Abstract
Dose-dependent effects of ketamine on processed electroencephalographic depth-of-hypnosis indices have been reported. Limited data are available for the NeuroSENSE WAVCNS index. Our aim was to establish the feasibility of closed-loop propofol-remifentanil anesthesia guided by the WAVCNS index in the presence of an analgesic dose of ketamine. Thirty ASA I-II adults, 18–54 years, requiring general anesthesia for anterior cruciate ligament surgery were randomized to receive: full-dose [ketamine, 0.5 mg kg−1 initial bolus, 10 mcg kg−1 min−1 infusion] (recommended dose for postoperative pain management); half-dose [ketamine, 0.25 mg kg−1 bolus, 5 mcg kg−1 min−1 infusion]; or control [no ketamine]. After the ketamine bolus, patients received 1.0 mcg kg−1 remifentanil over 30 s, then 1.5 mg kg−1 propofol over 30 s, followed by manually-adjusted propofol-remifentanil anesthesia. The WAVCNS was > 60 for 7/9 patients in the full-dose group at 7 min after starting the propofol infusion. This was inconsistent with clinical observations of depth-of-hypnosis and significantly higher than control (median difference [MD] 17.0, 95% confidence interval [CI] 11.4–26.8). WAVCNS was median [interquartile range] 49.3 [42.2–62.6] in the half-dose group, and not different to control (MD 5.1, 95% CI − 4.9 to 17.9). During maintenance of anesthesia, the WAVCNS was higher in the full-dose group compared to control (MD 14.7, 95% CI 10.2–19.2) and in the half-dose group compared to control (MD 11.4, 95% CI 4.7–20.4). The full-dose of ketamine recommended for postoperative pain management had a significant effect on the WAVCNS. This effect should be considered when using the WAVCNS to guide propofol-remifentanil dosing.
Trial Registration ClinicalTrails.gov No. NCT02908945.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Processed electroencephalogram (pEEG) monitors such as the Bispectral Index (BIS, Medtronic), M-Entropy (GE Healthcare), SedLine (Masimo) or NeuroSENSE monitor (NeuroWave Systems) use signal processing techniques to derive a single measure of depth-of-hypnosis (DOH) from frontal EEG. The use of a pEEG monitor can reduce the risk of intraoperative awareness, reduce drug overdosing, and improve postoperative recovery [1]. However, pEEG indices may not be reliable when multiple drugs with different EEG effects are used [2], such as when low-dose (analgesic) ketamine is given during multimodal anesthesia to reduce postoperative pain and opioid requirements [3,4,5,6,7,8,9].
The NeuroSENSE WAVCNS index (NeuroWave Systems, Cleveland Heights, OH) is a pEEG monitor that was developed specifically for use during closed-loop controlled anesthesia. Its fully disclosed deterministic algorithm uses wavelet technology [10] and the WAVCNS index is predominantly determined by EEG in the γ-band (32–64 Hz) [10]. Its dynamics are delay-free, linear and known. This monitor has successfully guided closed-loop propofol and remifentanil infusion in adults [11] and children [12].
The effect of ketamine on frontal EEG depends on dosage (or, more specifically, the resulting effect-site concentration) and on co-administered drugs. Low-dose ketamine, administered as a sole agent, introduces oscillations in the high β- and low γ-range (24–32 Hz) [13], while most other anesthetic agents increase power at lower EEG frequencies [14]. When ketamine is co-administered with propofol, the EEG signature resembles that of propofol, with a reported shift in the peak α-spindle frequency [15].
Studies evaluating the effect of ketamine on pEEG monitors vary in drug dosing, timing of ketamine administration and co-administration of propofol. Due to the different signal processing algorithms, the expected response of a pEEG monitor in the presence of additional drugs cannot be inferred from other monitors. The entropy monitor did not reliably reflect the DOH during single agent ketamine anesthesia [16]. Increases in the BIS index (Medtronic, Dublin, Ireland) were observed following ketamine infusion during intravenous anesthesia [17,18,19,20,21], while no effect was reported for low dose ketamine [22]. Quantification of the effect of ketamine on the WAVCNS is limited to one (unpublished) study [23], with WAVCNS values calculated offline from EEG data collected in a previous study. No significant effect was observed.
