao, J., Huang, Gd. et al. The impact of a lung-protective ventilation mode using transpulmonary driving pressure titrated positive end-expiratory pressure on the prognosis of patients with acute respiratory distress syndrome. J Clin Monit Comput (2024). https://doi.org/10.1007/s10877-024-01198-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10877-024-01198-3