Abstract
Numerous factors could contribute to sleep disturbances in women with breast cancer. We hypothesized that stellate ganglion block (SGB) during surgery would preserve sleep after surgery and increase intraoperative regional cerebral oxygen saturation (rSO2) on the blocked side in patients undergoing breast cancer surgery. A randomized, double-blinded, controlled trial was conducted at the First Hospital of China Medical University from January 2016 to September 2016. Ninety-six patients who underwent radical breast cancer surgery requiring general anaesthesia were randomly assigned to one of two study groups: a control group that received a saline SGB and a block group that received a 0.25% ropivacaine hydrochloride SGB. The primary outcome measure was the postoperative sleep profile, which was assessed using the bispectral index on the first postoperative night. The secondary outcome measure was the intraoperative rSO2, monitored was throughout surgery using near-infrared spectroscopy. A total of 91 female patients (mean age: 45 years; range 24–51 years) were included in the study. The duration of sleep was significantly increased by 66.3 min in the ropivacaine-SGB group compared with the saline-SGB group. No differences in rSO2 were observed on either the left or right side of the patients in either group 50 min after anaesthesia induction. We conclude that ropivacaine-SGB combined with general anaesthesia might increase the first postoperative sleep duration without influencing the intraoperative rSO2 in female patients undergoing elective breast cancer surgery.
Clinical trials.gov identifier NCT02651519
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sleep disturbances are prevalent in women with breast cancer [1], and, along with depression and fatigue, have been identified as a symptom cluster among these patients [2]. Numerous factors could contribute to sleep disturbances in women who undergo chemotherapy, radiotherapy, surgical treatment or hormonal therapy. However, few studies have evaluated the relationship between postoperative sleep disturbances and anaesthesia management in women who have undergone surgical treatment.
Ultrasound-guided C6 stellate ganglion block (SGB) was first described in 1995 [3]. This technique may improve the safety of the procedure and provide valuable therapeutic benefits for patients with chronic pain [4], postoperative pain [5], and CO2-pneumoperitoneum-induced sympathetic neural excitation [6]. Furthermore, SGB has been suggested to provide breast cancer survivors with relief from hot flashes and sleep dysfunction with few or no side effects [7, 8].
Near-infrared spectroscopy (NIRS) is a technique that has been clinically applied to monitor regional cerebral oxygen saturation (rSO2) since the 1980s [9]. Researchers have investigated the influence of SGB on bilateral cerebral oxygenation using NIRS [10, 11]. Both studies concluded that SGB decreases cerebral blood flow in the non-blocked hemisphere.
Little information is available regarding the effects of ultrasound-guided SGB on postoperative sleep and intraoperative rSO2 in patients undergoing breast cancer surgery. Therefore, we hypothesized that performing ultrasound-guided SGB during surgery would preserve sleep quality after breast cancer surgery and increase the intraoperative rSO2 on the blocked side in these patients.
2 Methods
2.1 Study design
This study is a prospective, randomized, controlled, and double-blinded trial. Major assessments were made during the operation and the first postoperative night. We followed the Consolidated Standards of Reporting Trials recommendations in designing and reporting the findings of our study. The trial was approved by the Ethics Committee of the First Hospital of China Medical University (protocol number 2015110302, Chairman Prof. Xing-hua Gao, December 14, 2015, Trial registration: NCT02651519 Principal investigator’s name: Wen-fei Tan, Date of registration: 2016-01-05 https://clinicaltrials.gov/ct2/show/NCT02651519?term=NCT02651519&rank=1) and was registered with the Clinical Trials Registry (NCT02651519). All participants provided written informed consent in accordance with the Declaration of Helsinki.
