Abstract
PEEP is regulated by the internal PEEP/maximum peak inspiratory pressure limit (Pmax) valve. Malfunctioning of the PEEP/Pmax valve can result in the creation of unintentional or unstable PEEP, and a reduction of inspired tidal volume. Some of our Dräger Fabius® anesthesia machines were noted to exhibit changes in expiratory waveforms and unstable PEEP during general anesthesia. We considered that the cause was associated with PEEP/Pmax valve malfunction, and then investigated the problems in collaboration with the manufacturer. Seven of the 22 Dräger Fabius® anesthesia workstations at our department exhibited problems with their PEEP/Pmax valves. We replaced the PEEP membrane and sealing washers in these seven anesthesia machines, and the problems were temporarily resolved. After a short interval, however, one of the seven machines began to show a similar phenomenon. We then asked the manufacturer to overhaul the PEEP/Pmax valve and the entire breathing circuit of the machine. On close investigation, we found that the valve components and the internal surface of the breathing circuit were contaminated with unexpected deposits. The build-up of deposits occurred within a year after the previous regular inspection. Our troubleshooting process determined the issue with the PEEP/Pmax valve, which could go unnoticed because the valve is encased inside the breathing circuit, and requires disassembly for close inspection. Our findings should raise awareness regarding the importance of the preventive maintenance cycle as a safety precaution to keep the anesthetic circuit free of unexpected contamination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The application of positive end-expiratory pressure (PEEP) during mechanical ventilation in modern anesthesia practice has become increasingly common with the widespread concept of lung-protective ventilation that employs lower tidal volumes with appropriate PEEP levels in attempts to minimize ventilator-associated lung injury [1,2,3]. In the Dräger workstation, PEEP is regulated by the internal PEEP/maximum peak inspiratory pressure limit (Pmax) valve, which provides a pressure threshold that allows expiratory flow to occur only when airway pressure equals or exceeds the selected PEEP [4]. Recently, some of our Fabius® anesthesia machines were noted to exhibit changes in pressure and flow waveforms and unstable PEEP during general anesthesia. Given the nature of the problems, we considered that these changes were associated with PEEP/Pmax valve malfunction. We then conducted a thorough investigation in collaboration with the device manufacturer.
2 Methods
We carried out an unscheduled inspection of the 22 Fabius® workstations at our department. When unexpected changes in expiratory flow and pressure were detected, we replaced PEEP/Pmax valve components (the PEEP membrane and sealing washers; Fig. 1, Nos. 1 and 5, respectively) as a remedy that could restore the ventilator performance to normal. The inspection reports, in combination with other information available from the manufacturer, were reviewed to provide our troubleshooting process. The current investigation was approved by our Institutional Review Board [#2203-(6)].
3 Results
3.1 Ventilation-related problems with our anesthesia workstations
Our inspection revealed that seven of the 22 anesthesia machines exhibited change in pressure and flow waveforms, unstable PEEP, or change in tidal volume (Table 1), even though they passed the electric self-check and manual leak testing prior to general anesthesia. Four of the machines triggered alarms indicating the presence of gas leaks on the expiratory side of the breathing circuit (Table 1, Nos. 2, 3, 5, and 6); however, the source could not be identified by examination of the breathing system.
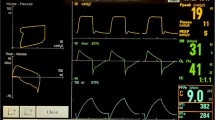
We also experienced a similar problem with one of the anesthesia machines (Table 1, No. 1) in the course of anesthetizing three different patients, all of whom presented with an American Society of Anesthesiologists physical status of I or II: a 49-year-old man, a 32-year-old woman, and a 70-year-old man. None of these patients had a history of respiratory disease including asthma, and only the third patient had slight breathlessness (Hugh-Jones Grade 1). The patients underwent elective surgery (abdominal, dental, and urological surgery, respectively) under general anesthesia in the supine position. Following tracheal intubation or laryngeal mask airway insertion, either volume- or pressure-controlled ventilation was delivered with a respiratory rate of 10–15 breaths/min, an inspiratory pressure of 15–20 cmH2O, a tidal volume of 8–12 mL/kg, and an I:E ratio of 1:2 (Fig. 2). In each instance, a pre-use check of the anesthesia machine was completed by the anesthesia provider. A PEEP of 4–5 cmH2O was applied in the presence of hemodynamic stability, but shortly afterwards there was a decrease in expiratory flow accompanied by a delayed decrease in expiratory airway pressure (Fig. 3). Possible causes of change in pressure and flow waveforms, including asthma attack, bronchospasm, and occlusion or obstruction of the breathing circuit, were quickly ruled out. The waveform returned to normal when PEEP was switched to zero end-expiratory pressure, but the same change was observed upon reapplication of PEEP. Thereafter, we maintained mechanical ventilation without PEEP. The patients recovered from general anesthesia uneventfully.
