Abstract
Poor sleep quality is associated with autonomic dysfunctions and altered pain perception and tolerance. To investigate whether autonomic dysregulations related to insomnia would still exist under general anesthesia, we adopt heart rate variability (HRV) analysis to evaluate ANS activity and surgical pleth index (SPI) to compare nociceptive/anti-nociceptive balance. We enrolled 61 adult females scheduled for gynecological surgeries under general anesthesia. All the subjects were ASA Class I to III without using medicines affecting HRV. We used the Insomnia Severity Index to evaluate sleep qualities. ECG data were recorded and signals which denote four different surgical stages were extracted (baseline, incision, mid-surgery, and end of surgery). We analyzed the HRV changes across the whole surgical period and differences among good and poor sleepers. We also compared the SPI differences among groups. For baseline HRV analysis, we found significant differences in the RMSSD (p = 0.043), pNN50 (p = 0.029), VLF power (p = 0.035), LF power (p = 0.004), and HF power (p = 0.037) between the good and poor sleeper groups. However, all intergroup differences disappeared after anesthesia induction. Temporal HRV changes significantly among different perioperative stages (RMSSD, p < 0.001; pNN50, p = 0.004; LF, p < 0.001; and HF, p < 0.001). Patients with different sleep qualities did not exhibit different SPI levels in all four periods. Poor sleepers exhibited attenuated parasympathetic activities at the baseline but no differences after the induction. Nociceptive/anti-nociceptive balance seems not be altered by poor sleep condition under general anesthesia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Insomnia is a prevalent disease which affects 20–30% of the general population in the developed countries [1,2,3] It has been reported that insomnia is associated with autonomic dysfunctions and altered pain perception and tolerance [4,5,6,7]. Even in healthy subjects, partial sleep deprivation may lead to hyperalgesia, deleterious pain inhibitory functions and changes in the activity of the autonomic nervous system (ANS) [8, 9]. However, we don’t know much about how insomnia and poor sleep quality affect ANS system when patients are under anesthesia in the surgery, neither do we know whether these insomnia patients have altered nociceptive/anti-nociceptive balance under surgical stresses.
To get a further understanding of the influence of sleep quality on the ANS activity and nociceptive/anti-nociceptive balance under general anesthesia, we adopt heart rate variability (HRV) analyses and surgical pleth index (SPI) as our targets of study. Among methodologies for ANS studies, HRV analysis of electrocardiogram (ECG) is one of the widely-used and well-known methods to evaluate the activity and modulation of the different components in ANS [10, 11]. As for the SPI, which was originally called “surgical stress index”, is an index derived from normalized heart beat interval and pulse wave amplitude of plethysmography [12, 13]. So far, there is no gold standard test or measure for “adequacy of analgesia” under general anesthesia, however, SPI has been proposed to be an indicator of nociceptive/anti-nociceptive balance under general anesthesia, and it is associated with the intensity of surgical stress and the analgesic effects of remifentanil [13,14,15]. In a prior study comparing SPI and ANS modulation of cardiac activity, changes in sympathetic activity were associated with SPI scores [16].
In our study, we hypothesized that the ANS activity and pain perception in patients with poor sleep quality may be dysregulated not only during awake but also under general anesthesia, compared with normal sleepers. Therefore our aim is to apply HRV analyses and SPI as quantitative measures to investigate the change in ANS activity and the nociceptive/anti-nociceptive balance during different stages of surgery in patients with different sleep quality.
2 Methods
The study protocol was approved by the Institutional Review Boards of the Chi Mei Medical Center on March 26, 2018 (IRB serial number: 10703-005). This observational study was conducted at Chi-Mei Medical Center in Tainan, Taiwan, with a data collecting period from March 30, 2018 to December 5, 2018. Written informed consents were obtained from all patients enrolled the study. This manuscript adheres to the applicable STROBE guidelines [17].
2.1 Participants and study design
Adult female patients (aged 20 years or more) scheduled for gynecological surgeries under general anesthesia were enrolled in this study. All the subjects were classified as American Society of Anesthesiologists Classification (ASA) I, II, or III without hypertension, arrhythmia, severe peripheral, or cardiac neuropathy as well as without any history of consuming prescription medicine that interfere with cardiac rhythm or autonomic nervous system activity. Long-term opioid users (Oral morphine equivalent > 30 mg for more than 6 weeks) were excluded.
