Abstract
Purpose
The Patient State Index (PSI) is a newly introduced electroencephalogram-based tool for objective and continuous monitoring of sedation levels of patients under general anesthesia. This study investigated the potential correlation between the PSI and the Richmond Agitation‒Sedation Scale (RASS) score in intensive care unit (ICU) patients and established the utility of the PSI in assessing sedation levels.
Methods
In this prospective observational study, PSI values were continuously monitored via SedLine® (Masimo, Irvine, CA, USA); the RASS score was recorded every 2 h for patients on mechanical ventilation. Physicians and nurses were blinded to the PSI values. Overall, 382 PSI and RASS score sets were recorded for 50 patients.
Results
The PSI score correlated positively with RASS scores, and Spearman’s rank correlation coefficient between the PSI and RASS was 0.79 (95% confidence interval [CI]: 0.75‒0.83). The PSI showed statistically significant difference among the RASS scores (Kruskal‒Wallis chi-square test: 242, df = 6, P < 2.2-e16). The PSI threshold for distinguishing light (RASS score ≥ − 2) sedation from deep sedation (RASS score ≤ − 3) was 54 (95% CI: 50–65; area under the curve, 0.92 [95% CI: 0.89‒0.95]; sensitivity, 0.91 [95% CI: 0.86‒0.95]; specificity, 0.81 [95% CI: 0.77–0.86]).
Conclusions
The PSI correlated positively with RASS scores, which represented a widely used tool for assessing sedation levels, and the values were significantly different among RASS scores. Additionally, the PSI had a high sensitivity and specificity for distinguishing light from deep sedation. The PSI could be useful for assessing sedation levels in ICU patients.
University Hospital Medical Information Network (UMIN000035199, December 10, 2018).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sedatives and analgesics are commonly administered to critically ill patients who require mechanical ventilation in an intensive care unit (ICU) [1, 2]. However, insufficient sedation and/or analgesia increases patient stress, whereas inappropriate deep sedation is related to worse clinical outcomes, such as prolonged mechanical ventilatory management and ICU stay [1,2,3,4]. Clinical guidelines recommend the avoidance of deep sedation in ICU patients, and healthcare professionals in the ICU are required to evaluate the sedation levels of patients accurately and to optimize these levels [1].
Currently, the sedation levels in ICU patients are assessed by an observer’s subjective impression, such as by means of the Richmond Agitation‒Sedation Scale (RASS) [5,6,7]. The RASS is a 10-stage scoring system with the lowest possible score of − 5 (unarousable) and the highest possible score of + 4 (combative). The target sedation level for most ICU patients is between RASS − 2 and 0 for stable patients [1]. However, routine assessment with a sedation-level scale can be laborious, and intermittent evaluation using the scale makes it difficult to obtain continuous information on sedation levels in patients [8,9,10] (Table 1).
Procedure
-
1.
Observe patient. Is patient alert and calm (score 0)? Does patient have behavior that is consistent with restlessness or agitation (score + 1 to + 4 using the criteria listed above, under Description)?
-
2.
If patient is not alert, in a loud speaking voice, state the patient’s name and direct the patient to open eyes and look at the speaker. Repeat once if necessary. Can prompt patient to continue looking at the speaker. Patient opens eyes and makes eye contact, which is sustained for more than 10 s (score − 1). Patient opens eyes and makes eye contact, but this is not sustained for 10 s (score − 2). Patient has any movement in response to a voice, but makes no eye contact (score − 3).
-
3.
If patient does not respond to voice, physically stimulate the patient by shaking the shoulder and then rubbing the sternum if there is no response to shaking the shoulder. Patient has any movement to physical stimulation (score − 4). Patient has no response to voice or physical stimulation (score − 5).
