Abstract
Electrical Impedance Tomography (EIT) is a novel real-time lung imaging technology for personalized ventilation adjustments, indicating promising results in animals and humans. The present study aimed to assess its clinical utility for improved ventilation and oxygenation compared to traditional protocols. Comprehensive electronic database screening was done until 30th November, 2023. Randomized controlled trials, controlled clinical trials, comparative cohort studies, and assessments of EIT-guided PEEP titration and conventional methods in adult ARDS patients regarding outcome, ventilatory parameters, and P/F ratio were included. Our search retrieved five controlled cohort studies and two RCTs with 515 patients and overall reduced risk of mortality [RR = 0.68; 95% CI: 0.49 to 0.95; I2 = 0%], better dynamic compliance [MD = 3.46; 95% CI: 1.59 to 5.34; I2 = 0%] with no significant difference in PaO2/FiO2 ratio [MD = 6.5; 95%CI -13.86 to 26.76; I2 = 74%]. The required information size except PaO2/FiO2 was achieved for a power of 95% based on the 50% reduction in risk of mortality, 10% improved compliance as the cumulative Z-score of the said outcomes crossed the alpha spending boundary and did not dip below the inner wedge of futility. EIT-guided individualized PEEP titration is a novel modality; further well-designed studies are needed to substantiate its utility.
Highlights
Question: Is the EIT-guided PEEP titration in ARDS universally beneficial and effective?
Findings: This systematic review found better survivability, dynamic compliance, in ARDS patients, as the required information size was achieved for a power of 95%. There was no significant improvement in oxygenation and successful weaning incidence compared to conventional methods. However, the required information size for these contexts is yet to be achieved.
Meaning: EIT-guided PEEP titration in ARDS patients showed promising results and warranted further clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electrical impedance tomography (EIT) has been increasingly utilized in various clinical conditions, including ARDS, acute hypoxemia, [1,2,3,4] general anaesthesia, [5, 6] and postoperative cardiac surgery [7, 8].
It is an efficient, non-invasive,radiation-free, real-time bedside lung imaging technology to identify atelectasis, overdistension, and recruitment. It reconstructs impedance changes through the application of imperceptible currents and measurement of changes in voltage around the thorax, thereby allowing for individualized PEEP titration. It assesses regional respiratory system compliance (Crs) changes by identifying cyclic opening & closing, regional overdistension, and early lung recruitment changes. EIT-derived regional ventilation delay inhomogeneity (SDRVD) correlates with alveolar cycling, offering a potential tool for adjusting ventilation settings. Its ability to estimate ventilation and perfusion depending upon impedance variations and real-time assessment of regional Crs changes, identifying atelectrauma and overdistension, makes it a comprehensive tool [9,10,11,12].
Mechanical ventilation is vital in acute respiratory distress syndrome (ARDS), which has morphological features such as regional atelectasis, overdistension, and lung inhomogeneities. Cyclic opening and closing of lung units pose risks like ventilator-induced lung injury (VILI) [13,14,15].
The stress–strain concept quantifies the harm caused by high lung volumes. Adjusting positive end-expiratory pressure (PEEP) based on global Crs aims to balance overdistension and cyclic opening/closing but lacks consistent benefits, possibly because global Crs inadequately predicts recruitability, especially in atelectasis dynamics [16,17,18,19].
Studies demonstrate that EIT-guided positive end-expiratory pressure (PEEP) titration is valuable for optimizing PEEP in animals and humans [20,21,22]. However, the question remains whether EIT-guided PEEP titration can enhance ventilation and optimize global oxygenation, which requires further investigation in this specific clinical context.
Thus, the current study aimed to assess the clinical utility of EIT-guided PEEP titration compared to traditional protocols in terms of better ventilation and oxygenation in adherence to the "Preferred reporting items of systematic review and meta-analysis" (PRISMA) statement [23].
2 Methods
2.1 Literature search
The comprehensive search spanned various electronic databases (PubMed, Medline, and Embase), Google Scholar (https://scholar.google.com), preprint platforms MedRxiv (https://www.medrxiv.org), and Clinical trial database (https://ClinicalTrials.gov) until November 30, 2023, using the following keywords "EIT" OR " Electrical Impedance Tomography" AND " PEEP" OR " Positive End-Expiratory Pressure" AND " Acute Respiratory Distress Syndrome" OR "ARDS" OR "General Anesthesia" OR "Surgery". Using the above MeSH terminology, all the articles were screened & reviewed by BY and SS. Disagreements were sorted out by taking PK's opinions.
