Abstract
The Oxygen Reserve Index (ORi™) is a non-invasive variable that reflects oxygenation continuously. The aims of this study were to examine the relationship between arterial partial pressure of oxygen (PaO2) and ORi during general anesthesia, and to investigate the usefulness of ORi as an indicator to avoid hyperoxia. Twenty adult patients who were scheduled for surgery under general anesthesia with arterial catheterization were enrolled. After induction of general anesthesia, inspired oxygen concentration (FiO2) was set to 0.33, and arterial blood gas analysis was performed. The PaO2 and ORi at the time of blood collection were recorded. After that, FiO2 was changed to achieve an ORi around 0.5, 0.2, and 0, followed by arterial blood gas analysis. The relationship between ORi and PaO2 was then investigated using the data obtained. Eighty datasets from the 20 patients were analyzed. When PaO2 was less than 240 mmHg (n = 69), linear regression analysis showed a relatively strong positive correlation (r2 = 0.706). The cut-off ORi value obtained from the receiver operating characteristic curve to detect PaO2 ≥ 150 mmHg was 0.21 (sensitivity 0.950, specificity 0.755). Four-quadrant plot analysis showed that the ORi trending of PaO2 was good (concordance rate was 100.0%). Hyperoxemia can be detected by observing ORi of patients under general anesthesia, and thus unnecessary administration of high concentration oxygen can possibly be avoided.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Almost all anesthesiologists observe percutaneous oxygen saturation (SpO2) as an indicator to adjust inspired oxygen concentration (FiO2) during general anesthesia. Currently, SpO2 is indispensable in clinical practice as a monitor that can estimate the oxygen saturation of arterial blood noninvasively. SpO2 is useful for adjusting FiO2 to detect and treat hypoxemia [1]. On the other hand, when SpO2 approaches 100% (equivalent to an arterial partial pressure of oxygen [PaO2] greater than about 128 mmHg), there can be no further increase in saturation, regardless of how high the PaO2 rises [2]. Therefore, SpO2 alone is not enough to monitor oxygenation in a hyperoxic range.
In the field of general anesthesia and intensive care, there have been many reports of adverse effects related to hyperoxia. They have reported that hyperoxia is associated with the development of atelectasis [3] and elevated mortality rates of serious diseases in intensive care unit [4, 5]. Additionally, acute lung injury is also related to hyperoxia [6]. Although hyperoxia-related side effects have been reported, several studies have suggested that hyperoxia occurs frequently in the clinical setting [7, 8]. Arterial blood gas analysis is the only way to quantify hyperoxemia in the hyperoxic range [9]; however, this measurement requires arterial catheterization and oxygenation is not continuously monitored.
The Oxygen Reserve Index (ORi™) (Masimo Corp., Irvine, CA, USA) is a variable that is related to real-time oxygenation reserve status in the mild hyperoxic range (PaO2 of about 100 to 200 mmHg) [10]. ORi can be measured noninvasively by applying a sensor that is multi-wavelength pulse co-oximeter adhesive sensor. ORi is a nondimensional index that changes according to the oxygenation reserve status, from 0.00 (no reserve) to 1.00 (maximum reserve). Although some studies have examined ORi as an indicator for early detection of hypoxemia [11,12,13,14], there have been few clinical reports that focused on ORi to avoid hyperoxia in the clinical setting. We hypothesized that if the relationship between ORi and PaO2 is elucidated, it may be possible to avoid excessive hyperoxia by monitoring ORi. The aim of this study was to examine the relationship between PaO2 and ORi during general anesthesia, and investigate the usefulness of ORi as an indicator of hyperoxemia.
2 Materials and methods
2.1 Study design
This prospective study was approved by the Ethics Committee of Fukushima Medical University (No.2636), and the trial was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN000021118). Written informed consent was obtained from all individual participants.
2.2 Patient population
Twenty patients who were scheduled for surgical procedures under general anesthesia between April, 2016 and May, 2017 were enrolled. The inclusion criteria were: (1) an age of 20 years or older; (2) an American Society of Anesthesiologists physical status classification of 1 or 2; and (3) an arterial catheter insertion is required for the surgery to monitor blood pressure continuously and/or analyze arterial blood gas. The exclusion criteria were: (1) an inability to wear the sensor due to deformity of fingers; (2) an inadequate signal due to hypoperfusion of the fingers; (3) cardiac or pulmonary disease (e.g. chronic obstructive pulmonary disease, interstitial pneumonia); and/or (4) preoperative anemia due to hemoglobinopathies (e.g. sickle cell disease, thalassemia). In cases where one-lung ventilation was performed, the data were collected before or after the ventilation periods.