The recording of electrocardiogram (ECG) and plethysmography signals started right after all standard monitors were deployed on patients in the operation rooms. After acquiring the baseline signal for a minimum of 5 min, general anesthesia was induced by administering intravenous anesthetics (propofol or thiopental), fentanyl, and muscle relaxant. Anesthesia was maintained by administering inhalation anesthetics (desflurane or sevoflurane), analgesics (fentanyl or morphine), and muscle relaxants. All patients who received anticholinergics, ketamine, or any other drugs that affect heart rate before the emergence of anesthesia were excluded. Patients who suffered from massive blood loss or who were hemodynamically unstable were also excluded.
2.2 Sleep quality assessment
We used the Insomnia Severity Index (ISI) to evaluate patients’ sleep qualities. The ISI is a seven-item self-reporting instrument that subjectively targets the symptoms, consequences, and concerns of insomnia. According to the literature, the ISI exhibits adequate internal consistency and validity in detecting insomnia cases [18]. Total scores of less than or equal to 7, 8–14, 15–21, and greater than 22 are interpreted clinically as absence of insomnia, sub-threshold insomnia, moderate insomnia, and severe insomnia, respectively. However, in this study, we divided the patients into two groups (good sleeper if the score was equal to or less than 7 and poor sleeper if the score was equal to or greater than 8). Subsequently, our study sample could be grouped into good sleepers (N = 31; mean age = 42.9 ± 10) and poor sleepers (N = 30; mean age = 44.8 ± 12.7).
2.3 ECG acquisition and analysis
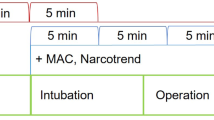
We collected data by using a personal computer (PC) with the S/5 iCollect data acquisition software (GE Healthcare Finland Oy, Helsinki, Finland). The PC was connected to a GE Datex-Ohmeda S/5 Anesthesia Monitor, and it acquired data simultaneously as the signals were displayed on a screen. The sampling rate of the ECG signal was 300 Hz. All the signals were processed offline. From the raw data file, 5-min baseline data and 10-min data from three other periods (incision, mid-surgery, and end) were analyzed. We partly adopted the open-source mhrv matlab package provided by the PhysioZoo platform for computing the inter-beat (RR) interval and HRV indices [19].
Time-domain analysis of the HRV involves determining the average of the normal interbeat intervals (AVNN), standard deviation of the normal interbeat intervals (SDNN), root-mean-square successive difference between adjacent normal interbeat intervals (RMSSD), and percentage of adjacent intervals that varied by greater than 50 ms (pNN50). Frequency-domain measures of the HRV include the high-frequency (HF) power (0.15–0.40 Hz), low-frequency (LF) power (0.04–0.15 Hz), very-low-frequency (VLF) power (0.003–0.04 Hz), and LF/HF ratio. HF power is generally interpreted as a marker of vagal modulation and is respiratory-related, whereas LF power is modulated by the sympathetic and parasympathetic systems. LF power indicates the baroreflex in response to spontaneous changes in blood pressure modulated either by the sympathetic or parasympathetic systems. The LF/HF ratio refers to the global sympatho-vagal balance, and it normally ranges between 1 and 2 in resting adults. VLF power is a major determinant of physical activity; however, its origin is controversial. The RMSSD and pNN50 in time-domain measures correlate with the HF power in frequency-domain measures, which is related to parasympathetic modulation [10, 11].
2.4 SPI analysis and of numeric rating scale (NRS)
SPI values are automatically calculated from plethysmography waveforms by a built-in software in GE monitors. We calculated the mean value of SPI in the four distinct periods (baseline, incision, mid-surgery, end) and compared the results between groups. We also recorded the NRS of each patient for pain perception after they were sent to POR once he/she were able to communicate.
2.5 Statistical analysis
The pilot sample size was 12 for each group, and this data was used to estimate power and sample size by the sample size calculator (https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html) based on the HF power during baseline period with an alpha of 0.05 and the value of power of 0.8. The power analysis showed that at least 28 subjects required for each group and our final sample consists of 31 good sleepers and 30 poor sleepers.