2.2 Patients
One hundred five patients undergoing radical breast cancer surgery were enrolled to the treatment intervention with general anaesthesia and ultrasound-guided SGB from January 2016 to September 2016 at the First Hospital of China Medical University. The objective of the trial was to evaluate the intraoperative rSO2 and postoperative sleep quality of patients undergoing radical breast cancer surgery, which includes an SGB administered with ropivacaine hydrochloride (n = 48) or saline solution (n = 48). All patients were monitored with NIRS during the entire perioperative period and a bispectral index (BIS)-Vista monitor during the first postoperative night.
2.3 Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) age between 18 and 55 years old (pre-menopausal); (2) scheduled to undergo elective radical breast cancer surgery; and (3) American Society of Anaesthesiologists (ASA) risk classification I–II.
The exclusion criteria were as follows: (1) patient refusal; (2) known hypersensitivity to the study medication (ropivacaine); (3) long-term use of opioids; (4) a history of psychiatric or neurological disease; and (5) a preoperative Pittsburgh Sleep Quality Index (PSQI) global score higher than 6.
2.4 Randomization and masking
The study patients were randomly assigned via computer-generated sequences placed into sealed envelopes to the following two groups: a general anaesthesia + saline SGB (control group) and a general anaesthesia + 0.25% ropivacaine hydrochloride SGB (block group). Treatment allocation was revealed by opening the envelope on the morning of surgery. All patients, the anaesthesiologist performing the block, and the staff involved in postoperative data collection and analyses were blinded to the group allocations. The trial was monitored by an independent data and safety monitoring organization. The group allocations were not revealed until the final statistical analysis was completed.
2.5 Interventions
2.5.1 Before anaesthesia
All patients were assessed with the PSQI 1 day before surgery [12]. The PSQI differentiated between good sleepers (PSQI global score < 6) and poor sleepers (PSQI global score ≥ 6). No additional requirements or preoperative oral analgesics were permitted. After patients were moved into the operating room, standard monitoring was performed, including systolic blood pressure, diastolic blood pressure, heart rate, electrocardiography, and blood oxygen saturation. Regional cerebral oxygen saturation was monitored throughout surgery with NIRS (FORE-SIGHT™, CAS Medical Systems, Branford, CT, USA). Sensors to detect rSO2 were placed on the right and left forehead and covered with opaque tape to prevent light interference. RSO2 values from the right and left sides were recorded directly to determine cerebral oxygenation from the FORE-SIGHT data every 2 s.
2.5.2 General anaesthesia
General anaesthesia was induced as follows: 2 mg/kg intravenous (IV) propofol, 2 mg IV midazolam, 0.4 μg/kg IV sufentanil, and 0.2 mg/kg IV cisatracurium. The patients’ lungs were ventilated with intermittent positive pressure. After intubation, the tidal volume was adjusted to 6–8 ml/kg, and the ventilator rate was adjusted to maintain an end-tidal CO2 of 35–45 mmHg. To maintain anaesthesia, sevoflurane (Baxter Healthcare of Puerto Rico, Guayama, Puerto Rico) was used at an end-tidal concentration of 2–2.5%, and an air-oxygen (FiO2: 50%) mixture. An additional IV dose of sufentanil and 0.05 mg/kg cisatracurium were administered as needed. At the end of surgery, the trachea was extubated after the return of spontaneous respiration and neuromuscular function, and patients were transferred to the post-anaesthesia care unit (PACU).
2.5.3 SGB technique
Before surgery and 15 min after the induction of anaesthesia (after rSO2 had returned to baseline), right-side single-shot SGB was performed under ultrasound guidance by an anaesthesiologist. A broadband (5–12 MHz) linear array ultrasound probe (S-Nerve Ultrasound System, Sonosite, Bothell, WA, USA) was used with an imaging depth of 2–4 cm. An insulated PAJUNK (PAJUNK, GmbH, Medizin Technologie, Geisingen, Germany) needle (50 mm, 21 gauge) was introduced a few millimetres from the probe using an in-plane technique. At the C6 level, the linear probe was placed at the anterior scalene muscle between the carotid sheath and the brachial plexus. Under direct vision, the needle tip was placed posterior to the carotid artery, anterior to the longus colli muscle and under the transverse short axis, and 6 ml 0.25% ropivacaine hydrochloride or 6 ml saline was injected. This injection was performed by a single investigator using a completely aseptic technique.