The Patient State Index (PSI) is a tool for continuous and objective monitoring of the depth of sedation in the operating room and is considered to be reliable for assessing the sedation level during general anesthesia [11,12,13,14,15,16,17,18,19]. The PSI analyzes the electroencephalogram (EEG), obtained with a sensor attached to the forehead [11, 12]. A PSI value closer to 0 indicates deep sedation, whereas values closer to 100 indicate wakefulness [11]. However, there have been only a few reports on the use of the PSI for monitoring sedation levels in ventilated patients in the ICU, and the assessment of the PSI in the ICU is not consistent [11, 20,21,22]. Furthermore, no studies have examined the correlation between the PSI and the RASS, which is recommended by clinical guidelines for sedation assessment in the ICU.
Therefore, the present study investigated the reliability of using the PSI in the ICU by examining the potential correlation between the PSI and RASS and evaluated the usefulness of the PSI as an indicator of the sedation level in the ICU.
2 Materials and methods
2.1 Study design
This single-center, single-blind, prospective, observational study was conducted in an ICU of the Tokyo Women’s Medical University Hospital (Tokyo, Japan). The study was registered in the University Hospital Medical Information Network (UMIN000035199, December 10, 2018) after approval from the Tokyo Women’s Medical University Ethics Committee (Approval No. 4994, November 23, 2018).
2.2 Study setting
The study was conducted in an 18-bed medical/surgical ICU of a university hospital with a 1,235-bed capacity. Intensive care is provided by a multidisciplinary team of attending physicians from the departments of internal medicine or surgery, full-time intensive care physicians, nurses, pharmacists, physical therapists, clinical engineers, and dieticians. The targeted sedation level and general management of the patient, including mechanical ventilatory management, weaning, spontaneous breathing trials, and extubation plans, are discussed and determined in team meetings.
2.3 Study population
This study targeted patients aged ≥ 20 years who were admitted to the ICU and were predicted to require mechanical ventilation with tracheal intubation for ≥ 12 h. Written informed consent was obtained from all patients or their legal representatives. The exclusion criteria were as follows: (1) previous neurosurgery; (2) diagnosis of acute cerebral hemorrhage/cerebral infarction, or a history thereof; (3) previous cardiopulmonary resuscitation; (4) impaired consciousness (defined as < 14 points on the Glasgow Coma Scale even before administration of a sedative); (5) hepatic encephalopathy; (6) drug toxicity; (7) patients who would find medical care provided in Japanese difficult, (8) requirement for continuous administration of muscle relaxants in the ICU; (9) patients deemed highly likely to die within 24 h of ICU admission; (10) patients for whom the sensor could not be attached properly to the forehead due to skin infections; and (11) patients whose informed consent could not be obtained.
2.4 Sedation protocol
The RASS evaluations were conducted every 2 h during routine clinical practice by adequately trained nurses [7]. The target sedation level during mechanical ventilation was determined by the attending physicians, intensive care physicians, and nurses according to the patient’s condition, and the physicians adjusted the dose of analgesics and sedatives to achieve this target level. Propofol (0–3 mg/kg/h), dexmedetomidine (0–0.7 mcg/kg/h), midazolam (0–0.18 mg/kg/h), and/or fentanyl (0–2 mcg/kg/h) were administered in various combinations.
2.5 Monitoring and data collection
To measure and record the PSI, we used the brain-function-monitoring module SedLine® v2010 (Masimo, Irvine, CA, USA) and the patient monitoring platform Root® (Masimo). The SedLine® sensor was attached to the forehead and the electrode impedance checked, and the monitor screen was covered entirely to ensure blinding of both nurses and physicians.
The PSI was measured continuously until extubation or for up to 24 h after the start of measurement, whichever came first. The nurses in charge evaluated the RASS every 2 h according to the institutional protocol. RASS evaluation was performed 10 min after treatments involving stimulation, such as postural change, when oral or tracheal suctioning were performed, or when the dose of sedatives was changed. The PSI value immediately before RASS evaluation was collected from the data automatically stored inside the equipment.
In postoperative patients, PSI measurements were started 6 h after ICU admission and confirmed that the train-of-four (TOF) ratio on the muscle relaxant monitor TOF-Watch SX® (Organon, Ireland) had recovered to ≥ 0.9 in order to exclude the influence of general anesthesia. Similarly, in patients who were intubated using muscle relaxants in the ICU, measurements were started after confirming that the effect of the muscle relaxant had waned.