We adopted the PICO format for structuring the findings, where "P" denoted the population (Adults requiring mechanical ventilation due to ARDS), "I" referred to interventions (EIT-guided PEEP titration), "C" pertained to comparisons (conventional treatment), and "O" encompassed outcomes (P/F ratio, driving pressure, PEEP optimization, successful weaning).
2.2 Inclusion and exclusion criteria
Randomized controlled trials, controlled clinical trials, prospective and retrospective comparative cohort studies, case-control studies, comparing the clinical utility of EIT-guided PEEP titration with conventional management in patients with ARDS were included.
Case reports, narrative reviews, expert opinions, studies other than those in English, without appropriate control groups, were excluded.
2.3 Study selection and data extraction
SS and BY conducted independent screenings of abstracts to remove duplication and eliminate irrelevant articles. Eligible studies underwent full-text screening for inclusion criteria. A pre-designed data extraction sheet facilitated information collection such as the first author, publication year, study nature, country, patient count, P/F ratio, driving pressure, optimized PEEP, and successful weaning. Discrepancies were resolved through discussions with PK.
2.4 Risk of bias assessment
SS & PK independently determined any potential bias in the included RCTs using the “RoB 2.0″ [24] assessment tool, and non-randomized trials using the "Risk of Bias in Non-randomized Studies—of Interventions (ROBINS-I)” [25]. The disagreements were settled by discussing with MB.
2.5 Quality of the evidence
PK and SS evaluated each outcome individually as either "High" or "Moderate" or "Low" or "Very low" with the "Grading of Recommendations Assessment, Development and Evaluation (GRADE)" [26, 27] tool, comprising five downgrading factors: "study limitations, indirectness, imprecision, consistency of effect, and publication bias" and three upgrading factors: "dose-response relation, large magnitude of the effect, and plausible confounders or biases", and disagreements were resolved by MB.
2.6 Data synthesis
The meta-analysis was carried out by SS using Review Manager Software (RevMan V.5.4.1) & Trial sequential analysis (TSA) Copenhagen Trial Unit (Version 9.5.10 Beta, Copenhagen, Denmark) [28], The mean difference was utilized as the outcome measure for continuous variables & the log odds ratio for dichotomous variables. Outcome heterogeneity (tau2) was estimated using I2 statistic. Mild heterogeneity was considered for I2 < 30%, moderate for I2 = 30% to 70%, and significant for I2 > 70%
Sensitivity analysis was conducted by removing one study at a time, noting effects on outcome heterogeneity. Funnel plot was used to assess publication bias.
3 Results
3.1 Basic characteristics
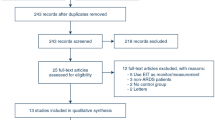
This review comprised five controlled cohort studies [29, 30, 33,34,35] and two randomized controlled trials, [31, 32] out of 341 screened publications. (Fig. 1) While three studies [29, 32, 35] included severe ARDS patients, two of them [30, 33] included mild, moderate, and severe ARDS, and one [31] had moderate and severe ARDS patients, as per Berlin definition. One of the studies included chronic obstructive pulmonary disease (COPD) [33], and the other one incorporated coronavirus disease (COVID-19) [34]-related ARDS patients.
While one study described 49% of patients required prone positioning, [30] in two studies, the prevalence was 2–4% [31, 35] in the EIT group compared to 41%, 0–3% in the control group. Two studies describe the use of inhaled nitric oxide in 54% and 66% of the patients who received EIT-guided PEEP optimization and 51.1% and 96.8% in the control group. 33.3% to 38.1% of patients in the EIT group received ECMO compared to 11.1 to 16.1% of patients in the control [31, 35].
Prevalence of tracheotomized patients ranges from 6- 11% in the control group and 14–28% in patients with EIT guided PEEP optimization group. The protocol for EIT application also varied across the studies (Table 1).
None of them had any serious concerns about the risk of bias (Fig. 2).