The purpose of this investigation was to establish feasibility of closed-loop propofol-remifentanil anesthesia guided by the WAVCNS index, in the presence of a low-dose (analgesic) ketamine administration scheme. Optimal ketamine dosing remains uncertain [24]. Guidelines from the American Pain Society on the management of postoperative pain recommend a loading bolus (0.5 mg kg−1) followed by a continuous intraoperative infusion (10 mcg kg−1 min−1), with or without a postoperative infusion [24]. None of the studies evaluating the effect of ketamine on pEEG indices include a ketamine loading dose prior to induction of propofol anesthesia, or include transient responses during induction of anesthesia and as a result of varying propofol and remifentanil administration during continuous ketamine infusion. This study therefore aimed to establish feasibility of WAVCNS guided closed-loop induction and maintenance of intravenous anesthesia in the presence of this recommended dose of ketamine, targeting the 40–60 range recommended for general anesthesia [25, 26].
Hence, this study evaluated the behaviour of the WAVCNS index during intravenous anesthesia in the presence of the recommended dose of ketamine (full-dose: 0.5 mg kg−1 bolus, 10 mcg kg−1 min−1 infusion) and half the recommended dose [24] (half-dose: 0.25 mg kg−1 bolus, 5 mcg kg−1 min−1 infusion), versus a control group (no ketamine) and compared the WAVCNS trends to traditional measures of DOH such as drug requirements, blood pressure, heart rate, and unwanted intraoperative events. The primary outcome was the WAVCNS index trends. Post-hoc analysis of the EEG signal was performed to generate hypotheses for the observed response to the full-dose, and to provide data to guide future studies and algorithm development.
2 Methods
2.1 Study design and approval
A randomized, parallel-group, feasibility study (clinicaltrials.gov NCT02908945, registration date 2016-09-21, PI Richard Merchant) was conducted with Fraser Health Research Ethics Board approval (FHREB 2016-054, approval date 2016-08-16), and written informed participant consent. This manuscript adheres to the CONSORT guidelines for reporting randomized trials.
2.2 Study participants
We recruited 30 American Society of Anesthesiologists (ASA) physical status I-II adults aged 18–54 years scheduled for elective anterior cruciate ligament repair surgery, requiring general anesthesia, at Eagle Ridge Hospital in Port Moody, BC, Canada. This procedure is associated with significant postoperative pain [27], and patients may benefit from low-dose intraoperative ketamine [8]. Criteria for exclusion were contraindication to ketamine, propofol, or remifentanil, known or suspected neurological disease, acquired scalp or skull abnormalities, psychiatric illness, history of drug misuse/abuse within the past 30 days, required preoperative sedative, pregnancy or nursing, catecholamines or thyroid hormones, anticipated use of nitrous oxide, and current enrollment in any other study involving investigational drugs or devices.
2.3 Randomization and blinding
Participants were randomized to one of three groups, in blocks of six (1:1:1 ratio). The randomization was generated in Excel (Microsoft Cooperation, Redmond, WA) by a staff member not involved in the study and the randomized group allocation was stored in sealed opaque envelopes. Participants in the full-dose group received a 0.5 mg kg−1 loading dose of racemic ketamine hydrochloride (Sandoz Canada Inc., Boucherville, QC), followed by a continuous 10 mcg kg−1 min−1 infusion throughout maintenance of anesthesia. Participants in the half-dose group received a 0.25 mg kg−1 loading dose of ketamine, followed by a continuous 5 mcg kg−1 min−1 infusion. Participants in a control group received no ketamine. Participants were blinded to their group assignment, but their anesthesiologists were not. The NeuroSENSE screen was covered to ensure the anesthesiologist was blinded to the WAVCNS.
2.4 Equipment
The NeuroSENSE pEEG monitor evaluates the normalized EEG signal in the gamma frequency band, using wavelet analysis. For each 1-second epoch, the output of this analysis is compared against (i) the isoelectric state with full cortical depression and (ii) the fully awake state with normal EEG activity. The resulting Wavelet Anesthetic Value for Central Nervous System Monitoring (WAVCNS) index quantifies the depth-of-hypnosis between these two extreme states, ranging from 0 (isoelectric EEG) to 100 (fully awake) [10]. A WAVCNS within the 40–60 range is recommended for general anesthesia [25, 26]; a WAVCNS <40 suggests deep anesthesia, and a WAVCNS >60 indicates light anesthesia. Rapid increases in WAVCNS are expected in response to stimulation. The device records the EEG data at 256 Hz, collects the WAVCNS values calculated per 1 s epoch (raw WAVCNS) and provides a filtered WAVCNS index. The device’s default filtering of the WAVCNS was used (auto mode), which uses an automatic filter selection algorithm that varies from 5 to 60 s (shorter during rapid changes and longer in the presence of noise).
