Abstract
Previous studies have shown that sugammadex decreases the anesthetic depth when administered to reverse the neuromuscular blockade produced by rocuronium/vecuronium. The aim of the present study was to investigate the effect of sugammadex alone on anesthetic depth and hemodynamics. Sixty patients scheduled for abdominal surgery participated in the study. Anesthesia was induced with thiopental/fentanyl and maintained with N2O/oxygen and sevoflurane concentrations adjusted to maintain Entropy and Bispectral Index (BIS) values between 40 and 50. Cis-atracurium 0.2 mg/kg was administered for neuromuscular blockade which was monitored with a TOF-Watch® SX acceleromyograph. State entropy (SE), response entropy (RE), Bispectral Index (BIS), systolic (SAP) and diastolic blood pressure (DAP), heart rate (HR), SpO2, end-tidal CO2 and sevoflurane concentrations were recorded every 3 min intraoperatively. Sugammadex 2 mg/kg (Group-2), 4 mg/kg (Group-4) or 16 mg/kg (Group-16) was given intravenously when a count of two responses of the train-of-four (TOF) or a post-tetanic count (PTC) 1-3 appeared or when no response at all (PTC = 0) was observed, respectively. The overall SE values, thus the primary outcome of the study, were 44 ± 11, 43 ± 10 and 43 ± 11 for Group-2, Group-4 and Group-16, respectively (p = 0.812). Also, the secondary endpoints, namely RE, BIS, SAP and DAP, HR and SpO2 did not differ between the three groups. Comparisons between Group-2 versus Group-4, Group-2 versus Group-16 and Group-4 versus Group-16 showed no differences (p > 0.05) for all the studied variables. Sugammadex alone at low, medium or high clinical doses has no effect on anesthetic depth as assessed by Entropy and BIS or on hemodynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sugammadex, a γ cyclodextrin, reverses the variable degrees of neuromuscular block produced by rocuronium and vecuronium and enhances their elimination rate [1].
Several studies suggest that along with reversal of the neuromuscular block, sugammadex also decreases the depth of anesthesia. This effect has been demonstrated for the doses of 200 mg and 4 mg/kg which are recommended for reversal of moderate or deep neuromuscular block [2, 3]. Also doses of 1, 2, 4, 6, or 8 mg/kg of the drug given 3, 5, or 15 min after 0.6 mg/kg of rocuronium decreased the depth of anesthesia in about one-fifth of anesthetized patients compared to the control group [4].
Neuromuscular block has been associated with decreased anesthetic requirements [5]. A possible explanation is that the muscle stretch receptors send signals via muscle afferents stimulating arousal centers in the brain [6]. Therefore reversal of neuromuscular block restores the proprioceptive input arising from these receptors, arousing so the brain. Other investigators reported conflicting data on the effect of reversal of neuromuscular block on the depth of anesthesia [7]. As sugammadex does not penetrate the intact blood brain barrier, it is expected to be devoid of central or peripheral neural and muscular effects, when given alone.
The cardiovascular safety profile of sugammadex when administered to reverse the neuromuscular block induced by rocuronium has been assessed in cardiac and noncardiac patients [8, 9]. However, as our study investigates the effect of sugammadex alone on depth of anesthesia, a possible lightening of anesthesia is assumed to be accompanied by increases in heart rate and blood pressure.
Our hypothesis was that sugammadex alone administered at the recommended clinical doses will not affect the Entropy values, BIS values, hemodynamics, pulse oximetry and end-tidal CO2 during moderate, deep and intense neuromuscular block.
The aim of the present study was to investigate the effect of sugammadex alone, at doses applicable to the clinical setting, on state entropy (SE), response entropy (RE) and BIS, as well as on hemodynamics excluding the effects produced by the reversal of neuromuscular block. For this reason a benzylisoquinoline neuromuscular blocker was used.
2 Patients and methods
The study was approved by the Research and Ethics Committee of Aretaieio University Hospital Athens, Greece (No Σ-150/03-08-10) and was registered in the ClinicalTrials.gov registry under the number NCT01301261. Written informed consent was obtained from all patients participating in the study. The study followed the CONSORT guidelines.
Patients of both sexes, aged between 30 and 80 years with physical status ASA I-III, scheduled for elective abdominal surgery were eligible for this prospective, randomized, double blind study. The first patient entered the study on December 29, 2010 and the last patient on April 24, 2014.
Exclusion criteria were hypertension, central nervous system disease and treatment with antihypertensive drugs, sedatives, tranquilizers or other central nervous system depressants, thus factors which might influence measurements of the primary and secondary outcomes of the study. Other reasons for exclusion were severe hepatic or renal impairment.