3.2 Meta-analysis
3.2.1 Mortality
The mortality risk was lower with EIT-guided PEEP optimization in five studies with 298 patients [Risk ratio (RR) = 0.68; 95% CI: 0.49 to 0.95; I2 = 0%; p = 0.02). (Fig. 3a).
Trial sequential analysis: The required information size was estimated at 276 for a power of 95% based on the 50% relative risk reduction for mortality. The cumulative Z-score crossed the alpha spending boundary and did not dip below the inner wedge of futility, indicating a significant effect size has been achieved. (Supplementary Fig. 1).
3.2.2 Dynamic compliance
A meta-analysis of seven studies involving 515 patients found better dynamic compliance with EIT-guided PEEP optimization by an average of 3.46 ml/cm H2O (95% CI: 1.59 to 5.34; I2 = 0%; p < 0.003) compared to the conventional methods. (Fig. 3b).
Trial sequential analysis: The required information size was estimated at 510 for a power of 95% based on the 10% improved dynamic compliance. The cumulative Z-score crossed the alpha spending boundary and did not dip below the inner wedge of futility, indicating a significant effect size has been achieved. (Supplementary Fig. 2).
3.2.3 Oxygenation
There is no substantial improvement in PaO2/ FiO2 with EIT-guided PEEP optimization compared to conventional methods, [Mean Difference (MD) = 6.5; 95%CI -13.86 to 26.76; I2 = 74%; p = 0.53] found in 515 patients of seven studies (Fig. 4a).
Trial sequential analysis: The required information size was estimated at 879 for a power of 95% based on the observed change of mean PaO2/ FiO2 by at least 10. (Supplementary Fig. 3).
3.2.4 Successful weaning
There is no substantial improvement in successful weaning incidence with EIT-guided PEEP optimization than conventional methods, [Odds Ratio (OR) = 6.5; 95%CI 0.07 to 3.01 to, I2 = 51%; p = 0.32] found in 259 patients of three studies (Fig. 4b).
3.2.5 Driving Pressure
No significant difference in the requirement of driving pressure was found in the groups of 515 patients from seven studies. [MD = -0.3; 95%CI -0.99 to 0.37; I2 = 82%; p = 0.38] (Supplementary Fig. 4a).
3.2.6 Optimized PEEP
No significant difference in the requirement of PEEP was found in the groups across 461 patients in six studies. [MD = 0.95; 95%CI -0.40 to 2.29; I2 = 96%] (Supplementary Fig. 4b).
3.3 Quality of evidence
The evidence regarding the utility of EIT-guided PEEP titration in ARDS patients in terms of mortality and dynamic compliance is of low quality owing to considerable indirectness, and PaO2/FiO2, successful weaning, driving pressure, optimized PEEP, is of very low quality additionally due to inconsistency, imprecision. (Table 2).
3.4 Publication bias
An assessment of publication bias regarding dynamic compliance suggests its unlikelihood, as indicated by the absence of Funnel plot asymmetry through the Egger's Regression and Begg & Mazumdar Rank Correlation (p = 0.98 and p = 0.56, respectively). (Supplementary Fig. 5).
4 Discussion
This systematic review found reduced risk of mortality, better dynamic compliance with no significant improvement in oxygenation and successful weaning with EIT-guide PEEP titration in ARDS patients than conventional methods.
A recent systematic review of 202 participants found a higher PaO2/FiO2 ratio [standardized mean difference (SMD) = 0.636; 95% CI 0.364 to 0.908.] with no significant change in compliance compared to alternative PEEP titration strategies [SMD = -0.085; 95% CI -0.342 to 0.172.]. However, it needed to assess the generalizability of the findings and the adequate size of the population for identifying the effect with optimum power calculation [36].
An impressive 94% positive predictive value for better oxygenation during prone ventilation was reported using electrical impedance tomography to monitor collapse in dependent lung areas [37].
Another multicentric study on COVID-19 patients reported that median EIT-based PEEP varied across groups: 10, 13.5, and 15.5 cm H2O for low, medium, and high recruit ability, respectively (P < 0.05) [38].