A study laptop collected data every second from the NeuroSENSE monitor, the CARESCAPE B850 patient monitor (GE Healthcare, Chicago, IL), and three Alaris TIVA infusion pumps (Becton, Dickinson and Company, Franklin Lakes, NJ), one each for propofol, remifentanil, and ketamine. These drugs were only administered via these programmable pumps, so that timing and doses could be accurately logged.
2.5 Study procedures
Preoperative medications and intravenous fluids were administered as per routine anesthetic and surgical care. The attending anesthesiologist monitored the patient as per routine practice and could modify the anesthetic/analgesic drug administration at any time. Induction of anesthesia was conducted as follows. The ketamine bolus dose was given over 60 s for the full-dose and half-dose groups; no placebo was given to the control group. Once this bolus was completed, a 1.0 mcg kg−1 bolus of remifentanil was then given over 30 s. A 1.5 mg kg−1 bolus of propofol was given over 30 s once the remifentanil bolus was completed. Additional bolus doses of 0.5 mg kg−1 propofol were administered at the anesthesiologist’s discretion based on clinical response. Loss of consciousness was defined and recorded as loss of eyelash reflex (LoE) as assessed by the anesthesiologist. Bag/mask ventilation was followed by laryngoscopy and insertion of the airway device (endotracheal tube or laryngeal mask airway) when deemed clinically appropriate. Neuromuscular blocking agents were used at the anesthesiologist’s discretion.
Anesthesia was maintained by manually adjusted continuous infusions of propofol and remifentanil, starting at 150 mcg kg−1 min−1 and 0.1 mcg kg−1 min−1 respectively. The full-dose and half-dose groups received a continuous ketamine infusion. Propofol and remifentanil boluses could be administered at the anesthesiologist’s discretion. Throughout the procedure, the patient’s vital signs were monitored in routine fashion and hemodynamic variability (hyper- or hypotension, tachy- or bradycardia) was managed at the anesthesiologist’s discretion. All interventions (e.g. vasoactive drug administration, fluid boluses) were recorded, as well as the justification for the intervention and any other relevant clinical comments. Any instances of somatic response (patient movement, eye opening, coughing, swallowing, grimacing, lacrimation, or sweating) were documented, along with any consequent interventions.
The ketamine infusion was turned off once the last suture was placed. For emergence of anesthesia, propofol and remifentanil infusions were tapered and turned off at the anesthesiologist’s discretion.
2.6 Sample size
The sample size was based on previous studies examining the influence of ketamine on pEEG monitors [17, 18, 22]. This study was not powered to test a specific hypothesis.
2.7 Data analysis
Previously available data on the effect of ketamine on the WAVCNS are limited and do not include induction of anesthesia [23]. Consequently, the WAVCNS response to a clinical analgesic ketamine scheme was unknown and no specific hypotheses or a priori data/time points of interest were defined. Data analysis was performed in an exploratory, post-hoc manner, i.e. analysis of the WAVCNS was determined after reviewing the data.
The average of the left and right WAVCNS values was calculated, and is referred to as the WAVCNS going forward. Median and interquartile range (IQR) were calculated each second (corresponding to the sampling time) per group for the WAVCNS and predicted propofol effect site concentration, according to the Schnider model [28]. The median and IQR were plotted, and WAVCNS trends in the full-dose and half-dose groups were compared to the trends in the control group.
Based on visual analysis of these WAVCNS trends, and of the power spectral density of the EEG signals from the start of the ketamine infusion until procedure start, the following time-points of interest were identified for comparison of the WAVCNS: (T1) start of the propofol infusion, when ketamine and remifentanil may have potentially affected the EEG, but not in combination with propofol, (T2) loss of eyelash reflex LoE, (T3) 7 min after the start of the propofol infusion (when the WAVCNS >60 for 7/9 patients in the full-dose group), and (T4) procedure start.
During maintenance of anesthesia, defined as procedure start to end of the propofol infusion, median WAVCNS, median propofol and remifentanil infusion rates, and the percentage time the WAVCNS remained within 40–60 were calculated for each patient. Median (IQR) values were calculated per group and results from the full-dose and half-dose groups were compared to the control group. Comparisons were reported with median differences (MD) and their 95% confidence interval (CI), performed using the Wilcoxon rank-sum test.