2.1 Randomization and blinding
Patients were randomly assigned to three groups of 20 patients each, by means of a computer generated program.
Group-2 (G2) received 2 mg/kg of sugammadex when a count of two responses of the train of four (TOF) appeared. The patients received an equal volume of normal saline 3–5 min after cis-atracurium injection (when PTC was 0) and when a post-tetanic count (PTC) 1-3 appeared.
Group-4 (G4) received 4 mg/kg of sugammadex when a PTC 1-3 appeared. These patients received the same volume of normal saline 3–5 min after cis-atracurium injection when PTC was 0, and also when two of the four responses of the TOF appeared.
Group-16 (G16) received 16 mg/kg of sugammadex 3–5 min after cis-atracurium injection when the PTC was 0. The patients received an equal volume of normal saline when a PTC 1-3 was obtained and also when two of the four responses of the TOF appeared.
To assure blinding of investigators, three identical syringes containing normal saline or sugammadex according to group allocation were prepared for each patient. The syringe barrel was covered with tape and labelled as “PTC 0”, “PTC 1-3” and “TOF 2” by a nurse not involved in the study. The syringe volumes were calculated as if containing sugammadex at the appropriate dose, according to the group. This way all syringes seemed identical to the blinded anesthesiologist involved in the study.
2.2 Anesthetic technique and monitoring
Premedication was omitted. All patients received standardized anesthesia with fentanyl 2 μg/kg and thiopental 4–5 mg/kg for induction, followed by cis-atracurium 0.2 mg/kg to facilitate tracheal intubation and provide surgical muscle relaxation. Anesthesia was maintained with sevoflurane in an oxygen/nitrous oxide mixture and incremental doses of fentanyl 2–4 μg/kg. Sevoflurane inspired concentration was adjusted to maintain BIS and Entropy values between 40 and 50. After that, all measurements were performed under steady-state conditions of anesthesia.
Electrocardiogram (ECG), non invasive arterial blood pressure (systolic—SAP- and diastolic—DAP), heart rate (HR), pulse oximetry (SpO2) and end-tidal CO2 (ETCO2) were monitored through the procedure using the S/5 Anaesthesia Monitor, (Datex-Ohmeda, Helsinki Finland). The S/5 Entropy-Module of the above Anesthesia Monitor was used for monitoring the SE and RE as evaluative parameters of the anesthetic depth. The SE mainly includes components from the electroencephalogram (EEG) and is determined at a frequency range of 0.8 and 32 Hz, while RE registers frontal electromyography (EMG) and EEG and operates at a frequency range between 0.8 and 47 Hz [10]. Depth of anesthesia was also assessed by BIS monitor (BIS A-2000; Aspect Medical Systems, Newton, MA, USA) connected to a BIS™ sensor attached to patient’s forehead. Both Entropy and BIS provide dimensionless numbers (from 0 to 100), with higher values denoting wakefulness.
Neuromuscular transmission monitoring of the adductor pollicis muscle was also implemented. The TOF-Watch® SX acceleromyograph (Organon Ltd, Dublin, Ireland) was used. Two surface electrodes were attached to the volar area of the wrist over the ulnar nerve. After induction of anesthesia the transducer of the acceleromyograph was calibrated by pressing the appropriate button for more than 1 s. The TOF response (2 Hz stimulus of 0.2 ms duration—at 60 mA-delivered every 15 s) and PTC were recorded every 3 min until a 25 % recovery of TOF was obtained. Skin temperature in the adductor pollicis muscle was also recorded every 3 min. The appropriate dose of sugammadex and the relevant two placebos according to group allocation were administered as indicated by the degree of the neuromuscular block (moderate, deep or intense).
When a 25 % of TOF was obtained the study protocol was accomplished and a second dose of cis-atracurium depended upon the surgery demands, and was given at the discretion of the anesthesiologist. At the end of surgery residual neuromuscular block was reversed by neostigmine with atropine according to the clinical practice implemented in our department.
2.3 Measurements
The SE, RE, BIS, SAP, DAP, HR, SpO2, ETCO2, inspired and end-tidal sevoflurane concentration (SEVOinsp and SEVOet, respectively) and temperature of the palmar surface of the hand were recorded as soon as a PTC 0 number was obtained after the cis-atracurium injection (baseline) and every 3 min thereafter up to a 25 % TOF recovery. Immediately after baseline recordings the first syringe labelled “PTC 0”—containing sugammadex or normal saline according to group allocation—was administered to the patient.
