Abstract
During low-flow oxygen therapy, the true value of inspired oxygen fraction (FiO2) is generally unknown. Knowledge of delivered FiO2 values may be useful as well as to adjust oxygen therapy, as well as to predict patient deterioration. This study proposes a New FiO2 Prediction Formula (NFiO2) for low-flow oxygenation and compares its predictive value to precedent formulas. In a bench study, the O2 Flow rate was delivered through a T-piece connected to a dual-compartment artificial lung controlled by a mechanical ventilator. To test the NFiO2 formula, a set of ventilatory parameters were tested: Tidal Volume was set from 400 to 600 ml, Respiratory Rate (RR) was set from 18 to 30 CPM, Ti/Ttot was set at 0.33 and 0.25, and O2 flow rates from 3 to 10 L/min. A data acquisition system measured all parameters. To quantify the accuracy of the NFiO2 compared to other FiO2 prediction formulas, Bland and Altman agreement analyses were performed. To make use of the Duprez Formula 2018 in clinical practice, we simplified the formula to estimate the FiO2 during oxygenation at low flow. This NFiO2 formula makes use of only O2 Flow Rate and RR. Bias and limits of agreement between predicted FiO2 and benchtop FiO2 highlighted consistent differences between different FiO2 prediction formulas. The NFiO2 and the Duprez Formula 2018 seemed to be the most accurate formulas, followed by the Vincent Formula, and lastly the Shapiro Formula. A New FiO2 Prediction Formula was developed using clinical readily available variables (RR and O2 Flow rate) which showed good accuracy in predicting FiO2 during oxygenation at low flow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background
Oxygen therapy is the principal treatment of hypoxemia. Oxygen administration should be closely monitored and adapted to the patient’s clinical conditions during therapy. Knowledge of delivered FiO2 values may be useful as well as to adjust therapy, as well as to predict patient deterioration. During low-flow oxygen therapy (usually administered through a nasal cannula), FiO2 values are generally unknown or difficult to estimate [1]. For many years, to estimate FiO2, various formulas, based on theoretical reasoning, have been proposed. The two mainly used formulas to estimate FiO2 at low flow are the Vincent and Shapiro Formulas [2,3,4,5,6].
Both formulas estimate FiO2 on the amount of delivered O2 Flow only. However, at low-flow, FiO2 will depend on the mixture of inspired air (at 21% of oxygen) by the patient and the delivered O2 Flow by the system [5,6,7]. Therefore, FiO2 will greatly depend on the patient’s breathing pattern [8]. Thus, in 2018, based on a bench study, we proposed a formula to predict FiO2 during oxygenation at low flow (Duprez Formula 2018). This formula considers: O2 Flow, Minute Ventilation (MV) and Ti/Ttot ratio [4]. While encompassing inspiratory flow, Duprez Formula 2018 has been shown to greatly increase FiO2 prediction accuracy compared to former FiO2 prediction formulas. Unfortunately, Duprez Formula 2018 remains of limited clinical use because the value of MV and Ti/Ttot ratio are unknown during low-flow oxygen therapy. Therefore, this study aims:
-
(1)
To simplify the Duprez Formula 2018 in a New Formula that only uses bedside readily available variables (i.e. RR and LPMO2).
-
(2)
To test the predictive performance of this new formula experimentally.
-
(3)
To compare the accuracy of this new formula against the precedent formulas.
2 Methods
2.1 Part 1: theoretical simplification of the Duprez Formula 2018
The original Duprez Formula 2018 is equal to:
Ti—Inspiratory time (sec), Ttot—Total breathing cycle (sec), LPM O2: Oxygen Flow Rate (L/min), MV—Minute Ventilation (RR×Vt), Vt—Tidal Volume (L), RR—Respiratory Rate (CPM).