Instances of the WAVCNS indicating insufficient depth of anesthesia (> 60) were compared with clinical indicators of depth of anesthesia, including blood pressure and heart rate trends, marked instances of somatic response, and the administration of additional boluses of propofol required to achieve/maintain adequate anesthesia. Bolus administration data are reported with the odds ratio and 95% CI, and p-value as calculated using Fisher’s exact test.
Analyses were performed and data were plotted using MATLAB (The Mathworks, Natick, MA). Statistical comparisons were conducted using R (R Foundation for Statistical Computing, Vienna, Austria).
2.8 Post-hoc EEG analysis
Post-hoc analysis of the EEG signal was performed to generate hypotheses and to guide future studies. Characteristics of the raw EEG signals, sampled at 256 Hz, were analysed; the power spectral density was calculated using the multi-taper method (window length 3 s, overlap 2 s) [29]. Total power was analysed using the average over both channels and over 10 s. Power between 58 and 62 Hz (line interference) was removed from the estimated power spectrum in the frequency domain by setting the power to zero for these frequencies. Artefacts, defined as total power in the 0–64 Hz range exceeding an artefact-free threshold, were excluded. Normalized spectra were analysed using the average over both channels, normalized over the 0–64 Hz frequency range. The frequency at which the maximal amplitude α-spindles occurred (peak frequency) was calculated for each instant, as was the relative power in the γ-band (32–64 Hz). The resulting time series were filtered using a 30-second moving average, and the median (IQR) plotted over the first 30 min following the start of the propofol infusion.
3 Results
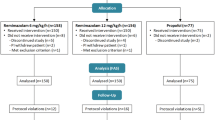
Data were collected for 30 cases (24 males) of median [IQR] age 29 [26–37] years, weight 83.5 [75.3–92.3] kg, and height 180.2 [175.9–185.3] cm, between 2016-10-04 and 2017-04-07. One participant in the full-dose group received a different ketamine dose in error, and was excluded; LoE was not recorded for one patient in the full-dose group, and was excluded from the LoE results, but retained for other analyses. One case in the half-dose group was excluded from the WAVCNS analysis due to a data synchronization issue, but included in the analysis of other outcomes (Fig. 1). Characteristics were similar between groups (Table 1).
3.1 WAVCNS trends
At the start of propofol infusion (T1) and at LoE (T2), the WAVCNS in the ketamine full-dose and half-dose groups was not different from the control group (Table 2; Fig. 2). At 7 min after the start of the propofol infusion (T3), the WAVCNS in the full-dose group was significantly higher than the control group (MD 17.0, 95% CI 11.4–26.8), but the WAVCNS in the half-dose group was not different from control (Table 2). At procedure start (T4), the WAVCNS in the full-dose group was higher than control (MD 15.6, 95% CI 10.4–19.1) and was also higher in the half-dose group compared to control (MD 7.7, 95% CI 1.2–18.1).
Depth-of-hypnosis (WAVCNS) and propofol effect-site concentration (Ce) over time, showing the full-dose group (F-D, red) and half-dose group (H–D, blue) compared to the control group (C, black) during the first 50 min of anesthesia (left). WAVCNS is also shown in detail during the first 10 min of anesthesia (right). Thick lines show the median values over time, the interquartile range is indicated with thin lines. Note that the drop in median WAVCNS after ~ 6 min in the half-dose group represents the response of one participant to an additional propofol bolus, and does not reflect a transient increase following induction of anesthesia, as observed in the full-dose group
During maintenance of anesthesia, the WAVCNS was higher in the full-dose group compared to the control group (MD 14.7, 95% CI 10.2–19.2) and was also higher in the half-dose group compared to control (MD 11.4, 95% CI 4.7–20.4) (Table 2).
In the control group, the WAVCNS followed a typical pattern for propofol induction of anesthesia, characterized by a rapid decrease to ~ 50 and consequent stable WAVCNS indices (Fig. 2). The full-dose response to the start of propofol infusion showed an initial rapid decrease followed by a gradual increase in the WAVCNS index, prior to a gradual decrease to maintenance values. The half-dose group did not show this initial decrease and gradual increase, but was characterized by variability in response.
3.1.1 Propofol dosing and clinical observations
To complete induction of anesthesia, additional propofol boluses were required in 8/10 (80%) participants in the control group, 3/9 (33%) participants in the full-dose group (odds ratio compared to control = 0.14, 95% CI 0.01–1.37), and 6/10 (60%) in the half-dose group (odds ratio compared to control = 0.39, 95% CI 0.03–3.89). The total propofol dose administered up to LoE was not statistically different between groups (Table 2). One participant in the half-dose group and two in the control group received rocuronium in anticipation of, or following, a difficult airway.