Spectral HRV indices were log-transformed to produce a normal distribution. SPSS Statistics software version 23 (IBM Corp. Released 2015. Version 23.0. Armonk, NY) was used for statistical analyses. The Student’s t test was used to compare numerical demographic data, and the chi-square test was used to compare nominal items between good and poor sleepers. Next, multiple linear regression with the stepwise method was used to identify demographic predictors of the SPI index. We used repeated measures analysis of variance (ANOVA) with the Greenhouse–Geisser correction to analyze the effects of anesthesia and surgery on HRV perioperatively. Furthermore, we used the Student’s t test to examine the differences in the HRV and SPI indices between good and poor sleepers. We performed partial correlation analysis to evaluate the association of the total scores on the ISI questionnaire with the HRV indices by controlling for age. A p value of less than 0.05 (two-tailed) was considered significant for all the statistical comparisons.
3 Results
3.1 Demographic data
87 women were enrolled in this study initially. Of these patients, 17 were excluded as atropine was administrated perioperatively, six were excluded because remifentanil was chosen for analgesia under general anesthesia. Of the remaining subjects, three were excluded due to too much artificial interferences in the acquiring data at data preprocessing stage. There were total 61 patients included in our final analysis, in which 31 were good sleepers and 30 were poor sleepers.
The demographic data of patients with different sleep qualities are provided in Table 1. No differences were observed in the age, body mass index, total blood loss, mean end-tidal minimum alveolar concentration (MAC) of inhalation anesthetics, surgical time, and ASA classification between the “good sleeper” and “poor sleeper” groups. The ratio of laparoscopic surgery versus open surgery exhibited no significant intergroup differences. Because we only enrolled patients scheduled for gynecological surgery, all the recruited participants were female. The only two opioids used during the intraoperative periods were fentanyl and morphine. The amounts of fentanyl and morphine consumption were the same for both the groups. The SPI index was not correlated with the ISI score or sleep quality status in all the four analysis periods. Age was the only demographic variable that correlated with the SPI (r = − 0.433, p < 0.001), and this correlation was valid only before anesthesia induction. NRS scores collected upon arrival to the post-operative recovery (POR) unit were also not correlated with sleep quality.
3.2 Temporal changes in the HRV within subjects during perioperative periods
All the HRV measures differed significantly among different perioperative periods. Posthoc tests conducted using the Bonferroni correction indicated substantial reductions in the RMSSD, pNN50, LF, and HF measures from the baseline to the incision period (p < 0.001, p = 0.004, p < 0.001, and p < 0.001, respectively). The spectral power values of the RMSSD, pNN50, and HF indices remained low and stable in the following periods, and they differed significantly from the values in the baseline period but not from the values in the incision period. After remaining stable in the incision and mid-surgery periods, another reduction occurred in the LF measure from the mid-surgery period toward the endpoint (p = 0.001). (Fig. 1) With regard to the AVNN and SDNN, the stimuli of incision elicited an increase from the baseline level to a peak value in the incision period (p < 0.001 and p = 0.024, respectively), followed by a decrease toward the endpoint. The LF/HF ratio exhibited a similar trend to the SDNN; however, it did not differ significantly across perioperative periods.
3.3 HRV in the perioperative periods between the good and poor sleeper groups
In the baseline (awake) HRV analysis, we found significant differences in the RMSSD (p = 0.043), pNN50 (p = 0.029), VLF power (p = 0.035), LF power (p = 0.004), and HF power (p = 0.037) measures between the good and poor sleeper groups (Tables 2 and 3). Good sleepers were inclined to have stronger parasympathetic activities than poor sleepers did before anesthesia induction. However, all the differences between groups disappeared after anesthesia induction. We observed depression in sympathetic and parasympathetic activity for both the groups; however, we could not detect between-group differences.
3.4 Correlation between the ISI score and HRV variables
There existed some correlations between the ISI scores and HRV variables at the baseline. The RMSSD and ISI scores were negatively correlated (r = − 0.26, p = 0.049). A negative correlation was also observed between pNN50 and the ISI scores (r = − 0.29, p = 0.024). In the frequency domain, both HF power (r = − 0.27, p = 0.038) and LF power (r = − 0.37, p = 0.004) were negatively correlated with the ISI scores. No correlation was found among the data collected in the “incision,” “mid-surgery,” and “end” periods.
3.5 The correlation between SPI and HRV variables
At the baseline, the AVNN and SPI were negatively correlated after adjusting for age (r = − 0.38, p = 0.004). Moreover, the VLF power was positively correlated with the SPI after incision (r = 0.29, p = 0.033). Toward the end of the surgery, the SDNN was positively correlated with the SPI score (r = 0.27, p = 0.048).