2.5.4 Postoperative analgesia, nausea and vomiting
Regardless of group allocation, all patients received flurbiprofen axetil 50–100 mg for postoperative analgesia, and postoperative nausea and vomiting was treated with 5 mg IV tropisetron (administered in the ward).
2.5.5 Postoperative nocturnal sleep: the BIS-Vista monitor
Postoperative nocturnal sleep was evaluated with the BIS-Vista monitor (Medtronic-Covidien, Dublin, Ireland), and the following three outcome measures were measured in this study: duration of sleep, sleep efficiency index (SEI), and area under the curve (AUC) [13]. Sleep was defined as a BIS less than 80 [14]. Duration of sleep was defined as the duration of all BIS data less than 80 during the 10 h of monitoring (from 20:00 to 06:00). SEI was defined as the ratio of a patient’s total sleep time over the time available for “nocturnal” sleep (10 h). The BIS-AUC was calculated using the trapezoidal rule, which uses trapeziums to approximate the region under a curve and to calculate its area (GraphPad Prism version 5.01). Each night, the AUC values were set to missing if the recordings were less than 10 h in duration. Each patient was in a private room when they were transferred to the ward. All family members who were present were aware of the rules of the trial.
2.6 Follow-up visits
When the patients were awake in the PACU, signs of Horner’s syndrome and changes in temperature were recorded to assess whether sympathetic block was appropriately achieved. The patients in this trial were visited the next morning to assess sleep quality and analgesic effects.
2.7 End of participation in the study
Patients were excluded from the study for any of the following reasons: (1) refusal to participate; (2) an anaesthesia time exceeding 4 h; or (3) treatment with additional sedatives or analgesics during the postoperative BIS-Vista monitoring period.
2.8 Criteria for removal from the study
During the study, patients who met any of the following criteria were removed from the study: (1) the loss of more than 500 ml of blood during surgery; (2) an operation time exceeding 3 h; (3) a violation of the trial protocol; or (4) a desire to withdraw from the study.
2.9 Study outcomes
The primary outcome measure was postoperative sleep, which was assessed using BIS data for the first postoperative night between the two groups. The secondary outcome was to compare intraoperative rSO2, which was monitored throughout surgery using NIRS.
2.10 Sample size
The sample size was calculated based on the average (mean ± standard deviation [SD]) sleep durations on the first postoperative night calculated in the pilot study (control group: 223.2 ± 113.1 min; ropivacaine group: 256.3 ± 56.3 min). The formula for determining sample size [15] was n = 15.7/ES2 + 1, where ES is the effect size, which is defined as the difference between the groups divided by the mean of the SD between the groups, with α = 0.05 and power = 0.8. The study was adequately powered with n = 48 in each group.
2.11 Statistical analysis
Statistical analyses were performed using SPSS software, version 22 for Windows (IBM, Armonk, NY, USA). A fully specified statistical analysis protocol was written in an independent manner. Before statistical testing, each continuous variable was analysed to determine whether it had a normal distribution using the Kolmogorov–Smirnov test. Continuous data are described as the mean (± SD) or median (25% and 75% percentiles) and were analysed with the independent t test, the Mann–Whitney U test or the Friedman signed-rank test. Categorical data are described as a frequency or percentage and were analysed by the Chi square test. The R package (http://cran.r-project.org/) was used for figure creation. A P value < 0.05 was considered significant.