The first development of the new FiO2 Formula is based on the hypothesis that the Vt of a resting adult varies approximatively between 0.4 and 0.6 L [9]. We therefore replaced Vt with a mean value of 0.5 L. The Duprez Formula 2018 can be rewritten as:
As the \( \frac{{{\text{Ti}}}}{{{\text{Ttot}}}} \) ratio is unknown in clinical practice, a new constant (k) must be found:
This simplifies the formula as follow:
Since Duprez Formulas 2018 were proposed for two Ti/Ttot (0.25 and 0.33), this constant (k) should vary between ± 0.40 and ± 0.50 respectively, (more easily used as \(\frac1{\text k}\) and thus ± 2.5 to ± 1.9 )
2.2 Part 2: Experimental set-up
(1) To determine a new constant (k) value:
(2) To test the New FiO2 Prediction Formula
The parameters analyzed on bench were:
-
Ti/Ttot: 0.25 and 0.33
-
LPM O2: from 3 to 10 L/min (step: 1 L/min)
-
Vt: 0.4/0.5/0.6 L
-
RR: 18/22/26/30 CPM
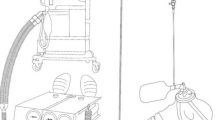
Spontaneous breathing was generated with a mechanical test lung (Dual Test Lung—Michigan Instruments, Inc. Grand Rapids Model 5600i) including two independent experimental lungs. With a special coupling clip, the first lung is used to drive the second lung in order to achieve a breathing simulation (inspiratory and expiratory flow). The settings of the experimental lung were: resistance: ± 5 cm H2O/L/sec and compliance ± 0.06 L/cm H2O. The first lung was connected to a mechanical ventilator Servo-i® set to volume control mode with descending ramp flow waveform (25%), time pause and inspiratory rise time at 0%, peep of 0 cm H2O, the trigger was set at − 15 cm H20 in order to avoid self-triggering. O2 Flow rate from a wall-mounted Thorpe Tube (Air Liquide™ RTM3; 0 to 15 L/min) was delivered through a T piece (with a very small material dead space). The T piece was directly fixed to the flow sensor (Fig. 1). An O2 analyzer was located of the O2 port of the second lung.
The main outcome variable was FiO2 (expressed as the volumetric percentage of O2 in the steady-state experimental lung). FiO2 was measured with a GA-200, O2 Gas Analyzer (Iworx®, United States). The O2 Monitor was calibrated with air room (21%) then at 100% with certified O2 gas. FiO2 was measured as the mean of 10 breaths after a stabilization period of at least one minute.
To test the predictive value of the New FiO2 Prediction Formula (NFiO2) across different breathing patterns and experimental settings, we defined 1080 (3 × 360) experimental set-ups by modifying different parameters: O2 Flow, Vt, Respiratory rate (RR), and the ratio between inspiratory time (Ti) upon the total breathing cycle (Ttot).
Ti/Ttot, Tidal Volume, and RR were measured with a data acquisition system IX-214 (Iworx®, United States) which included an SP-304 flow sensor and a data-acquisition hardware connected to a Software Labscribe 3™ (Iworx®, United States). The flow sensor was calibrated using a 1-liter calibration syringe (Hans Rudolph 5540™—United States) and ambient air. During this step, the gap between the required value and measured value was of maximum ± 30 mL.
O2 Flow was measured continuously with a Thermal O2 Mass Flow Meter (Red Y Vögtlin™ Instruments GmbH, Switzerland: Accuracy ± 1.5% of full scale/Repeatability +/– 0.1% of full scale). Because the accuracy of measurements could become aleatory at very low flow, only the O2 Flows rate from 3 to 10 L/min were measured.
During the experiment, all measurements were performed in triplicate with recalibration on each occasion. Each experiment was blinded for previously obtained results to avoid a Pygmalion effect.
2.3 Part 3: comparison of the NFiO2 accuracy against precedent formulas
The following formulas were analyzed:
Duprez Formula 2018: \({\text{FiO}}_2=0.21+0.79\times\left(\frac{\text{Ti}}{{\text{Ttot}}}\right)\times\left(\frac{{\text{LPMO}}_2}{{\text{MV}}}\right)\)
Vincent Formula: \({\text{FiO}}_2=0.21+\left(0.03\times{\text{LPMO}}_2\right)\)
Shapiro Formula: \({\text{FiO}}_2=0.20+\left(0.04\times{\text{LPMO}}_2\right)\)
2.4 Statistical analysis
Data were analyzed using Sigma Plot Software (Version 14.0 Systat Software Inc, UK). Mean values are expressed with their standard deviation. The repeatability of the Thermal O2 Mass Flow Meter and the SP-304 Flow Sensor was conducted by using the intraclass correlation coefficient (two-way mixed effects, absolute agreement, single rater, multiple measurements) [10].
-
Determination of the new constant (k):
-
Computing the mean value of “k” across the different experimental set-ups.
-
Using the Bland and Altman method [11], (computation of the agreement between the FiO2 calculated by the NFiO2 and the measured FiO2 obtained on the bench) different constant (k) values were tested to predict the most adequate FiO2. The following constants (k) were tested: from 0.40 to 0.55.
-
-
NFiO2 comparison against precedent formulas.
To compare the accuracy of NFiO2, we analyzed the FiO2 obtain for each experimental set-up (n = 360) and compare it with other prediction formulas: Duprez Formula 2018, Vincent and Shapiro Formulas [2, 3].
As such, the bias (measured FiO2—predicted FiO2), the standard deviation (SD), and the Limits of Agreement (LoA) are reported for each prediction formula with their 95% confidence interval.