One of the reasons behind the conflicting outcome regarding oxygenation in our study is multifactorial. Primarily, the disease severity of included patients varied from mild to severe ARDS along with COPD and COVID-19. The use of the prone position was also not uniform and standardized. This itself can influence a wide variety of primary outcomes. ARDS is an acute hypoxemic state caused by the sudden development of diffuse injury to the terminal respiratory units with exudative pulmonary oedema. The application of PEEP to recruit the collapsed alveoli is crucial. However, determining the optimal PEEP striking a balance between preventing over-distension and de-recruitment while avoiding hemodynamic compromise remains a complex task.
Thirdly, the duration of EIT intervention was also variable, ranging from a few hours to two days. Frequent EIT-guided PEEP titration was not done, unlike ARDS net tables, where PEEP was variable according to the FiO2. Moreover, application EIT-guided PEEP titration was based on finding the intercept point between the overall collapse and distension curves, and the selection of this point was subjective, which can be a source of PEEP discrepancy. At the same time, the control groups included either the ARDS net tables or pressure-volume loops (by calculating the maximal hysteresis/setting the PEEP above the lower inflexion point).
Another critical issue is the difference in the operator experience. EIT is a complex technique compared to other techniques for setting optimal PEEP. Secondly, the position of the EIT belt affects its measurement. The impedance changes are measured in a lens-shaped slice of the thorax. As a result, it cannot visualize the ventilation distribution of the whole lung, which is a major drawback. The PEEP level with the best regional compliance differs for cranial and caudal lung regions in mechanically ventilated patients. While performing a decremental PEEP trial to find the optimal PEEP in the caudal lung regions, the diaphragm can enter the measurement field, producing artefacts and causing erroneous results [39].
Various methods are available for titrating PEEP through EIT, which include the overexpansion and collapse (OD/CL) method, end-expiratory lung impedance (EELI) method, GI index method and regional ventilation delay (RVD) method [33]. However, a consensus on the best EIT-derived parameter has yet been reached.
Zhao et al. demonstrated that the global inhomogeneity (GI) index positively correlated with PEEP adjustment with global dynamic compliance and intra-tidal compliance method [20].
EIT reflects regional changes better, i.e. recruitment of dependent and non-dependent lung areas can be visualized separately, compared to the latter techniques, which do not provide any information on ventilation distribution. For the same reason, overall lung compliance is expected to increase, corresponding to the decline in driving pressures.
Heines et al. concluded that the difference in EIT-based PEEP and clinician set PEEP was clinically relevant in 28% of the patients, whereas the EIT-based PEEP disagreed with the PEEP settings according to the ARDS network [39].
PEEP plays an integral part in the acute phase of ARDS by preventing the collapse of recruited airways, thereby improving oxygenation. It minimizes ventilator-associated lung injury (VALI) in the long term by preventing repeated alveolar collapse/distension cycle (atelectotrauma). As a result of this effect, optimal PEEP can contribute to weaning success by minimizing VALI. The studies included in our review did not demonstrate weaning success by EIT-guided PEEP titration. This can be explained by these studies being underpowered to demonstrate a significant difference.
5 Strengths & limitation
The present study comprises the most considerable population reported so far with the necessary minimum population required size for identifying the effect.
Diverse populations and protocols contribute to significant heterogeneity; some of the studies have retrospective control and are prone to selection bias. Moreover, the minimum population required size was not achieved to identify a relevant change in oxygenation and successful weaning.
6 Conclusion
Electrical impedance tomography-guided individualized lung protective ventilation strategies are required to improve the overall outcome, with further requirements of prospective multicenter randomized control trials to demonstrate its utility.
Data availability
Data openly available in a public repository & derived data is available on request.
References
Liu S, Tan L, Möller K, et al. Identification of regional overdistension, recruitment and cyclic alveolar collapse with electrical impedance tomography in an experimental ARDS model. Crit Care. 2016;20(1):119. Published 2016 May 3. https://doi.org/10.1186/s13054-016-1300-y.
Franchineau G, Bréchot N, Lebreton G, et al. Bedside contribution of electrical impedance tomography to setting positive end-expiratory pressure for extracorporeal membrane oxygenation-treated patients with severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(4):447–57. https://doi.org/10.1164/rccm.201605-1055OC.