3 Results
3.1 Patient characteristics
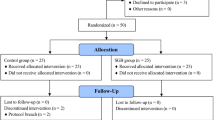
Of the 105 potential patients assessed for eligibility, nine patients were excluded; thus, 96 patients were randomly assigned to one of two groups. One patient in the saline-SGB group was lost to follow-up because of a BIS equipment error, and one patient in the saline-SGB group was excluded from the analysis because of sedative treatment. One patient in the ropivacaine-SGB group was lost to follow-up because of refusal to undergo BIS monitoring, and two patients in the ropivacaine-SGB group were excluded from the analysis because of sedative treatment (Fig. 1). A total of 91 female patients (mean age: 45 years; range 24–51 years) were included in the final study analysis. Table 1 presents the results of the demographic and preoperative characteristics of the two groups. There was no significant difference in these characteristics between the two groups.
3.2 BIS-AUC, duration of sleep and BIS-SEI during the entire nocturnal sleep period
The BIS-AUC in the ropivacaine-SGB group was significantly lower than in the saline-SGB group, and the SEI was significantly higher in the ropivacaine-SGB group, indicating “better” sleep [13] (Table 2). Furthermore, sleep duration was significantly increased by 66.3 min in the ropivacaine-SGB group compared with the saline-SGB group. One example of the first postoperative night BIS data in the two groups is shown in Fig. 2.
3.3 Changes in intraoperative rSO2
The analysis incorporated 1500 observation points, which consisted of measurements of the examined variables obtained every 2 s for 50 min on both the left and right side of all patients (Fig. 3). The average-rSO2-AUC was calculated using the trapezoidal rule, which uses trapeziums to approximate the region under a curve and calculate its area (GraphPad Prism version 5.01) (Fig. 3). There were no changes in the rSO2 on either the left or right side of the patients in both groups 50 min after anaesthesia induction (Fig. 3). Examples of the intraoperative rSO2 of a patient in the saline-SGB group (Fig. 4a) and a patient in the ropivacaine-SGB group (Fig. 4b) are shown.
Time course analysis of the regional cerebral tissue oxygen saturation data of 50 min. Thick red lines indicate median values. Thick black lines indicate the upper and lower boundaries of the 95% confidence interval. ‘Time 0’ is the beginning of anaesthesia induction. Data were collected every 2 s from induction until 50 min after stellate ganglion block (SGB). Average-rSO2-AUC, area under the curve; the values represent the median [25th percentile, 75th percentile]; P = 0.598 by the Friedman signed-rank test
4 Discussion
The results of this study suggest that patients who receive ropivacaine-SGB may have longer sleep duration after elective breast cancer surgery compared with patients treated with saline-SGB. Intraoperative rSO2 did not differ between the ropivacaine-SGB and saline-SGB patients, and no difference in intraoperative rSO2 was observed between the block side and non-block side.
SGB is used in pain treatment for atypical facial pain, migraines, and complex regional pain syndrome of the chest, is a selective sympathetic block that influences the ipsilateral head, neck, and upper extremity. The efficacy of SGB is assessed based on the presence of Horner’s syndrome. The mechanism of action of SGB is not completely clear but may involve peripheral vasodilation, resulting in neural inhibition in the ganglion’s sphere of innervation [16]. The first postoperative night sleep may be preserved because the parasympathetic nervous system is dominant. Furthermore, SGB is a safe procedure and may provide extended relief for all clusters of post-traumatic stress disorder symptoms, including sleep disturbance [17]. SGB can be safely used to treat hot flashes and sleep dysfunction in survivors of breast cancer [7, 8, 18]. BIS-Vista has been used in our previous sleep measurement studies [19,20,21], and a recent study suggested that BIS monitoring can provide a useful measure of natural physiological sleep depth [22]. Although the exact mechanism by which SGB influences sleep is unclear, selective sympathetic block may be responsible for the results reported above as well as our current results.