In the full-dose group, WAVCNS >60 were observed in 7/9 (78%) cases and WAVCNS was > 70 in 3/9 (33%) cases (Fig. 2) in the period between the start of propofol infusion and procedure start. No clinical signs of inadequate anesthesia were reported in the full-dose group during this period and blood-pressure and heart-rate trends showed no response to stimulation. In the half-dose group, variability in WAVCNS was high. Median WAVCNS was > 60 during the first 6 min following the start of the propofol infusion. Clinical signs of light anesthesia were reported during this period for some patients in this group: response to stimulation (n = 2), requiring additional induction bolus (n = 1), and suspected light anesthesia (n = 1). The control group showed a consistent decrease to WAVCNS in the 40–60 range; response to stimulation was reported for one patient.
During maintenance of anesthesia, the median propofol dose was not different in the full-dose group than the control group (MD − 22.8 mcg kg−1 min−1, 95% CI − 40.0 to 0.0) and was lower in the half-dose group compared to control (MD − 30.0 mcg kg−1 min−1, 95% CI − 50.0 to − 10.0) (Table 2). Remifentanil dosing was not different in either the full-dose or half-dose group compared to control (Table 1). The WAVCNS was within the 40–60 range for 94.7% [86.7–99.7] of the maintenance period in the full-dose group, and for 95.2% [26.4–99.5] in the half-dose group; these values were consistent with clinical assessment. In the control group, the median [IQR] WAVCNS was 38.6 [36–41.8], with only 33.8% [9.9–83.3] of time spent within the 40–60 range; these values reflect relatively deep anesthesia.
3.2 EEG analysis
Spectra in the full-dose group showed lower power at the start of the propofol infusion than the control group (Fig. 3, T1). This EEG effect was not reflected in the WAVCNS, which was not different at the start of propofol infusion (T1) for the full-dose group compared to the control group. Power of the half-dose group was similar to the control group. After 7 min (T3) and at procedure start (T4), the peak frequency increased with increased ketamine dose (Fig. 3, T3 and T4). The elevated WAVCNS (Fig. 2) during the first 20 min after the start of the propofol infusion in the full-dose group coincided with elevated peak frequency and elevated relative γ-band power, exceeding their respective steady-state values during maintenance of anesthesia (Fig. 4). This trend with overshoot in the peak frequency was not observed in the half-dose group (median) or control.
EEG power spectral density (Power) and corresponding unfiltered depth of hypnosis (WAVCNS) indices. Spectra of the full-dose group [F-D] are shown in red, the half-dose group [H-D] in blue, and the control group [C] in black. Thick lines show the median, the interquartile range is indicated with dashed lines. T1: Power at the start of propofol infusion, and the boxplots of the corresponding depth-of-hypnosis index (WAVCNS.). T2: Power and WAVCNS at loss of consciousness. T3: Power and WAVCNS at 7 min after the start of propofol infusion. T4: Power and WAVCNS at procedure start. The figures are scaled to 0–50 Hz to illustrate the characteristics in the α-band and the peak frequency
Trends of peak frequency (a, c) and relative power in the γ-band (b, d), filtered using a 30-second moving average filter. Trends for the full-dose (F-D) group are shown in red, the half-dose (H-D) group in blue, and the control group in black. Thick lines show the median, the interquartile range is indicated with thin lines. The elevated WAVCNS in the first 10 min after the start of the propofol infusion observed in the full-dose group coincide with a transient increase in the peak frequency and γ-band power, exceeding their respective steady-state values during maintenance of anesthesia. This trend is not seen in the control group. The median of the peak frequency in the half-dose group shows some, but less pronounced, overshoot. Note that the drop in γ-power in the half-dose group after ~ 6 min represents the response of one participant to an additional propofol bolus, and does not reflect a transient increase following induction of anesthesia, as observed in the full-dose group
4 Discussion
4.1 WAVCNS trends
The WAVCNS was higher in both the full-dose and half-dose ketamine groups compared to the control group at procedure start (T4) and during maintenance of anesthesia. The WAVCNS was also higher in the full-dose group compared to control at 7 min after the start of the propofol infusion (T3). While these higher WAVCNS values were achieved with fewer propofol boluses for induction of anesthesia in the full-dose group, the total propofol induction dose was not different between groups (Table 2). In the full-dose group, the WAVCNS was inconsistent with clinical observations of the depth-of-hypnosis in the first 10 min after the start of propofol-remifentanil anesthesia; after an initial decrease, the WAVCNS increased to values normally associated with light anesthesia or even sedation (WAVCNS >70 in 33% of patients), while no clinical signs of insufficient anesthesia were reported. This increasing WAVCNS is not typically seen during propofol anesthesia, where increases due to stimulation are commonly more rapid and resolved more quickly (as observed in the control group). These results suggest that the dose of ketamine recommended for the management of postoperative pain [24] has a significant effect on the NeuroSENSE WAVCNS.