2.6 Statistical analyses
Quantitative and qualitative data are expressed as mean values with standard deviation (SD) or median values with interquartile range, and as numbers or percentages, respectively.
We used the Shapiro‒Wilk normality test to investigate if the numerical values for PSI followed a normal distribution. We used Spearman’s rank correlation coefficient (< 0.40 weak correlation, 0.40–0.69 moderate, > 0.70 strong), with 1000 bootstrap replicates, to examine if PSI scores correlated with RASS scores, and the Kruskal‒Wallis test to investigate if there were statistically significant differences in PSI among the RASS categories. For the correlation between PSI and RASS, we added post hoc subgroup analyses of younger and elderly patients (patients ≤ 65 years old vs. patients > 65 years old).
Furthermore, we used pairwise comparisons using the Wilcoxon rank sum test, with Benjamini‒Hochberg correction, to estimate the categories of RASS that showed significant differences in PSI values. Additionally, we fitted a linear mixed-effects model fit by REML, with RASS as a fixed effect and Patient ID as a random effect, to investigate the potential significant association of PSI with RASS, considering each patient’s records.
We used analysis of variance (ANOVA) tables for models to calculate the Wald chi-square and the significance (P-value) of the dependent PSI factor, with RASS as a fixed effect predictor in the model.
We defined RASS scores of ≤ − 3 (− 3, − 4, −5) as deep sedation and RASS scores of ≥ − 2 as light sedation. We then used these two new categories of RASS and constructed and plotted a receiver-operating characteristic (ROC) curve and estimated the optimal cut-off point of the PSI for distinguishing light from deep sedation.
The significance level was set at P < 0.05. For all statistical analyses, we used R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
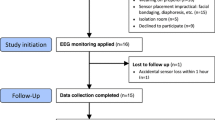
Of the 218 patients admitted to the ICU between December 10, 2018, and February 9, 2019, 168 patients were excluded because they were younger than 20 years of age, admitted without mechanical ventilation, expected to be extubated within 12 h after ICU admission, had undergone open-head surgery, had a history of cerebral hemorrhage/cerebral infarction, or did not meet the inclusion criteria. Consequently, 50 consecutive patients were enrolled in the study (Fig. 1).
The patient characteristics are shown in Table 2. The age range was 31–80 years and most of the patients were admitted to the ICU postoperatively. Propofol, dexmedetomidine, and fentanyl were administered as the sedative in most patients, and midazolam was used in a few patients.
A total of 382 sets of PSI and RASS data were obtained from 50 patients. The Power (post hoc analysis) of this sample size for the linear mixed effect model was 99.63%. The summarized values of PSI among the RASS categories are presented in Table 3.
The Shapiro‒Wilk normality test showed that the PSI (W = 0.88, P < 2.2e-16) data did not follow a normal distribution. Therefore, we used only non-parametric tests. Spearman’s rank correlation showed a strong correlation between the PSI and RASS scores (ρ = 0.79, 95% confidence intervals [CI]: 0.75–0.83) (Fig. 2). Also in subgroup analyses, Spearman’s rank correlation showed strong correlation between the PSI and RASS scores in both group, patients ≤ 65 years old (n = 20, ρ = 0.84, 95% CI: 0.79–0.89) and patients > 65 years old (n = 30, ρ = 0.78, 95% CI: 0.74–0.82). The Kruskal‒Wallis test showed that the PSI values differed statistically significantly (Kruskal‒Wallis chi-square = 242, df = 6, P < 2.2e-16) among the RASS categories (Fig. 2).