SGB may increase rSO2 in awake patients [11]. Our results suggest that there is no difference in intraoperative rSO2 between the block side and non-block side. This finding is the result of several factors, such as anaesthetic and flow-metabolism coupling. During anaesthesia induction, changes in rSO2, which increased to a peak value and then return to baseline rSO2 at 15 min. We performed the SGB procedure 15 min after anaesthesia induction to avoid the influence of anaesthesia induction on rSO2 (Fig. 4a, b). In contrast to pulse oximetry, NIRS-based rSO2 does not differentiate between arterial and venous blood but continuously and noninvasively measures relative concentrations of oxyhemoglobin and deoxyhemoglobin. Under most circumstances, the contribution from the cerebral venous saturation predominates; therefore, rSO2 does not provide an indicator of oxygen delivery but rather provides information on the balance between regional oxygen supply and demand [23]. Although SGB is a selective sympathetic block and may involve peripheral vasodilation, it is not reliable enough to influence the balance between regional oxygen supply and demand. There were similar changes in the rSO2 for both the block and non-block side in our research.
This study has several limitations. The main deficiency was the lack of validation with a full polysomnogram. BIS is a method to monitor the effect of aesthetic drugs; the algorithm was not designed or validated for sleep stages. We also need to observe the postoperative sleep disturbance for a longer postoperative period. Finally, our study included only female patients and thus does not avoid gender bias. Considering these limitations, we conclude that ropivacaine-SGB combined with general anaesthesia might increase the first postoperative sleep duration without influencing intraoperative rSO2 in female patients undergoing elective breast cancer surgery.
Change history
01 June 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10877-023-01040-2
References
Costa AR, Fontes F, Pereira S, Goncalves M, Azevedo A, Lunet N. Impact of breast cancer treatments on sleep disturbances: a systematic review. Breast. 2014;23:697–709. doi:10.1016/j.breast.2014.09.003.
Ho SY, Rohan KJ, Parent J, Tager FA, McKinley PS. A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J Pain Symptom Manag. 2015;49:707–15. doi:10.1016/j.jpainsymman.2014.09.009.
Kapral S, Krafft P, Gosch M, Fleischmann D, Weinstabl C. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. A pilot study. Reg Anesth. 1995;20:323–8.
Perrine DC, Votta-Velis G, Borgeat A. Ultrasound indications for chronic pain management: an update on the most recent evidence. Curr Opin Anaesthesiol. 2016;29:600–5. doi:10.1097/aco.0000000000000369.
Kumar N, Thapa D, Gombar S, Ahuja V, Gupta R. Analgesic efficacy of pre-operative stellate ganglion block on postoperative pain relief: a randomised controlled trial. Anaesthesia. 2014;69:954–60. doi:10.1111/anae.12774.
Chen Y, Xie Y, Xue Y, Wang B, Jin X. Effects of ultrasound-guided stellate ganglion block on autonomic nervous function during CO2-pneumoperitoneum: a randomized double-blind control trial. J Clin Anesth. 2016;32:255–61. doi:10.1016/j.jclinane.2016.03.019.
Lipov EG, Joshi JR, Sanders S, Wilcox K, Lipov S, Xie H, Maganini R, Slavin K. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. Lancet Oncol. 2008;9:523–32. doi:10.1016/s1470-2045(08)70131-1.
Haest K, Kumar A, Van Calster B, Leunen K, Smeets A, Amant F, Berteloot P, Wildiers H, Paridaens R, Van Limbergen E, Weltens C, Janssen H, Peeters S, Menten J, Vergote I, Morlion B, Verhaeghe J, Christiaens MR, Neven P. Stellate ganglion block for the management of hot flashes and sleep disturbances in breast cancer survivors: an uncontrolled experimental study with 24 weeks of follow-up. Ann Oncol. 2012;23:1449–54. doi:10.1093/annonc/mdr478.
Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–7.
Park HM, Kim TW, Choi HG, Yoon KB, Yoon DM. The change in regional cerebral oxygen saturation after stellate ganglion block. Korean J Pain. 2010;23:142–6. doi:10.3344/kjp.2010.23.2.142.