In the half-dose group, WAVCNS values > 60 were observed following the initial ketamine bolus of 0.25 mg kg−1. However, data were inconclusive with respect to consistency with clinical observations; response to airway manipulation and suspected light anesthesia were observed in some cases. During maintenance of anesthesia, the WAVCNS was consistent with the clinically observed depth-of-hypnosis in both ketamine groups; the WAVCNS remained within the 40–60 ‘adequate anesthesia’ range, for median 94.7% of the time in the full-dose group and 95.2% in the half-dose group.
Inconsistency between clinical observations and the WAVCNS in the full-dose group indicate that controlling propofol infusion to a WAVCNS target between 40 and 60 may lead to overdosing following induction of anesthesia in the presence of this ketamine dosing scheme.
4.2 EEG power spectral analysis
The post-hoc analysis of EEG power spectra was performed to generate hypotheses following the unexpected WAVCNS trend observed in the full-dose group during the first 10–20 min after the start of the propofol infusion. The high β- and low γ-range (24–32 Hz) oscillations reported with low-dose ketamine, when administered as a sole agent [2, 13], were not apparent in our study. EEG power spectral estimates prior to propofol administration (reflecting the effect of ketamine and remifentanil without propofol) showed decreased power following the ketamine bolus of 0.5 mg kg−1 in the full-dose group, but not after the 0.25 mg kg−1 bolus in the half-dose group. This effect was not reflected in the WAVCNS.
Following propofol administration, the observed EEG signature indicates a ketamine dose-dependent shift in the peak frequency of the α-spindles, which has been previously reported when ketamine was co-administered with propofol [15]. A neurophysiological explanation for this α peak-frequency shift has been offered in a modeling study [30]. This change in EEG signature does not explain the observed WAVCNS response, since the WAVCNS is predominantly based on the γ-band. However, the shift in peak frequency was accompanied by an increase in relative γ-band power, which may explain the observed increase in the WAVCNS. Further investigation is required to quantify how these dose-dependent effects on the EEG signature affect the WAVCNS.
The transient increase of the WAVCNS to values inconsistent with clinically observed depth-of-hypnosis, following the ketamine bolus in the full-dose group, coincided with the transient increase in the peak frequency and relative γ-band power. We therefore speculate that the full-dose bolus of 0.5 mg kg−1 ketamine introduces a peak in ketamine EEG effect, which exceeds the effect observed for the corresponding maintenance infusion rate of 10 mcg kg−1 min−1. This would need to be confirmed by further pharmacodynamic study of the effects of ketamine on EEG, and by study of potential pharmacokinetic/pharmacodynamic interactions between propofol and ketamine.
4.3 Comparison with other pEEG monitors
The Bispectral Index (BIS), M-Entropy, and NeuroSENSE monitor use different signal processing techniques to derive a DOH index: M-Entropy uses the spectral entropy in the 0.8-32Hz frequency range, while NeuroSENSE monitor focuses on the γ-band (32–64 Hz) [10]. Both use normalization of the EEG signal or spectrum. The M-Entropy monitor did not reflect the effect of ketamine as a sole agent [16]. The findings of our study at T1 (after ketamine bolus administration, at the start of the propofol infusion) indicate that the NeuroSENSE monitor may not reflect the effect of ketamine as a sole anesthetic agent either. While the overall power decreased, the relative power and the EEG signature in the γ-band may not have changed.
The results of this study indicate a dose-dependent effect of ketamine on the WAVCNS during propofol-remifentanil anesthesia. Studies focussed on the BIS monitor also indicate a dose-dependent effect [17,18,19,20,21,22]. However, the signal processing used to derive these indices is not comparable (details of the BIS algorithm have not been published) and the specific EEG characteristics driving this effect may not be the same. Due to different study protocols, results from these studies cannot be compared quantitatively: The aim of our study was to establish feasibility of closed-loop propofol-remifentanil anesthesia guiding the WAVCNS to the 40–60 range, in the presence of a clinical ketamine infusion scheme. In contrast to prior studies quantifying the effect of ketamine on the BIS [17, 22], this clinical setting, and therefore our study protocol, involves varying ketamine dosing as well as varying propofol dosing. Differences between the ketamine and control groups may be confounded by the administered amount of propofol and the fact that the WAVCNS in the control group indicated relatively deep anesthesia.