Dargaville PA, Rimensberger PC, Frerichs I. Regional tidal ventilation and compliance during a stepwise vital capacity manoeuvre. Intensive Care Med. 2010;36(11):1953–61.
Wolf GK, Gómez-Laberge C, Rettig JS, et al. Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med. 2013;41(5):1296–304. https://doi.org/10.1097/CCM.0b013e3182771516.
Pereira SM, Tucci MR, Morais CCA, et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology. 2018;129(6):1070–81. https://doi.org/10.1097/ALN.0000000000002435.
He X, Jiang J, Liu Y, et al. Electrical impedance tomography-guided PEEP Titration in patients undergoing laparoscopic abdominal surgery. Medicine (Baltimore). 2016;95(14): e3306. https://doi.org/10.1097/MD.0000000000003306.
Krause U, Becker K, Hahn G, Dittmar J, Ruschewski W, Paul T. Monitoring of regional lung ventilation using electrical impedance tomography after cardiac surgery in infants and children. Pediatr Cardiol. 2014;35(6):990–7. https://doi.org/10.1007/s00246-014-0886-6.
Liu K, Huang C, Xu M, et al. PEEP guided by electrical impedance tomography during one-lung ventilation in elderly patients undergoing thoracoscopic surgery. Ann Transl Med. 2019;7(23):757. https://doi.org/10.21037/atm.2019.11.95.
Frerichs I, Amato MB, van Kaam AH, et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax. 2017;72(1):83–93. https://doi.org/10.1136/thoraxjnl-2016-208357.
Costa EL, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35(6):1132–7. https://doi.org/10.1007/s00134-009-1447-y.
Gómez-Laberge C, Arnold JH, Wolf GK. A unified approach for EIT imaging of regional overdistension and atelectasis in acute lung injury. IEEE Trans Med Imaging. 2012;31(3):834–42. https://doi.org/10.1109/TMI.2012.2183641.
Victorino JA, Borges JB, Okamoto VN, et al. Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med. 2004;169(7):791–800. https://doi.org/10.1164/rccm.200301-133OC.
Constantin JM, Grasso S, Chanques G, et al. Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit Care Med. 2010;38(4):1108–17. https://doi.org/10.1097/CCM.0b013e3181d451ec.
Gattinoni L, Carlesso E, Cressoni M. Selecting the “right” positive end-expiratory pressure level. Curr Opin Crit Care. 2015;21(1):50–7. https://doi.org/10.1097/MCC.0000000000000166.
Costa ELV, Slutsky AS, Brochard LJ, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204(3):303–11. https://doi.org/10.1164/rccm.202009-3467OC.
Chiumello D, Carlesso E, Cadringher P, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178(4):346–55. https://doi.org/10.1164/rccm.200710-1589OC.
Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, et al. Effgect of lung recruitment and titrated Positive End-Expiratory Pressure (PEEP) vs low peep on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. https://doi.org/10.1001/jama.2017.14171.
Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775–86. https://doi.org/10.1056/NEJMoa052052.
Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55. https://doi.org/10.1056/NEJMsa1410639.
Zhao Z, Steinmann D, Frerichs I, Guttmann J, Möller K. PEEP titration guided by ventilation homogeneity: a feasibility study using electrical impedance tomography. Crit Care. 2010;14(1):R8. https://doi.org/10.1186/cc8860.
Erlandsson K, Odenstedt H, Lundin S, Stenqvist O. Positive end-expiratory pressure optimization using electric impedance tomography in morbidly obese patients during laparoscopic gastric bypass surgery. Acta Anaesthesiol Scand. 2006;50(7):833–9. https://doi.org/10.1111/j.1399-6576.2006.01079.x.
Luepschen H, Meier T, Grossherr M, Leibecke T, Karsten J, Leonhardt S. Protective ventilation using electrical impedance tomography. Physiol Meas. 2007;28(7):S247–60. https://doi.org/10.1088/0967-3334/28/7/S18.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Published 2021 Mar 29. https://doi.org/10.1136/bmj.n71.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Sterne JAC, Hernán MA, Reeves BC, Savović J, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355: i4919. https://doi.org/10.1136/bmj.i4919.
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94.
Norris SL, Meerpohl JJ, Akl EA, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol. 2016;79:150-158.e1.
Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention research, Copenhagen, Denmark. 2011:1–115. Available from www.ctu.dk/tsa. Accessed 29 Dec 2023.
Becher T, Buchholz V, Hassel D, et al. Individualization of PEEP and tidal volume in ARDS patients with electrical impedance tomography: a pilot feasibility study. Ann Intensive Care. 2021;11(1):89. Published 2021 Jun 2. https://doi.org/10.1186/s13613-021-00877-7.
He H, Chi Y, Yang Y, et al. Early individualized positive end-expiratory pressure guided by electrical impedance tomography in acute respiratory distress syndrome: a randomized controlled clinical trial. Crit Care. 2021;25(1):230. Published 2021 Jun 30. https://doi.org/10.1186/s13054-021-03645-y.
Hsu HJ, Chang HT, Zhao Z, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve: a randomized trial in moderate to severe ARDS. Physiol Meas. 2021;42(1):014002. Published 2021 Feb 6. https://doi.org/10.1088/1361-6579/abd679.
Jimenez JV, Munroe E, Weirauch AJ, et al. Electric impedance tomography-guided PEEP titration reduces mechanical power in ARDS: a randomized crossover pilot trial. Crit Care. 2023;27(1):21. Published 2023 Jan 17. https://doi.org/10.1186/s13054-023-04315-x.
Liu X, Liu X, Meng J, et al. Electrical impedance tomography for titration of positive end-expiratory pressure in acute respiratory distress syndrome patients with chronic obstructive pulmonary disease. Crit Care. 2022;26(1):339. Published 2022 Nov 4. https://doi.org/10.1186/s13054-022-04201-y.
Somhorst P, van der Zee P, Endeman H, Gommers D. PEEP-FiO2 table versus EIT to titrate PEEP in mechanically ventilated patients with COVID-19-related ARDS. Crit Care. 2022;26(1):272. Published 2022 Sep 12. https://doi.org/10.1186/s13054-022-04135-5.
Zhao Z, Chang MY, Chang MY, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):7. Published 2019 Jan 17. https://doi.org/10.1186/s13613-019-0484-0.
Yu M, Deng Y, Cha J, Jiang L, Wang M, Qiao S, Wang C. PEEP titration by EIT strategies for patients with ARDS: a systematic review and meta-analysis. Med Intensiva (Engl Ed). 2023;47(7):383–90. https://doi.org/10.1016/j.medine.2022.06.020.
Cardinale M, Boussen S, Cungi PJ, et al. Lung-dependent areas collapse, monitored by electrical impedance tomography, may predict the oxygenation response to prone ventilation in COVID-19 acute respiratory distress syndrome. Crit Care Med. 2022;50(7):1093–102. https://doi.org/10.1097/CCM.0000000000005487.
Jonkman AH, Alcala GC, Pavlovsky B, et al. Lung Recruitment Assessed by Electrical Impedance Tomography (RECRUIT): a multicenter study of COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. 2023;208(1):25–38. https://doi.org/10.1164/rccm.202212-2300OC.
Heines SJH, Strauch U, van de Poll MCG, Roekaerts PMHJ, Bergmans DCJJ. Clinical implementation of electric impedance tomography in the treatment of ARDS: a single centre experience. J Clin Monit Comput. 2019;33(2):291–300. https://doi.org/10.1007/s10877-018-0164-x.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Dr Soumya Sarkar (SS): Search strategy, Study selection, Data extraction, Data synthesis, Risk of bias assessment, quality of the evidence assessment, Manuscript drafting and editing. Dr Bharat Yalla (BY): Search strategy, Study selection, Data extraction. Dr Puneet Khanna (PK): Conceptualization, study selection, Risk of bias assessment, quality of the evidence assessment and editing. Dr Madhurjya Baishya (MB): Risk of bias assessment, quality of the evidence assessment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional ethical committee approval
Not Applicable.
Prior presentations
Nil.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkar, S., Yalla, B., Khanna, P. et al. Is EIT-guided positive end-expiratory pressure titration for optimizing PEEP in ARDS the white elephant in the room? A systematic review with meta-analysis and trial sequential analysis. J Clin Monit Comput 38, 873–883 (2024). https://doi.org/10.1007/s10877-024-01158-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-024-01158-x