2.3 Data collection
In each case, after the patient entered the operating room, a sensor capable of measuring ORi (rainbow® sensor, R2–25, Revision L, Masimo Corp.) was applied to the third or fourth finger. The sensor was shielded from ambient light. The SpO2 and ORi values were displayed on the screen of Root® (software: v1593i) with Radical-7® (software: v1451i, Tech board: 7c07, Masimo Corp.).
The arterial catheter was inserted into the radial artery after induction of general anesthesia. First, the FiO2 was set at 0.33, approximately 30 min after induction of general anesthesia was completed. At each step, after changing FiO2, we waited for approximately 15 min or longer until the desired ORi value was reached and a stable equilibrium was obtained. Then, arterial blood gas analysis was performed using 1 mL blood collected from the catheter. PaO2 was obtained from arterial blood gas co-oximetry (SIMENS RAPIDLAB®1265, Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA), and the PaO2 and ORi at the time of blood collection were recorded.
Next, the FiO2 was adjusted to achieve an ORi of approximately 0.5, followed by arterial blood gas analysis and recording of the ORi and PaO2. After that, FiO2 was then adjusted again to achieve an ORi of approximately 0.2, followed by arterial blood gas analysis. If the PaO2 was less than 100 mmHg at this point, the measurement for the current study was terminated and the FiO2 was returned to optimum concentration. Otherwise, the FiO2 was adjusted to achieve an ORi of approximately 0, followed by arterial blood gas analysis and recording of the ORi. The measurement was then terminated and the FiO2 was optimized.
2.4 Statistical analysis
Data were stored using Microsoft Excel 2013, and all statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [15], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequency used in biostatistics.
For analyzing the data of PaO2 < 240 mmHg (n = 69), correlations were performed using Pearson’s correlation coefficient, and a simple linear regression analysis was performed. The threshold of PaO2 < 240 mmHg was chosen according to a previous study that investigated the relationship between ORi and PaO2 [16]. In addition, a 95% prediction intervals were determined in the data of PaO2 < 240 mmHg (n = 69).
To obtain the optimal cut-off ORi value to detect PaO2 ≥ 150 mmHg, a receiver operating characteristic (ROC) curve analysis was used. In order to confirm the predictability and diagnostic ability of the ROC curve, the area under the curve (AUC) and its 95% confidence interval (95% CI) were also determined.
To assess the trending ability of ORi, four-quadrant plot analysis was performed. For the analysis, all 80 datasets, that is, 60 changes (3 changes per case) in PaO2 (ΔPaO2) and ORi (ΔORi), were used. The concordance rate (defined as the percentage of data points in the upper right or lower left quadrant of the four-quadrant plot, where a rate of > 92% is considered good [17]) was calculated. The exclusion zone was defined as an area of percentage change in ΔORi < 0.1 and/or PaO2 < 10 mmHg according to the previous research [18].
3 Results
We obtained 80 datasets from the patients (8 males and 12 females), who had a mean age of 63 ± 15 years, and a mean BMI of 23 ± 2 kg/m2. Their characteristics are described in Table 1. There were no cases where PaO2 was lower than 100 mmHg when FiO2 was set to achieve ORi around 0.2.
3.1 Relationship between ORi and PaO2
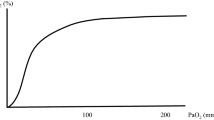
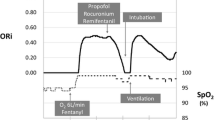
The ORi and SpO2 trends of a typical case are shown in Fig. 1. The individual differences in ORi per case and PaO2 at each four recording points are shown in Fig. 2. Figure 3a shows the relationship between the ORi and PaO2 of all 80 datasets, and Fig. 3b shows a scatter diagram of ORi obtained when PaO2 < 240 mmHg (n = 69). A linear regression analysis showed a relatively strong positive correlation (r2 = 0.706). The results of the simple linear regression analysis are shown in Table 2.
ORi and SpO2 trends during surgery (a typical case). A 24-year-old female. Height: 158 cm. Weight: 55 kg. She underwent proximal femur extensive resection and total hip arthroplasty due to left femur osteosarcoma. FiO2 was adjusted to achieve each goal of ORi, and arterial blood gas analysis was performed four times. (Four asterisks in the figure indicate the value of PaO2). Although the SpO2 remained approximately 100% during the surgery, ORi fluctuated dramatically as a consequence of the changing FiO2
a Scatterplot of all ORi and PaO2 values (n = 80). The 80 datasets, with the PaO2 plotted on the horizontal axis and the ORi plotted on the vertical axis. b The relationship between the ORi and PaO2 (PaO2 < 240 mmHg, n = 69) and 95% prediction intervals. The 69 data were showed with the PaO2 plotted on the horizontal axis and the ORi plotted on the vertical axis. A linear regression analysis (solid line) showed a relatively strong positive correlation (r2 = 0.706). Also shown are 95% prediction intervals (dashed lines)
3.2 Cut-off ORi value for hyperoxemia
Figure 4 shows the ROC curve to obtain the optimal cut-off ORi value to detect PaO2 ≥ 150 mmHg. The AUC was 0.932 (95% CI 0.875–0.979), and the cut-off value obtained from the ROC was 0.21 (sensitivity 0.950, specificity 0.755).