3 Results
The intraclass correlation coefficient of the O2 mass flow meter and SP-304 were > 0.90 (p < 0.01), respectively. The repeatability of experimental measurements was excellent.
Throughout the different experimental set-ups, the mean constant (k) value was 0.53 (SD 0.10). Therefore, for ease of use, the constant (k) value was rounded up to 0.5 and simplifies the FiO2 prediction formula as follow:
or
Meanwhile, different constants (k) were used and tested (Table 1). The constant k that showed the best agreement between calculated FiO2 and measured FiO2 using a Bland and Altman analysis was 0.53.
Compared to the other formulas, the bias and range of LoA were lowest for the NFiO2 (− 1.33/− 6.95 to 4.29) and Duprez Formula 2018 (− 3.61/− 8.68 to 1.46), followed by the Vincent Formula (4.15/− 4.65 to 12.96), and last by the Shapiro Formula (9.69/− 2.44 to 21.81) (Fig. 2; Table 2).
4 Discussion
At first, we simplified the Duprez Formula 2018 to estimate the FiO2 during oxygenation at low flow using clinical readily available variables i.e. LPMO2 and RR. The former Duprez Formula 2018 did indeed include variables (VM and \(\frac{{\text{Ti}}}{\text{Ttot}}\) ) that are unknown in clinical practice under low flow oxygen therapy and thus preventing its practical use. This experimental bench study could identify a new constant (k = 0.5 or 1/k = 2) value, encompassing unknown respiratory variables, that has allowed to reformulate the former Duprez 2018 formula as follow:
Despite this simplification, this formula did show to be robust even after comparison to the former Duprez Formula 2018 but also in comparison to previously used formulas (Shapiro and Vincent).
In our analysis, consistent differences were shown between prediction formulas. To estimate FiO2 during oxygenation at low flow, the NFiO2 and the Duprez Formula 2018 seemed to be the most accurate formulas, followed by the Vincent Formula, and lastly the Shapiro Formula.
For the Duprez Formula 2018 and NFiO2, if the minute ventilation (and/or RR) increases or Ti/Ttot decreases, then FiO2 decreases, and vice versa [12]. Therefore, following our experiences, the inspiratory flow rate (i.e., minute ventilation divided by Ti/Ttot) and the oxygen flow rate are the main parameters that determine the FiO2 value during oxygenation at low flow rate. Meanwhile, the Vincent and Shapiro Formulas do not include Minute Ventilation and Ti/Ttot ratio in their prediction formulas. This may be the reason why these formulas are less accurate. As for example, if O2 Flow is equal to 6 L/min and the RR is equal to 30 CPM, Shapiro FiO2 estimation, will predict an FiO2 of 44%, and the Vincent Formula will predict an FiO2 of 39%. With the NFiO2, the calculation will give a FiO2 value of 31%, either an absolute FiO2 difference of 13% and 8% between the former formulas and the NFiO2. Sometimes, in emergency situations, for a short period, the oxygen flow of oxygen through traditional nasal cannulas can exceed 6 L/min [13,14,15]. In this case, in patients with relatively high oxygen flow and high RR, the prediction of FiO2 with formula which do not take into account variables related to the Minute Ventilation (such as RR) could lead to an incorrect assessment of the degree of hypoxemia. For example, if the Oxygen flow reach 10 L/min and RR of 30 cpm, the FiO2 calculation with Shapiro Formula reach 60%, with Vincent Formula: 51% and 38% for NFiO2 either an absolute FiO2 difference of 22% and 13%. Nevertheless, when the Vt is around 500 mL and the RR around ± 15 CPM, the Vincent Formula seems adequate to accurately predict the FiO2 at low flow. The application of FiO2 prediction in low oxygenation with the New FiO2 Prediction Formula presented in this bench study should be further studied in clinical practice.
Beyond these considerations, it is of clinical interest to bring to the fore a standardized common denominator of the amount of oxygen a patient receives, such as FiO2. Given known factors affecting FiO2, formulas to determine FiO2 should be defined for each oxygen delivery device and adjusted for oxygen flow and the patient’s breathing pattern [16]. The present work illustrates that even in low-flow oxygen delivery settings, to enhance accuracy, it is important to integrate a breathing parameter, in this case, the RR, to estimate and adjust FiO2. Hence, FiO2 should be more accurate in specifying the degree of hypoxemia and, thus, oxygenation needs of a patient than specifying oxygen flow alone. Using such a unique denominator across different oxygen delivery devices has, in our opinion, different clinical impacts and advantages:
-
It could simplify communication between colleagues (between wards or institutions) regarding one patient’s oxygen needs.
-
It can facilitate and simplify data encoding and processing in electronic health records.
-
It could facilitate research work, such as severity score calculations.