4 Discussion
Three key findings were obtained in the present study. First, baseline HRV analysis indicated a decrease in the parasympathetic activity of poor sleepers. The ISI scores were negatively correlated with the RMSSD, pNN50, HF power, and LF power at the baseline. However, no differences were observed between the groups after anesthesia induction. Second, the baseline SPI was negatively correlated with age; however, the age effect disappeared after anesthesia induction. Third, the sleep quality was not correlated with the SPI levels, opioid consumption, and average end-tidal inhalation anesthetic concentrations.
4.1 Effect of sleep disturbance on anesthesia
Although anesthesia is usually described by patients as being in sleep, it behaviorally and physiologically differs from sleep. Anesthesia and sleep share a few common traits; however, the interactions of anesthesia and sleep have not been completely understood yet [20]. Until recently, studies pertaining to the effects of sleep disturbance on anesthesia were rare. A popular notion in the general population is that a patient with insomnia may require a high anesthesia dose. Erden et al. found an increased end-tidal concentration of sevoflurane during the maintenance phase of anesthesia in insomnia patients [21]. By contrast, our results did not exhibit any significant difference in entropy monitoring (Datex-Ohmeda) or the mean end-tidal concentration of inhalation anesthetics across different stages of anesthesia. Therefore, the present study does not support the general notion that insomnia patients would require a higher dose of anesthesia than people with normal sleep would. However, the insomnia patients in this study and Erden’s study did not take any hypnotics, and we cannot rule the possibility that severe insomnia patients who use hypnotics may require high anesthesia. Notably, the current general practice of anesthesia does not require discontinuation of hypnotics before anesthesia, and the risk of anesthesia in relation to the use of hypnotics is yet to be determined.
4.2 Pain tolerance during anesthesia
Sleep disturbance may reduce pain tolerance [5, 6]. Our results do not support our hypothesis that sleep quality affects HRV parameters under general anesthesia. Moreover, neither SPI values nor opioid dosages were statistically different between groups. These findings may imply that decreased pain tolerance or pain modulation in the central nervous system is associated with poor sleep quality only during the awake status. Furthermore, our findings may provide objective evidence to refute the assertion that insomnia patients require considerably higher doses of anesthetic or analgesic agents compared with normal sleepers.
The results of our study indicate decreased parasympathetic activity among poor sleepers before general anesthesia, which is compatible with studies suggesting an association between altered cardiac autonomic control and poor sleep [22, 23]. However, the between-group differences disappeared after anesthesia induction. The hypnotic effect of general anesthesia could suppress HRV in the time- and frequency-domain measures [24,25,26]. There has been known suppression effect of anesthesia on HRV [11, 25]. However, we speculate that the substantial ANS depressant effects of anesthesia agents may outweigh the HRV differences among patients with poor sleep quality and normal sleepers.
A study indicated that in cases of light anesthesia, the HF power decreased in response to nociceptive stimuli in a sensitive and reproducible manner [27]. However, in the present study, the HF power, LF power, and LF/HF ratio in the four surgical periods did not correlate with the SPI index, which is an indicator of the surgical stress level. Even in certain periods when the SPI indicated inadequate analgesia (e.g., SPI > 50), the HF power was not correlated with the SPI index. This finding is in contrast to the research of Colombo et al., who showed a significant correlation between the SPI and changes in all ANS indices [16]. Furthermore, the numbers and trends of the SPI under general anesthesia should be carefully interpreted because some confounding factors, such as fluid challenge [28] or posture change [29] could interfere with SPI responses.
5 Limitations
Our study has certain limitations. First, the proportions of moderate or severe insomnia patients in our participants were not sufficiently large. Therefore, in our study, we used a lower ISI score to define insomnia than that used in clinical settings (8 vs. 15). Therefore, the changes in HRV measures that can only be observed in moderate or severe insomnia patients may be masked by the mild insomnia patients in our analysis. Second, because our study was observational in nature, neither the SPI levels nor anesthesia agent usage could be controlled. The baseline data were limited to 5-min intervals to avoid interference with clinical anesthesia practice.