Box plot showing distribution of the PSI for each level of RASS. (Boxes represent the interquartile range (25th percentile [lower edge] and 75th percentile [upper edge]). The median PSI is denoted as the line across the box. Spearman’s rank correlation showed a strong correlation between the PSI and RASS scores (ρ = 0.79, 95% confidence intervals [CI]: 0.75–0.83). PSI, Patient State Index; RASS, Richmond Agitation-Sedation Scale)
Pairwise comparisons using the Wilcoxon rank sum test showed that the PSI differed significantly among almost all RASS categories; the only RASS categories that did not demonstrate a significant difference were between 0 and 1 (Table 4). An ANOVA showed that, in the first model we fitted, PSI was significantly associated with the RASS (Chi-square = 518; P < 0.001).
The ROC curve of the PSI for distinguishing light (RASS ≥ − 2) from deep sedation (RASS − 3, −4, − 5) is presented in Fig. 3. The area under the ROC curve was equal to 0.92 (95% CI: 0.89‒0.95). We calculated that the optimal cut-off point of the PSI for this curve was 54 (95% CI: 50–65), with a sensitivity = 0.91 (95% CI: 0.86‒0.95) and specificity = 0.81 (95% CI: 0.77–0.86) (Fig. 3).
Receiver operating characteristic (ROC) curve for Patient State Index (PSI) for detecting deep sedation (RASS − 3, −4, − 5). (The area under this ROC curve was equal to 0.92 (95%CI: 0.89‒0.95). The optimal cut-off point of the PSI for this curve was 54 (95%CI: 50–65), with a sensitivity = 0.91 (95%CI: 0.86‒0.95) and specificity = 0.81 (CI: 0.77–0.86))
4 Discussion
Two critical aspects were identified in this study, which aimed to assess correlations between the PSI and RASS, and the clinical utility of the PSI. First, the PSI values measured using SedLine® and Root® strongly correlated with RASS scores in sedated, mechanically ventilated ICU patients, and the PSI values associated with each RASS score differed significantly. This suggests that the PSI was an accurate, objective, and continuous indicator that may be helpful to clinicians in adjusting sedatives and providing appropriate sedation levels. The PSI was useful for evaluating the sedation state of patients under general anesthesia, although previous research on the PSI in ICU patients on mechanical ventilation is scarce [11, 20,21,22]. The results of this study shows that the PSI correlated well with the RASS, and that it can be useful for monitoring sedation levels in critically ill ICU patients, although ICU patients differ from patients under general anesthesia in terms of the presence of body movement, muscle contraction, edema, or diaphoresis. This has not been reported previously.
Second, the PSI distinguished and detected light and deep sedation with high accuracy. Because inappropriate deep sedation has also been demonstrated to be related to worse clinical outcomes [1,2,3,4], our results suggest that the detection of unnecessarily deep sedation by means of the PSI may improve patient prognosis, such as shortening the duration of mechanical ventilation and of ICU stay. Although deep sedation is required in patients who need muscle relaxants for acute respiratory distress syndrome, it is difficult to assess their sedation state by using a subjective scoring tool, such as the RASS, because of the lack of body movement and eye opening of these patients. In these patients, inappropriately light sedation levels increase patient discomfort, while unnecessarily high doses of sedatives can lead to adverse events such as hypotension. In these cases, objective and continuous monitoring of the sedation level using the PSI may be helpful and clinicians can use the PSI value as an indicator to appropriately adjust the sedatives.
A few small studies evaluated the performance of the PSI as an indicator of sedation levels in the ICU. Schneider et al. [11] reported that the PSI correlated well with the Ramsay Sedation Scale in patients who required mechanical ventilation. Several authors reported that the PSI changed sequentially with changes in sedation level arising from auditory or sensory stimulation and ventilator settings, and that the sleeping stage and awake state of ICU patients could be continuously monitored by means of the PSI [21, 22]. However, the correlation between the PSI and RASS, and the ability of the PSI to detect deep sedation had not been investigated in the abovementioned studies.