Flow and pressure waveforms after application of PEEP. As compared with Fig. 2, expiratory flow is impaired during almost every respiratory cycle. It takes longer for the pressure to return to the set PEEP during expiration. Note that the picture was taken 3 min after application of a PEEP of 5 cmH2O, a time point when a new equilibrium was achieved
3.2 Troubleshooting with the manufacturer
We collaborated with the manufacturer to replace the PEEP membrane and sealing washers in the seven anesthesia machines that showed unexpected changes as described above. The situation was temporarily resolved, but within 3 months, a Dräger Fabius® GS anesthesia workstation (Table 1, No. 2) began exhibiting a delay in the decrease in expiratory airway pressure during general anesthesia. The phenomenon included an unusual expiratory flow waveform, a decrease in tidal volume, differences between set and measured values of PEEP, and occasional alarms suggesting a gas leak in the expiratory breathing circuit, whereas the ventilator was running flawlessly. Unlike the previous remedy, the problems were not addressed by replacing the valve components.
3.3 Close investigation by the manufacturer’s head office
In view of the potential threat to patient safety, we asked Dräger Medical Japan to overhaul the PEEP/Pmax valve and the entire breathing circuit of anesthesia machine No. 2. As the cause remained unidentified, a close investigation was carried out by the head office in Germany. This investigation determined that the PEEP/Pmax valve components, as well as the internal surface of the breathing circuit, were contaminated with large numbers of deposits (Fig. 4). The ventilator performance returned to normal after thoroughly cleaning every constituent part of the valve. Further analysis indicated that the deposits contained calcium that was not derived from the parts used in the manufacture of the Dräger anesthesia workstations. According to the manufacturer, more than 20 similar cases have been reported worldwide since 2006.
4 Discussion
4.1 Problems with the PEEP/Pmax valve during expiration
The internal PEEP/Pmax valve is made up of concentric layers of discs and diaphragm-like components, which are coupled to a cylinder that applies a variable amount of pressure by moving the guide pin to affect the expiratory diaphragm (Fig. 1). In the Dräger Fabius® GS and Tiro anesthesia machines, this electromechanical valve is placed in the expiratory limb of the anesthesia circuit (Fig. 5), so that the set PEEP can be transmitted through the expiratory diaphragm. During expiration, the active opening of the PEEP/Pmax valve allows gas from the lungs to be expelled passively into the breathing circuit [5]. Depending on the applied PEEP, the PEEP/Pmax valve partially opens to produce resistance at the end of expiration.
Diagram of the Dräger Fabius® GS and Tiro breathing circuit (from the Dräger Fabius® ventilator schematic). On the right is the expiratory limb where the PEEP/Pmax valve (marked as a red circle) and the flow sensor (indicated as a red arrow) are placed (Reproduced with permission of Dräger Medical Japan)
Malfunctioning of the PEEP/Pmax valve can lead to the creation of unintended or unstable PEEP during mechanical ventilation. Our investigation found numerous deposits in the PEEP/Pmax valve components. The accumulation of these contaminating deposits could result in excessive friction on the pin and hinder movement of the valve (jamming), causing a higher than intended pressure during expiration (Fig. 6). The application of unintentional PEEP due to jamming valves could be hazardous for patients with unstable hemodynamics during general anesthesia.
Unusual expiratory pressure curve caused by a jamming PEEP/Pmax valve. The left figure shows that expiratory pressure can be higher than the set PEEP as a result of jamming valves (Source: Dräger Medical Japan). Similar waveforms were observed in our anesthesia workstation, as presented in the right picture
4.2 Problems with the PEEP/Pmax valve during inspiration
The PEEP/Pmax valve is closed during the inspiratory phase to ensure that the circuit pressure does not exceed the set Pmax, preventing inspiratory gas from passing through the valve into the expiratory side during inspiration [5]. The inability of the valve to close during inspiration would allow gas to enter the expiratory breathing circuit and subsequently leave the expiratory limb through the partially open valve. The incompetent valve would be associated with loss of gas on the expiratory side during inspiration even when the set Pmax is not reached, resulting in a reduction in effective tidal volume. It could even lead to loss of gas from the circuit into the room air.
Our investigation revealed that three of the four machines that had displayed the gas leak alarm in the expiratory breathing circuit recovered after replacing the valve components (Table 1, Nos. 3, 5, and 6). In the Dräger workstation, the electric self-check detects a leak flow of greater than 250 mL/min prior to use, and a leak flow of approximately 15 mL/min in the expiratory limb triggers an alarm indicating that there is expiratory flow during inspiration. The PEEP/Pmax valve could have been leaking during inspiration in our cases, even when the circuit pressure was below the set Pmax. Interestingly, the leak was too small to be detected during the self-check and manual leak testing, but was large enough to trigger an alarm during mechanical ventilation. This phenomenon could suggest difficulty in detecting leaks that could occur because of the presence of fine gaps around the valve created by attachment of unanticipated deposits. The extent of such leaks could differ from machine to machine, depending on the amount or location of the accumulated deposits on the valve components.
There are a number of case reports detailing gas leaks that were undetected even though automated self-checks were performed [6,7,8,9]. In view of these cases and ours, it would be of practical use to extend problem-solving procedures to include consideration of problems arising from the PEEP/Pmax valve.