In this era of big data analysis, using an adjusted FiO2 will be more reliable than encoding, processing, and interpreting amounts of oxygen delivered through different devices. Ultimately, it could therefore facilitate automatic electronic severity score calculations through the electronic medical interfaces and software and adjust alarm settings for patients’ safety.
5 Limitations
First, given our experimental design, the New FiO2 Prediction Formula should be limited to an oxygen flow between 3 and 10 L/min (without material dead space), a RR between 18 and 30 CPM, two Ti/Ttot (0.25 and 0.33) and a Vt between 400 mL and 600 mL respectively (either adult conditions). Second, a patient’s tidal volume and inspiratory flow can vary from breath to breath. In this case, the FiO2 for a given breath can sometimes be difficult to predict [17]. Third, the effect of open mouth (or not) on FiO2 has not been evaluated and could result in a variation of FiO2 from previous bench results [6]. Finally, due to the bench study, we did not consider the effect of anatomical dead space on the FiO2. For this reason, it is possible that the FiO2 value itself differs slightly from that of real humans.
6 Conclusion
FiO2 assessed during low flow rate oxygen therapy in a spontaneous breathing lung model and compared to four FiO2 prediction formulas shows larger differences. This bench study highlights, compared to former prediction formulas, that the New FiO2 Prediction Formula for low-flow oxygen therapy, based on RR and oxygen flow rate, shows better accuracy in predicting FiO2.
This bench study highlights that including RR to oxygen flow rate in a FiO2 prediction formula at low flow may enhance accuracy compared to formulas including solely oxygen flow rate. Although many parameters influence FiO2, this study confirms that respiratory frequency influences FiO2 in an inversely proportional manner.
Data availability
On demand.
References
Bazuaye EA, Stone TN, Corris PA, Gibson GJ. Variability of inspired oxygen concentration with nasal cannulas. Thorax. 1992;47(8):609–11.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Shapiro BA, Peruzzi WT, Kozlowski-Templin R. Clinical application of blood gases. St. Louis: Mosby; 1994.
Duprez F, Mashayekhi S, Cuvelier G, Legrand A, Reychler G. A new formula for predicting the fraction of delivered oxygen during low-flow oxygen therapy. Respir Care. 2018;63(12):1528–34.
Markovitz GH, Colthurst J, Storer TW, Cooper CB. Effective inspired oxygen concentration measured via transtracheal and oral gas analysis. Respir Care. 2010;55(4):453–9.
Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005;50(5):604–9.
McCoy R. Oxygen-conserving techniques and devices. Respir Care. 2000;45(1):95–103.
Palmisano JM, Moler FW, Galura C, Gordon M, Custer JR. Influence of tidal volume, respiratory rate, and supplemental oxygen flow on delivered oxygen fraction using a mouth to mask ventilation device. J Emerg Med. 1993;11(6):685–9.
Haddad M, Sharma S. (2023) Physiology, Lung. In: StatPearls. Treasure Island (FL): StatPearls. https://pubmed.ncbi.nlm.nih.gov/31424761/. Accessed 26 Jun 2023.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8.
Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60.
Duprez F, Dupriez F, De Greef J, Gabriel J, Bruyneel A, Reychler G, et al. Performance of different low-flow oxygen delivery systems. Respir Care. 2022;67(3):322–30.
Whittle JS, Pavlov I, Sacchetti AD, Atwood C, Rosenberg MS. Respiratory support for adult patients with COVID-19. J Am Coll Emerg Physicians Open. 2020;1(2):95–101.
Duprez F, Bruyneel A, Vanhelleputte K, Meurant V, Raquet B, Dupriez F, et al. Comparison of the arterial oxygenation between two oxygen masks. Respir Care. 2022. https://doi.org/10.4187/respcare.09898.
Hess D. Respiratory care. Principles and practice. 4th ed. Burlington: Jones and Bartlett; 2021.
Duprez F, de Terwangne C, Bellemans V, et al. High-flow nasal cannula therapy, factors affecting effective inspired oxygen fraction: an experimental adult bench model. J Clin Monit Comput. 2022;36(5):1441–8. https://doi.org/10.1007/s10877-021-00784-z.
Wagstaff TaJ, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia. 2007;62(5):492–503.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
FD: conception experiment+wrote the main manuscript. BM experimentation+wrote the main manuscript. BL: conception of the experiment+wrote the main manuscript. MCM: Corrections and review. JBM: Corrections+wrote the main manuscript. SM: Corrections+conception. CD: Corrections, mathematical approach+wrote the main manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duprez, F., Mol, B., Lesire, B. et al. FiO2 prediction formula during low flow oxygen therapy in an adult model: a bench study. J Clin Monit Comput 38, 455–461 (2024). https://doi.org/10.1007/s10877-023-01109-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-023-01109-y