The PSI calculation was performed using the analysis protocol of the brain-function-monitoring module in SedLine®. For PSI measurement, the EEG is monitored with a four-channel EEG electrode sensor attached to the forehead, bilaterally, and the EEG is analyzed with a proprietary algorithm to offer a calculated value between 0 and 100 that indicates the sedation level [11]. The PSI is calculated by combining some factors, including changes in power in various EEG frequency bands, changes in symmetry and synchronization between key brain areas, inhibition of the prefrontal cortex, and quantitative parameters that reflect brain electrical activity in many dimensions [12].
EEG monitoring has markedly evolved since the introduction of the bispectral index (BIS) in 1996 [19, 23]. The PSI, BIS, Entropy (GE Healthcare, Chicago, IL, USA), and NeuroSENSE (NeuroWave Systems, Inc, Cleveland Heights, OH, USA) are well-known instruments for analyzing EEG and monitoring sedation level [19]. The correlation between the PSI and BIS, as well as the reliability, accuracy, and usefulness of the PSI under general anesthesia has been shown in several studies [13, 16, 18].
In the ICU, the BIS has been investigated as an objective index for evaluating sedation level in many studies [23,24,25]; however, the correlation between the BIS value and sedation level varies across studies [23,24,25]. As there are many factors that interfere with EEG monitoring and reliability, including body movement, EMG activity produced by facial muscle contraction, artifacts caused by interventions, and electrocardiogram artifacts, BIS usage in the ICU has been limited [1, 25,26,27].
However, with advances in the PSI measurement algorithm, the current version of the PSI is less influenced by EMG activity [12, 28,29,30]. The better tolerance of the PSI to the increase in EMG activity and artifacts from surgical manipulation, as compared with the BIS, has been shown in an operating theatre [30]. Despite confirming the disappearance of the effects of muscle relaxants in all patients, the correlation between the PSI and RASS was reasonably good in the present study. The influence of EMG activity on EEG monitoring cannot be completely eliminated, but better filtration of EMG activity noise might result in good PSI performance for ICU use.
This study had several strengths. First, this study investigated the correlation between the PSI and RASS in sedated, critically ill ICU patients, which has not been reported previously. Second, the types of sedatives and analgesics used were identified in this study. We did not create a sedation and analgesia protocol, but propofol, dexmedetomidine, and fentanyl were administered in most cases. The drug usage in this study adhered to the present recommendation for the use of sedatives and analgesics in the ICU setting, thus provided high external validity [1]. Third, RASS evaluations were routinely performed once every 2 h, and thus, the nurses were adequately trained to obtain an accurate evaluation. Fourth, the nurses who evaluated the RASS and physicians who adjusted the dose of analgesics and sedatives were both blinded to the PSI values, and the sedation level was adjusted to achieve the target RASS defined for each patient. Therefore, clinical data could be obtained without bias.
However, this study also had several limitations. First, this study was conducted in a single ICU where the majority of the patients were postoperative cases and only 8% were nonsurgical patients. Second, the Acute Physiology and Chronic Health Evaluation II score of patients was lower in this study than in previous reports [9, 21]. Differences in patient backgrounds highlight the need for further research that includes patients with more severe conditions, non-surgical patients, and post-neurosurgery patients. Third, nearly half of the PSI and RASS datasets were obtained from patients under deep sedation (RASS scores: −3, − 4, and − 5). Most of the deeply sedated patients underwent postoperative cardiovascular surgery (86.0%) and required deep sedation for postoperative stabilization of hemodynamics. Fourth, we did not compare PSI utility with that of other tools, including the BIS. However, given that the most objective indicator of sedation level has not been identified, the results of this study may be useful for future comparisons of the reliability of various tools.
It remains unclear whether sedation monitoring, including BIS and PSI, improves patient prognosis [25]. Therefore, we plan to conduct a future study to investigate the effect of PSI use on patient prognosis, such as on the dose of sedatives used, the duration of ventilation and of ICU stay, and cost, based on the results of this study. Finally, because we focused on PSI in this study, we did not evaluate spectral edge frequency (SEF) or suppression ratio (SR) as analyzed by SedLine®. Further studies are needed to validate their usefulness in ICU.