6 Conclusions
In summary, poor sleepers exhibited attenuated parasympathetic activity at the baseline; however, such a difference disappeared after general anesthesia induction. Overall, the trends of the ANS activity perioperatively exhibited a peak sympathetic tone at the incision period, followed by a decline to below-baseline levels. By contrast, the parasympathetic activity dropped rapidly after anesthesia induction and remained low in the following course. No significant differences were observed in the SPI levels between good and poor sleepers in all the four periods. Our results suggest that compared with normal sleepers, patients with poor sleep quality do not require higher opioid dosage or higher concentration of inhalation anesthetics during general anesthesia.
Abbreviations
- ANOVA:
-
Analysis of variance
- ANI:
-
Analgesic nociception index
- ANS:
-
Autonomic nervous system
- ASA:
-
American society of anesthesiologists
- AVNN:
-
Average of the normal interbeat intervals
- ECG:
-
Electrocardiogram
- HF:
-
High frequency
- HRV:
-
Heart rate variability
- ISI:
-
Insomnia severity index
- LF:
-
Low frequency
- MAC:
-
Minimum alveolar concentration
- NRS:
-
Numeric rating scale
- PC:
-
Personal computer
- POR:
-
Postoperative recovery
- pNN50:
-
Percentage of adjacent intervals that varied by greater than 50 ms
- RMSSD:
-
Root-mean-square successive difference between adjacent normal interbeat intervals
- RR:
-
Inter-beat
- SDNN:
-
Standard deviation of the normal interbeat intervals
- SPI:
-
Surgical pleth index
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- VLF:
-
Very low frequency
References
Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167–74. https://doi.org/10.1016/j.jsmc.2008.02.001.
Ford ES, Cunningham TJ, Giles WH, Croft JB. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16(3):372–8. https://doi.org/10.1016/j.sleep.2014.12.008.
Chaput JP, Yau J, Rao DP, Morin CM. Prevalence of insomnia for Canadians aged 6 to 79. Health Rep. 2018;29(12):16–20.
Farina B, Dittoni S, Colicchio S, Testani E, Losurdo A, Gnoni V, Di Blasi C, Brunetti R, Contardi A, Mazza S, Della Marca G. Heart rate and heart rate variability modification in chronic insomnia patients. Behav Sleep Med. 2014;12(4):290–306. https://doi.org/10.1080/15402002.2013.801346.
Sivertsen B, Lallukka T, Petrie KJ, Steingrimsdottir OA, Stubhaug A, Nielsen CS. Sleep and pain sensitivity in adults. Pain. 2015;156(8):1433–9. https://doi.org/10.1097/j.pain.0000000000000131.
Generaal E, Vogelzangs N, Penninx BW, Dekker J. Insomnia, sleep duration, depressive symptoms, and the onset of chronic multisite musculoskeletal pain. Sleep. 2017. https://doi.org/10.1093/sleep/zsw030.
Carter JR, Grimaldi D, Fonkoue IT, Medalie L, Mokhlesi B, Cauter EV. Assessment of sympathetic neural activity in chronic insomnia: evidence for elevated cardiovascular risk. Sleep. 2018;41(6):1–9. https://doi.org/10.1093/sleep/zsy048.
Wehrens SM, Hampton SM, Skene DJ. Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scand J Work Environ Health. 2012;38(2):171–81.
Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–52. https://doi.org/10.1016/j.jpain.2013.08.007.
Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93(5):1043–65.
Ernst G. Heart rate variability. London: Springer; 2014. https://doi.org/10.1007/978-1-4471-4309-3.
Ahonen J, Jokela R, Uutela K, Huiku M. Surgical stress index reflects surgical stress in gynaecological laparoscopic day-case surgery. Br J Anaesth. 2007;98(4):456–61. https://doi.org/10.1093/bja/aem035.
Huiku M, Uutela K, van Gils M, Korhonen I, Kymäläinen M, Meriläinen P, Paloheimo M, Rantanen M, Takala P, ViertioOja H, Yli-Hankala A. Assessment of surgical stress during general anaesthesia. Br J Anaesth. 2007;98(4):447–55. https://doi.org/10.1093/bja/aem004.
Wennervirta J, Hynynen M, Koivusalo AM, Uutela K, Huiku M, Vakkuri A. Surgical stress index as a measure of nociception/antinociception balance during general anesthesia. Acta Anaesthesiol Scand. 2008;52(8):1038–45. https://doi.org/10.1111/j.1399-6576.2008.01687.x.