4.4 Limitations
This feasibility study was not powered to test specific hypotheses. Due to the limited sample size and complexity of models required to describe confounding variables adequately (e.g., propofol and remifentanil dosing), no adjustments were implemented for confounding variables. Due to the exploratory nature of the analysis, all significant differences need to be confirmed in future studies.
Adequacy of anesthesia was evaluated by the anesthesiologist. Fifteen anesthesiologists participated. While anesthesia was considered adequate in all cases, there is no guarantee that the achieved depth-of-hypnosis or opioid-hypnotic balance was the same between participants or groups.
Finally, clinicians were not blinded to the ketamine dose (for safety reasons), and may have adjusted propofol and remifentanil dosing accordingly. While the WAVCNS during maintenance of anesthesia was consistent with the clinically observed adequacy of anesthesia, this study cannot conclude whether the WAVCNS is truly affected or increased by ketamine, or if it failed to represent deepening of anesthesia due to the synergy between propofol and ketamine.
5 Conclusions
The dose of ketamine recommended for management of postoperative pain (0.5 mg kg−1 bolus, 10 mcg kg−1 min−1 infusion) [24] has a significant effect on the NeuroSENSE WAVCNS during propofol-remifentanil anesthesia; during the first 15 min of anesthesia, clinical observations reflected adequate anesthesia, while the WAVCNS indicated light anesthesia or sedation. This inconsistency was not observed in the half-dose group. Anesthesiologists should be aware of the effect of this dose of ketamine when using the WAVCNS as a guide to the administration of propofol-remifentanil anesthesia and consider adjusting propofol-remifentanil dosing accordingly. Power spectral analysis indicated dose-dependent effects of ketamine on the EEG. Further investigation quantifying the effect of these EEG effects on the WAVCNS may be warranted.
References
Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2014;6:CD003843.
Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical electroencephalography for anesthesiologists: Part I: background and basic signatures. Anesthesiology. 2015;123:937–60.
Elia N, Tramèr MR. Ketamine and postoperative pain – a quantitative systematic review of randomised trials. Pain. 2005;113:61–70.
Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102:211–20.
De Kock M, Lavand’homme P, Waterloos H. “Balanced analgesia” in the perioperative period: Is there a place for ketamine? Pain. 2001;92:373–80.
Remérand F, Le Tendre C, Baud A, Couvret C, Pourrat X, Favard L, et al. The early and delayed analgesic effects of ketamine after total hip arthroplasty: a prospective, randomized, controlled, double-blind study. Anesth Analg. 2009;109:1963–71.
Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–46.
Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–23.
Jouguelet-Lacoste J, La Colla L, Schilling D, Chelly JE. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: a review of the current literature. Pain Med. 2015;16:383–403.
Zikov T, Bibian S, Dumont GA, Huzmezan M, Ries CR. Quantifying cortical activity during general anesthesia using wavelet analysis. IEEE Trans Biomed Eng. 2006;53:617–32.
West N, van Heusden K, Görges M, Brodie S, Rollinson A, Petersen CL, et al. Design and evaluation of a closed-loop anesthesia system with robust control and safety system. Anesth Analg. 2018;127:883–94.
West N, Dumont GA, van Heusden K, Petersen CL, Khosravi S, Soltesz K, et al. Robust closed-loop control of induction and maintenance of propofol anesthesia in children. Paediatr Anaesth. 2013;23:712–9.
Lee U, Ku S, Noh G, Baek S, Choi B, Mashour G. a. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–75.
John ER, Prichep LS. The anesthetic cascade: a theory of how anesthesia suppresses consciousness. Anesthesiology. 2005;102:447–71.
Hayashi K, Tsuda N, Sawa T, Hagihira S. Ketamine increases the frequency of electroencephalographic bicoherence peak on the α spindle area induced with propofol. Br J Anaesth. 2007;99(3):389–95.
Maksimow A, Särkelä M, Långsjö JW, Salmi E, Kaisti KK, Yli-Hankala A, et al. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin Neurophysiol. 2006;117:1660–8.