In conclusion, the PSIs measured by the SedLine® correlated well with the sedation levels evaluated using the RASS in mechanically ventilated ICU patients and were significantly different among the RASS scores. Additionally, the PSI can distinguish between deep and light sedation with high sensitivity and specificity, which may be useful to avoid inappropriately light sedation or deeper sedation than necessary, especially in patients taking concomitant muscle relaxants.
Our study suggests that PSI monitoring allows objective and continuous evaluation of the sedation level and can be useful for optimizing sedation in critically ill ICU patients. Future studies focusing on the clinical outcomes during sedation, guided by the PSI, are needed to validate these findings.
Availability of data and material
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code availability
not applicable.
References
Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–73. https://doi.org/10.1097/CCM.0000000000003299.
Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. https://doi.org/10.1097/CCM.0b013e3182783b72.
Treggiari MM, Romand JA, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. 2009;37:2527–34. https://doi.org/10.1097/CCM.0b013e3181a5689f.
Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–34. https://doi.org/10.1016/S0140-6736(08)60105-1.
De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med. 2000;26:275–85. https://doi.org/10.1007/s001340051150.
Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the intensive care unit: a systematic review. JAMA. 2000;283:1451–9. https://doi.org/10.1001/jama.283.11.1451.
Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. https://doi.org/10.1164/rccm.2107138.
Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32:106–12. https://doi.org/10.1097/01.CCM.0000098033.94737.84.
Ely EW, Truman B, Manzi DJ, Sigl JC, Shintani A, Bernard GR. Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004;30:1537–43. https://doi.org/10.1007/s00134-004-2298-1.
Almgren M, Lundmark M, Samuelson K. The Richmond Agitation–Sedation Scale: translation and reliability testing in a Swedish intensive care unit. Acta Anaesthesiol Scand. 2010;54:729–35. https://doi.org/10.1111/j.1399-6576.2009.02173.x.
Schneider G, Heglmeier S, Schneider J, Tempel G, Kochs EF. Patient State Index (PSI) measures depth of sedation in intensive care patients. Intensive Care Med. 2004;30:213–6. https://doi.org/10.1007/s00134-003-2092-5.
Drover D, Ortega HR. Patient State Index. Best Pract Res Clin Anaesthesiol. 2006;20:121–8. https://doi.org/10.1016/j.bpa.2005.07.008.
Prichep LS, Gugino LD, John ER, et al. The Patient State Index as an indicator of the level of hypnosis under general anaesthesia. Br J Anaesth. 2004;92:393–9. https://doi.org/10.1093/bja/aeh082.
Schneider G, Mappes A, Neissendorfer T, Schabacker M, Kuppe H, Kochs E. EEG-based indices of anaesthesia: correlation between bispectral index and patient state index? Eur J Anaesthesiol. 2004;21:6–12. https://doi.org/10.1017/s0265021504001024.
Schneider G, Gelb AW, Schmeller B, Tschakert R, Kochs E. Detection of awareness in surgical patients with EEG-based indices–bispectral index and patient state index. Br J Anaesth. 2003;91:329–35. https://doi.org/10.1093/bja/aeg188.
White PF, Tang J, Ma H, Wender RH, Sloninsky A, Kariger R. Is the patient state analyzer with the PSArray2 a cost-effective alternative to the bispectral index monitor during the perioperative period? Anesth Analg. 2004;99:1429–35. https://doi.org/10.1213/01.ANE.0000132784.57622.CC.
Chen X, Tang J, White PF, et al. A comparison of patient state index and bispectral index values during the perioperative period. Anesth Analg. 2002;95:1669–74. https://doi.org/10.1097/00000539-200212000-00036.
Drover DR, Lemmens HJ, Pierce ET, et al. Patient State Index: titration of delivery and recovery from propofol, alfentanil, and nitrous oxide anesthesia. Anesthesiology. 2002;97:82–9. https://doi.org/10.1097/00000542-200207000-00012.
Shander A, Lobel GP, Mathews DM. Brain monitoring and the depth of anesthesia: another Goldilocks dilemma. Anesth Analg. 2018;126:705–9. https://doi.org/10.1213/ANE.0000000000002383.