Kim EM, Yoon KB, Lee JH, Yoon DM, Kim do H. The effect of oxygen administration on regional cerebral oxygen saturation after stellate ganglion block on the non-blocked side. Pain Physician. 2013;16:117–24.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi:10.1186/cc6871.
Tung A, Lynch JP, Roizen MF. Use of the BIS monitor to detect onset of naturally occurring sleep. J Clin Monit Comput. 2002;17:37–42.
Lerman J. Study design in clinical research: sample size estimation and power analysis. Can J Anaesth. 1996;43:184–91. doi:10.1007/bf03011261.
Klein RN, Burk DT, Chase PF. Anatomically and physiologically based guidelines for use of the sphenopalatine ganglion block versus the stellate ganglion block to reduce atypical facial pain. Cranio. 2001;19:48–55.
Lynch JH, Mulvaney SW, Kim EH, de Leeuw JB, Schroeder MJ, Kane SF. Effect of stellate ganglion block on specific symptom clusters for treatment of post-traumatic stress disorder. Mil Med. 2016;181:1135–41. doi:10.7205/milmed-d-15-00518.
Fisher WI, Johnson AK, Elkins GR, Otte JL, Burns DS, Yu M, Carpenter JS. Risk factors, pathophysiology, and treatment of hot flashes in cancer. CA Cancer J Clin. 2013;63:167–92. doi:10.3322/caac.21171.
Tan WF, Guo B, Ma H, Li XQ, Fang B, Lv HW. Changes in postoperative night bispectral index of patients undergoing thoracic surgery with different types of anaesthesia management: a randomized controlled trial. Clin Exp Pharmacol Physiol. 2016;43:304–11. doi:10.1111/1440-1681.12530.
Jin F, Li XQ, Tan WF, Ma H, Lu HW. Preoperative versus postoperative ultrasound-guided rectus sheath block for improving pain, sleep quality and cytokine levels of patients with open midline incisions undergoing transabdominal gynaecological operation: study protocol for a randomised controlled trial. Trials. 2015;16:568. doi:10.1186/s13063-015-1096-0.
Tan WF, Wang ZL, Ma H, Jin F, Lu HW. Changes in the first postoperative night bispectral index of patients after thyroidectomy with different types of primary anesthetic management: a randomized controlled trial. J Clin Monit Comput. 2017. doi:10.1007/s10877-016-9974-x.
Benissa MR, Khirani S, Hartley S, Adala A, Ramirez A, Fernandez-Bolanos M, Quera-Salva MA, Fauroux B. Utility of the bispectral index for assessing natural physiological sleep stages in children and young adults. J Clin Monit Comput. 2015. doi:10.1007/s10877-015-9800-x.
Germon TJ, Evans PD, Barnett NJ, Wall P, Manara AR, Nelson RJ. Cerebral near infrared spectroscopy: emitter-detector separation must be increased. Br J Anaesth. 1999;82:831–7.
Funding
This work was funded by the Natural Science Foundation of Liaoning Province (2014021035) to Wen-fei Tan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest arising from this study.
Ethics approval
The trial was approved by the Ethics Committee of the First Hospital of China Medical University (protocol number 2015110302, Chairman Prof. Xing-hua Gao, December 14, 2015, Trial registration:NCT02651519 Principal investigator’s name: Wen-fei Tan, Date of registration: 2016-01-05 https://clinicaltrials.gov/ct2/show/NCT02651519?term=NCT02651519&rank=1) and was registered with the Clinical Trials Registry (NCT02651519).
Informed consent
All participants provided written informed consent in accordance with the Declaration of Helsinki.
About this article
Cite this article
Jin, F., Li, Xq., Tan, Wf. et al. RETRACTED ARTICLE: Effects of ultrasound-guided stellate-ganglion block on sleep and regional cerebral oxygen saturation in patients undergoing breast cancer surgery: a randomized, controlled, double-blinded trial. J Clin Monit Comput 32, 855–862 (2018). https://doi.org/10.1007/s10877-017-0074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-017-0074-3