4.3 Maintenance cycle
Our department provides anesthesia for over 8000 procedures annually to deal with a variety of challenging surgeries that often require long operating times. The calcium found in the deposits might have been derived from the carbon dioxide (CO2) absorbents (Amsorb® Plus, Armstrong Medical), which contain calcium hydroxide that reacts with CO2, as well as a lesser amount of calcium chloride and calcium sulphate to make granules harder and more chemically stable [10]. The build-up of contaminating deposits could occur inside the anesthetic circuit gradually over time according to duration and frequency of use of anesthesia workstations.
The accumulation of deposits of unanticipated materials can result in PEEP/Pmax valve malfunction even under regular maintenance [11]. Our maintenance program included scheduled annual testing and inspection of our anesthesia machines and monitors to ensure Pmax and PEEP accuracy as well as lung ventilator performance. The valve components were scheduled to be replaced biennially, followed by evaluation of pressure and flow waveforms during the test operation. Considering that the deposits built up within a year following maintenance, one potential strategy would be to introduce more frequent maintenance cycles in case of unexpected contamination. Replacing the valve components with frequency could also be a possible means of alleviating the problems.
4.4 Limitation
One limitation of the current investigation was that the overhaul of the PEEP/Pmax valve and breathing circuit was carried out in only a single anesthesia workstation. The extent of contamination was not ascertained in other machines, but a careful disassembly and cleaning of the valve would be needed to ensure elimination of deposits if a similar phenomenon should occur again.
5 Conclusion
The issue with the PEEP/Pmax valve, which can lead to changes in flow and pressure during mechanical ventilation, could go unnoticed because the valve is encased inside the breathing circuit, and requires disassembly for close inspection. Our findings highlight the importance of keeping the anesthetic circuit, including the internal components of the PEEP/Pmax valve, free of unexpected contamination through more thorough preventive maintenance cycles.
Data availability
The datasets used and analyzed during the current study may be made available from the corresponding author on reasonable request.
Abbreviations
- PEEP:
-
Positive end-expiratory pressure
- Pmax:
-
Maximum peak inspiratory pressure limit
- CO2 :
-
Carbon dioxide
References
Hess DR, Kondili D, Burns E, Bittner EA, Schmidt UH. A 5-year observational study of lung-protective ventilation in the operating room: a single-center experience. J Crit Care. 2013;28(4):533.
Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, Dionigi G, Novario R, Gregoretti C, de Abreu MG, Schultz MJ, Jaber S, Futier E, Chiaranda M, Pelosi P. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118(6):1307–21.
Barbosa FT, Castro AA, de Sousa-Rodrigues CF. Positive end-expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev. 2014;12(6):CD007922.
Sulemanji D, Kacmarek RM, Jiang Y. Manual and mechanical ventilators. In: Sandberg W, Urman R, Ehrenfeld E, editors. MGH textbook of anesthetic equipment. 1st ed. Philadelphia: Elsevier Saunders; 2011.
Ehrenwerth J, Eisenkraft JB, Berry JM. Anesthesia ventilators. Anesthesia equipment e-book. Principles and applications. 2nd ed. London: Saunders, Mosby Inc; 2013. p. 148–178.
Eng TS, Durieux ME. Case report: automated machine checkout leaves an internal gas leak undetected: the need for complete checkout procedures. Anesth Analg. 2012;114(1):144–6.
Liew WL, Jayamaha J. Anaesthetic machine leak from desflurane vaporiser. Anaesthesia. 2011;66(5):399–400.
Glen J, Marshall S. Gas leak related to Draeger Primus anaesthetic machine. Anaesthesia. 2010;65(7):750.
Patil V, Mackenzie IM. Hidden gas leak on a Datex-Ohmeda Aestiva/5 anesthetic machine. Anesth Analg. 2007;104(1):234–5.
Yamakage M, Takahashi K, Takahashi M, Satoh JI, Namiki A. Performance of four carbon dioxide absorbents in experimental and clinical settings. Anaesthesia. 2009;64(3):287–92.
Solanki SL, Doctor JR, Patil VP, Rana M. Positive end-expiratory pressure valve malfunctioning detected by capnography and airway pressure waveform. Ann Card Anaesth. 2014;17(3):255–7.
Acknowledgements
We thank Dräger Medical Japan for the use of data presented in the current article. We thank them for their contribution and support for conducting investigations to identify the cause of the failure of our anesthesia machines. We also thank Dr. Hisayoshi Tamai, Department of Anesthesiology, Toranomon Hospital, Tokyo, Japan, for critically reading the manuscript and suggesting substantial improvements.
Funding
The current study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
RO and MI collected all data. RO, MI, TI, and KU analyzed the data. TI wrote the draft of the manuscript. RO and YY supervised the investigation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and informed consent
This investigation was approved by the institutional review board of the University of Tokyo [#2203-(6)]. Informed consent was obtained in advance from all surgery patients for use of data for scientific research, including publication of deidentified patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ikeda, T., Orii, R., Iwakiri, M. et al. Unexpected deposits in the anesthetic circuit: a possible cause of PEEP/Pmax valve malfunction. J Clin Monit Comput 35, 943–948 (2021). https://doi.org/10.1007/s10877-020-00562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-020-00562-3