Grap MJ, Munro CL, Wetzel PA, et al. Sedation in adults receiving mechanical ventilation: physiological and comfort outcomes. Am J Crit Care. 2012;21:e53–63. https://doi.org/10.4037/ajcc2012301. quiz e64.
Grap MJ, Munro CL, Wetzel PA, et al. Stimulation of critically ill patients: relationship to sedation. Am J Crit Care. 2016;25:e48–55. https://doi.org/10.4037/ajcc2016269.
Vacas S, McInrue E, Gropper MA, et al. The feasibility and utility of continuous sleep monitoring in critically ill patients using a portable electroencephalography monitor. Anesth Analg. 2016;123:206–12. https://doi.org/10.1213/ANE.0000000000001330.
Karamchandani K, Rewari V, Trikha A, Batra RK. Bispectral index correlates well with Richmond Agitation Sedation Scale in mechanically ventilated critically ill patients. J Anesth. 2010;24:394–8. https://doi.org/10.1007/s00540-010-0915-4.
LeBlanc JM, Dasta JF, Kane-Gill SL. Role of the bispectral index in sedation monitoring in the ICU. Ann Pharmacother. 2006;40:490–500. https://doi.org/10.1345/aph.1E491.
Shetty RM, Bellini A, Wijayatilake DS, et al. BIS monitoring versus clinical assessment for sedation in mechanically ventilated adults in the intensive care unit and its impact on clinical outcomes and resource utilization. Cochrane Database Syst Rev. 2018;2:CD011240. https://doi.org/10.1002/14651858.CD011240.pub2.
Liu N, Chazot T, Huybrechts I, Law-Koune JD, Barvais L, Fischler M. The influence of a muscle relaxant bolus on bispectral and datex-ohmeda entropy values during propofol-remifentanil induced loss of consciousness. Anesth Analg. 2005;101:1713–8. https://doi.org/10.1213/01.ANE.0000184038.49429.8F.
Hemmerling TM, Migneault B. Falsely increased bispectral index during endoscopic shoulder surgery attributed to interferences with the endoscopic shaver device. Anesth Analg. 2002;95:1678–9. https://doi.org/10.1097/00000539-200212000-00038.
Priyadharsini SS, Rajan SE. Evolutionary computing based approach for the removal of ECG artifact from the corrupted EEG signal. Technol Health Care. 2014;22:835–46. https://doi.org/10.3233/THC-140860.
Caputo TD, Ramsay MA, Rossmann JA, et al. Evaluation of the SEDline to improve the safety and efficiency of conscious sedation. Proc (Bayl Univ Med Cent). 2011;24:200–4. https://doi.org/10.1080/08998280.2011.11928715.
Kim YS, Chung D, Oh SK, Won YJ, Lee IO. Unusual elevation in Entropy but not in PSI during general anesthesia: a case report. BMC Anesthesiol. 2018;18:22. https://doi.org/10.1186/s12871-018-0486-8.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Masafumi Idei, Yusuke Seino: Conceptualization, Methodology, Data curation, Validation, Investigation, Writing- Original draft preparation, Project administration.
Nobuo Sato, Yumi Saishu, Takuya Yoshida, Kimiya Fukui, Masahiro Iwabuchi, Junya Ishikawa, Kei Ota, Daigo Kamei: Conceptualization, Data curation, Writing- Original draft preparation, Writing- Reviewing and Editing.
Masashi Nakagawa, Takeshi Nomura: Conceptualization, Data curation, Supervision, Writing- Reviewing and Editing, Project administration.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest/competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The study was approved by the Tokyo Women’s Medical University Ethics Committee (Approval No. 4994, November 23, 2018). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Idei, M., Seino, Y., Sato, N. et al. Validation of the patient State Index for monitoring sedation state in critically ill patients: a prospective observational study. J Clin Monit Comput 37, 147–154 (2023). https://doi.org/10.1007/s10877-022-00871-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00871-9