Bonhomme V, Uutela K, Hans G, Maquoi I, Born JD, Brichant JF, Lamy M, Hans P. Comparison of the surgical Pleth Index with haemodynamic variables to assess nociception-anti-nociception balance during general anaesthesia. Br J Anaesth. 2011;106(1):101–11. https://doi.org/10.1093/bja/aeq291.
Colombo R, Raimondi F, Corona A, Rivetti I, Pagani F, Porta VD, Guzzetti S. Comparison of the Surgical Pleth Index with autonomic nervous system modulation on cardiac activity during general anaesthesia: a randomised cross-over study. Eur J Anaesthesiol. 2014;31(2):76–84. https://doi.org/10.1097/01.EJA.0000436116.06728.b3.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008.
Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307.
Joachim A, Behar AAR, Weiser-Bitoun I, Shemla O, Alexandrovich A, Konyukhov E, Yaniv Y. PhysioZoo: a novel open access platform for heart rate variability analysis of mammalian electrocardiographic data. Front Physiol. 2018;9:1390.
Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363(27):2638–50.
Erden V, Abitagaoglu S, Guler C, Dogan Z, Kirgezen S, Abut Y Insomnia may increase anesthetic requirement. J Clin Anesth 34:367–372. https://doi.org/10.1016/j.jclinane.2016.05.020.
Spiegelhalder K, Fuchs L, Ladwig J, Kyle SD, Nissen C, Voderholzer U, Feige B, Riemann D. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res. 2011;20(1 Pt 2):137–45. https://doi.org/10.1111/j.1365-2869.2010.00863.x.
Yang AC, Tsai SJ, Yang C-H, Kuo C-H, Chen T-J, Hong C-J. Reduced physiologic complexity is associated with poor sleep in patients with major depression and primary insomnia. J Affect Disord. 2011;131(1–3):179–85. https://doi.org/10.1016/j.jad.2010.11.030.
Widmark C, Olaison J, Reftel B, Jonsson LE, Lindecrantz K. Spectral analysis of heart rate variability during desflurane and isoflurane anaesthesia in patients undergoing arthroscopy. Acta Anaesthesiol Scand. 1998;42(2):204–10.
Matchett G, Wood P. General anesthesia suppresses normal heart rate variability in humans. Chaos. 2014;24(2):023129. https://doi.org/10.1063/1.4882395.
Venet T, Pichot V, Charier D, Scalabre A, Patural H. Autonomic cardiac regulation after general anesthesia in children. Paediatr Anaesth. 2018;28(10):881–7. https://doi.org/10.1111/pan.13468.
Jeanne M, Logier R, De Jonckheere J, Tavernier B. Heart rate variability during total intravenous anesthesia: effects of nociception and analgesia. Auton Neurosci. 2009;147(1–2):91–6. https://doi.org/10.1016/j.autneu.2009.01.005.
Hans P, Verscheure S, Uutela K, Hans G, Bonhomme V. Effect of a fluid challenge on the Surgical Pleth Index during stable propofol-remifentanil anaesthesia. Acta Anaesthesiol Scand. 2012;56(6):787–96. https://doi.org/10.1111/j.1399-6576.2011.02639.x.
Ilies C, Ludwigs J, Gruenewald M, Thee C, Hanf J, Hanss R, Steinfath M, Bein B. The effect of posture and anaesthetic technique on the surgical pleth index. Anaesthesia. 2012;67(5):508–13. https://doi.org/10.1111/j.1365-2044.2011.07051.x.
Funding
Dr. Ho is supported by CMMOST 10717 and CMFHR 10403 (Chi Mei Medical Center, Tainan, Taiwan). Prof. Yang is supported by funding from the Ovation Health Institute, USA.
Author information
Authors and Affiliations
Contributions
Chun Ning Ho: This author helped the design of the work, analysis and interpretation of data, drafting the manuscript. Pei-Han Fu: This author helped acquisition data and drafting the manuscript. Kuo-Chuan Hung: This author helped data acquisition and revising draft. Jen-Yin Chen: This author helped revising important content. Jia-Hui Chang: This author helped acquisition and interpretation of data. Chung-Kang Peng: This author helped revising important content. Albert C. Yang: This author contribute to analysis and interpretation of data, revising critical content, and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ho, CN., Fu, PH., Chen, JY. et al. Heart rate variability and surgical pleth index under anesthesia in poor and normal sleepers. J Clin Monit Comput 34, 1311–1319 (2020). https://doi.org/10.1007/s10877-019-00450-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00450-5