3.3 Correlation between PaO2 changes and ORi changes
To assess the trending ability of ORi values, we performed four-quadrant plot analysis and found that the concordance rate was 100.0% (Fig. 5).
4 Discussion
In the present study, the values of ORi and PaO2 were examined under different oxygen administration conditions during general anesthesia. We reported the individual differences in ORi, and found that there was a rather large variability in ORi values at certain PaO2 values (Figs. 2, 3a). Regarding the relationship between ORi and PaO2, Applegate et al. [16] compared 485 sets of ORi and PaO2 values, and found a positive correlation when PaO2 was lower than 240 mmHg (r2 = 0.536). Because the relationship between ORi and PaO2 could not assume as a simple linear relationship from the scatter plots of our all 80 datasets (Fig. 3a), we also analyzed data when PaO2 was less than 240 mmHg based on their report, and found a relatively strong positive correlation between PaO2 and ORi (r2 = 0.706). Moreover, Koishi et al. [14] also reported a positive correlation between PaO2 and ORi (r2= 0.671) in 101 datasets, including some data where PaO2 ≥ 240 mmHg. Although the sample size of the current study was smaller than that in the study by Applegate et al. these studies’ results showed that there is a positive correlation between ORi and PaO2. This indicates that value of ORi may suggest corresponding PaO2 values to some extent.
There are many reports on the adverse effects of hyperoxia and high concentration oxygen [3,4,5,6]; therefore, detection of unnecessary excessive hyperoxia is important. Currently, there is no gold standard for optimal PaO2 during general anesthesia. Even though their study was performed in an intensive care unit (ICU), de Jonge et al. [19] investigated the association between PaO2 and mortality in 36,307 patients who were treated with mechanical ventilation. They reported that the association of them was U-shaped, and that the nadir average mortality was at PaO2 of 15—20 kPa (113–150 mmHg), whereas mortality increased at PaO2 < 9 (68 mmHg) and > 30 (225 mmHg) kPa. In the current study, we defined hyperoxemia as PaO2 ≥ 150 mmHg to discuss cut-off ORi value for hyperoxemia. To determine the optimum ORi value for detecting hyperoxemia, we analyzed a cut-off ORi value. The AUC shown in this study was 0.932; therefore, the accuracy of the cut-off point (an ORi value of 0.21 for detecting PaO2 ≥ 150 mmHg) was high. In ordinary cases, maintaining an ORi of < 0.21 may be reasonable to prevent hyperoxia. In a recent study, Vos et al. [18] reported that the cut-off ORi value to detect PaO2 < 100 mmHg was 0.01 (sensitivity 0.99, specificity 0.82). Although they analyzed data obtained from healthy volunteers, based on the results of their study and the current study, unnecessary excessive hyperoxia might be avoided without frequent blood collection.
Regarding the variation between ORi and its matching PaO2, Applegate et al. [15] also reported that there was no correlation between ORi and PaO2 when PaO2 was 240 mmHg or higher (r2 = 0.0016). Moreover, in the current study, the variation of PaO2 was larger when in severe hyperoxemia with ORi of around 0.5, compared with when the ORi was around 0.0 or 0.2. Although ORi ranges between 0.00 and 1.00, ORi rose up to 0.6–0.7 under general anesthesia and in daily use in our facility. ORi was able to predict approximate PaO2: however, ORi value was not able to be used as an alternative to PaO2, especially in situations where ORi was relatively high. Improvements in ORi might be necessary to reduce the variation in the hyperoxic state in the future. On the other hand, regarding ORi trending ability, Vos et al. [18] reported a concordance rate of 94%. Although there were a few sample in small ΔPaO2 range due to the current study design, our results also showed a good concordance rate (100.0%). As the manufacturer reported, ORi is useful as a trending variable even in situations where ORi is relatively high.
The current study has some limitations. First is its small sample size, which affected the results. Additionally, due to the small sample size, we could not discuss factors that affect ORi or methods that more accurately predict PaO2 using ORi. It is plausible that a given patient’s factors (e.g. age, physique, body temperature, finger perfusion, hemoglobin concentration) affect their ORi value. Therefore, ORi might not reflect all changes in PaO2. Recently, the ORi scale has been updated although our dataset did not include this version. Clinicians should check the version when they interpret ORi value.