Vereecke HEM, Vanluchene AL, Mortier EP, Everaert K, Struys MMRF. The effects of ketamine and rocuronium on the A-Line auditory evoked potential index, Bispectral Index, and spectral entropy monitor during steady state propofol and remifentanil anesthesia. Anesthesiology. 2006;105:1122–34.
Hirota K, Kubota T, Ishihara H, Matsuki A. The effects of nitrous oxide and ketamine on the bispectral index and 95% spectral edge frequency during propofol-fentanyl anaesthesia. Eur J Anaesthesiol. 1999;16:779–83.
Wu CC, Mok MS, Lin CS, Han SR. EEG-bispectral index changes with ketamine versus thiamylal induction of anesthesia. Acta Anaesthesiol Sin. 2001;39:11–5.
Phillips W, Anderson A, Rosengreen M, Johnson J, Halpin J. Propofol versus propofol/ketamine for brief painful procedures in the emergency department: clinical and bispectral index scale comparison. J Pain Palliat Care Pharmacother. 2010;24:349–55.
Sakai T, Singh H, Mi WD, Kudo T, Matsuki A. The effect of ketamine on clinical endpoints of hypnosis and EEG variables during propofol infusion. Acta Anaesthesiol Scand. 1999;43:212–6.
Faraoni D, Salengros JC, Engelman E, Ickx B, Barvais L. Ketamine has no effect on bispectral index during stable propofol-remifentanil anaesthesia. Br J Anaesth. 2009;102:336–9.
Vereecke HEM, Agrawal G, Bibian S, Struys MMRF. The effect of ketamine and rocuronium on the neurosense WAVcns index during steady-state anesthesia with propofol and remifentanil. San Francisco: The International Society for Anaesthetic Pharmacology; 2013.
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain Elsevier. 2016;17:131–57.
Bibian S, Zikov T. NeuroSENSE Monitor with WAVcns Cortical Quantifier: A Deterministic Approach to EEG Analysis (white paper) [Internet]. 2018. http://www.neurowavesystems.com/pdfs/NeuroSENSE_White_Paper_September_2018.pdf.
Görges M, West N, Cooke E, Pi S, Brant RF, Dumont GA, et al. Evaluating NeuroSENSE for assessing depth of hypnosis during desflurane anesthesia: a randomized trial. Can J Anesth. 2019. https://doi.org/10.1007/s12630-019-01522-5.
Sahni N, Panda NB, Jain K, Batra YK, Dhillon MS, Jagannath P. Comparison of different routes of administration of clonidine for analgesia following anterior cruciate ligament repair. J Anaesthesiol Clin Pharmacol. 2015;31:491–5.
Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–82.
Babadi B, Brown EN. A review of multitaper spectral analysis. IEEE Trans Biomed Eng. 2014;61:1555–64.
Bojak I, Day HC, Liley DTJ. Ketamine, propofol, and the EEG: a neural field analysis of HCN1-mediated interactions. Front Comput Neurosci. 2013;7:1–14.
Acknowledgements
The authors would like to thank the anesthesiologists, surgeons, operating room staff, post-anesthetic care unit nurses, and surgical day care nurses at the Eagle Ridge Hospital for their interest and willingness to support study procedures, and the patients for their participation in the study.
Funding
This study was supported by internal funds. NeuroWave Systems Inc. provided loan equipment and sensors for this study.
Author information
Authors and Affiliations
Contributions
KvH contributed to study design and data analysis. EC contributed to study design, patient recruitment, data collection, and data analysis. SB contributed to study design and data analysis. NW contributed to data analysis. MG contributed to study design and data analysis. GAD contributed to study design. JMA contributed to study design. RNM contributed to study design and data collection. All authors contribution to manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
GAD is a co-inventor of the NeuroSENSE monitor (NeuroWave Systems Inc., Cleveland, OH), GAD and JMA have consulted for NeuroWave Systems Inc. KvH, MG, GAD, JMA, and RNM are party to a licensing agreement between UBC and NeuroWave Systems Inc. for control technology. GAD, JMA, and RNM have received funds for research sponsored by NeuroWave Systems Inc. The remaining authors have no interests to declare.
Informed consent
Written informed consent was obtained from all individual participants in this study.
Research involving Human Participants
This study was conducted with research ethics board approval (Fraser Health Research Ethics Board, FHREB 2016-054).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Heusden, K., Cooke, E., Brodie, S. et al. Effect of ketamine on the NeuroSENSE WAVCNS during propofol anesthesia; a randomized feasibility trial. J Clin Monit Comput 35, 557–567 (2021). https://doi.org/10.1007/s10877-020-00511-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-020-00511-0