ORi has unique characteristics reflecting the state of oxygenation in the hyperoxic range. The present study examined data under general anesthesia in the operating room, but ORi’s unique features can provide benefits not only in the operating room, but also in the ICU and other fields. In the future, in order to make the most use of ORi, further clinical study is required.
5 Conclusions
Our analysis of ORi in patients under general anesthesia demonstrated that PaO2 can be predicted to some extent in a mild hyperoxic range, and there is a possibility that unnecessary administration of high concentration oxygen can be avoided.
References
Pedersen T, Nicholson A, Hovhannisyan K, Moller AM, Smith AF, Lewis SR. Pulse oximetry for perioperative monitoring. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.cd002013.
Collins JA, Rudenski A, Gibson J, Howard L, O’Driscoll R. Relating oxygen partial pressure, saturation and content: the haemoglobin-oxygen dissociation curve. Breathe. 2015;11:194–201. https://doi.org/10.1183/20734735.001415.
Aboab J, Jonson B, Kouatchet A, Taille S, Niklason L, Brochard L. Effect of inspired oxygen fraction on alveolar derecruitment in acute respiratory distress syndrome. Intensiv Care Med. 2006;32:1979–86.
Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, Donati A. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18:711. https://doi.org/10.1186/s13054-014-0711-x.
Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, Jallo J, Pineda CC, Tzeng D, McBride W, Bell R. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42:387–96. https://doi.org/10.1097/CCM.0b013e3182a27732.
Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–41. https://doi.org/10.4187/respcare.01963.
Suzuki S, Eastwood GM, Peck L, Glassford NJ, Bellomo R. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. 2013;28:647–54.
de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensiv Care Med. 2011;37:46–51.
Saugel B, Belda FJ. The oxygen reserve index in anesthesiology: a superfluous toy or a tool to individualize oxygen therapy? Minerva Anestesiol. 2018;84:1010–2. https://doi.org/10.23736/S0375-9393.18.13103-8.
Scheeren TWL, Belda FJ, Perel A. The oxygen reserve index (ORI): a new tool to monitor oxygen therapy. J Clin Monit Comput. 2018;32:379–89. https://doi.org/10.1007/s10877-017-0049-4.
Szmuk P, Steiner JW, Olomu PN, Ploski RP, Sessler DI, Ezri T. Oxygen reserve index: a novel noninvasive measure of oxygen reserve—a pilot study. Anesthesiology. 2016;124:779–84. https://doi.org/10.1097/ALN.0000000000001009.
Yoshida K, Isosu T, Noji Y, Hasegawa M, Iseki Y, Oishi R, Imaizumi T, Sanbe N, Obara S, Murakawa M. Usefulness of oxygen reserve index (ORi™), a new parameter of oxygenation reserve potential, for rapid sequence induction of general anesthesia. J Clin Monit Comput. 2018;32:687–91. https://doi.org/10.1007/s10877-017-0068-1.
Niwa Y, Shiba J, Fujita H, Oka R, Takeuchi M. Oxygen reserve index (ORi™) contributes to prediction of hypoxemia and patient safety during tracheal stent insertion using rigid bronchoscopy: a case report. J Clin Monit Comput. 2018;1:2. https://doi.org/10.1007/s10877-018-0232-2.
Koishi W, Kumagai M, Ogawa S, Hongo S, Suzuki K. Monitoring the oxygen reserve index can contribute to the early detection of deterioration in blood oxygenation during one-lung ventilation. Minerva Anestesiol. 2018;84:1063–9. https://doi.org/10.23736/S0375-9393.18.12622-8.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Applegate RL 2nd, Dorotta IL, Wells B, Juma D, Applegate PM. The relationship between oxygen reserve index and arterial partial pressure of oxygen during surgery. Anesth Analg. 2016;123:626–33. https://doi.org/10.1213/ANE.0000000000001262.
Critchley LA, Lee A, Ho AM. A critical review of the ability of continuous cardiac output monitors to measure trends in cardiac output. Anesth Analg. 2010;111:1180–92.
Vos JJ, Willems CH, van Amsterdam K, van den Berg JP, Spanjersberg R, Struys MMRF, Scheeren TWL. Oxygen reserve index: validation of a new variable. Anesth Analg. 2018. https://doi.org/10.1213/ane.0000000000003706.
de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, Bosman RJ, de Waal RA, Wesselink R, de Keizer NF. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156. https://doi.org/10.1186/cc7150.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of Fukushima Medical University (No. 2636) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoshida, K., Isosu, T., Noji, Y. et al. Adjustment of oxygen reserve index (ORi™) to avoid excessive hyperoxia during general anesthesia. J Clin Monit Comput 34, 509–514 (2020). https://doi.org/10.1007/s10877-019-00341-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00341-9