The SE was defined as the primary outcome while RE, BIS, SAP, DAP, HR and SpO2 were the secondary endpoints of the study.
2.4 Power calculation and statistical analysis
Initial sample size estimation showed that approximately 20 patients were needed in each group to detect a clinical difference of SE by 20 % with a power of 0.80 and level of significance of 5 %.
To assess differences in patients’ characteristics between the three treatment groups, Kruskal–Wallis Test for non-normally distributed responses was carried out. A linear mixed model on the logarithm was fit in order to assess treatment differences over time. This model has time and treatment as fixed effects and a random effect for subject in order to allow for correlation between repeated measurements on the same individual. In all the above models, an interaction term between time and treatment was also included in order to assess whether the differences between two treatment groups were constant over time. If a significant effect was to be found, post hoc comparisons (adjusted for Bonferroni correction) would be carried out to assess at which point in time the two groups were significantly different.
3 Results
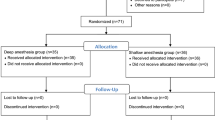
The three groups did not differ with regards to age, body weight or height (Table 1). The patients underwent gynecological or bowel surgery; the distribution of surgical procedures in each group is shown in Table 1. Of the 60 patients enrolled in the study, one patient in G4 group and one patient in G16 group did not complete the study. The excluded patients, the dropouts and the reasons are presented in the flow diagram (Fig. 1).
The overall mean values of SE, RE, BIS, SAP and DAP, HR, SpO2, ETCO2, SEVOinsp and SEVOet in the groups G2, G4 and G16 that received the three different doses of sugammadex were similar (p > 0.05 for all comparisons, Table 2). Also, comparisons over time between the three groups (G2 vs. G4, G2 vs. G16, and G4 vs. G16) regarding the SE, RE, BIS, SAP, DAP, HR and SpO2 revealed no significant differences (Tables 3, 4).
4 Discussion
The results of the present study demonstrate that sugammadex when given alone in the absence of neuromuscular block reversal does not affect the depth of anesthesia as assessed by entropy and BIS monitoring. Hemodynamics remained also unchanged.
Several studies support or refute the hypothesis that neuromuscular blockers change the depth of anesthesia, therefore reversal of neuromuscular block would also affect the anesthetic depth in the opposite direction. In humans pancuronium has been shown to decrease halothane requirements [5]. Reversal of neuromuscular block produced by atracurium in patients anesthetized with propofol and remifentanil increased the BIS and middle-latency auditory evoked potential values [11]. Sparr et al. [4] in a study of early reversal of deep rocuronium-induced neuromuscular block by sugammadex reported clinical signs of inadequate depth of anesthesia and increases in BIS values in about 20 % of patients after sugammadex administration, although depth of anesthesia was not a variable included in the study protocol. In another study with total intravenous anesthesia, the reversal of rocuronium-induced neuromuscular block with sugammadex or neostigmine resulted in increased BIS values [3].
In 13 patients under steady state sevoflurane anesthesia, tetanic electrical stimulation implemented as noxious stimulus after deep rocuronium neuromuscular block produced significantly smaller effect on the EEG and BIS responses compared to the pre-rocuronium effect. The investigators interpreted these results as possible inhibition of signals otherwise generated in the muscle spindles and reaching the brain [12]. Aho et al. [2] reported that reversal of rocuronium with sugammadex 200 mg produced increases in Entropy and BIS values in patients under light anesthesia with target control infusion of propofol and remifentanil. These results are not consistent with those reported by Illman et al. [7]. The investigators attributed the difference in their results after sugammadex administration to the different depths of anesthesia between the two studies and related the arousal effect of sugammadex to the higher BIS values.
However, some studies have shown conflicting results. In volunteers anesthetized with propofol and receiving mivacurium, Greif et al. [13] reported no differences in BIS values and frontal-temporal EMG intensities for the different degrees of neuromuscular block produced by mivacurium. Also, patients anesthetized deeply with propofol and remifentanil and received rocuronium as neuromuscular blocker did not exhibit increases in BIS or entropy values after reversal of the neuromuscular block with 2 mg/kg of sugammadex. The authors conclude that patients anesthetized with propofol/remifentanil who receive sugammadex to reverse the neuromuscular block are not at risk to experience premature awareness [7].
So far the effect of sugammadex on BIS, entropy or auditory evoked potentials levels was linked to reversal of the neuromuscular block, without discriminating between a possible effect produced by neuromuscular block reversal or sugammadex itself. Taking into account these contradictory results we investigated the effects of sugammadex alone on the depth of anesthesia and hemodynamics under steady state anesthesia by administering a neuromuscular blocker not being affected by the presence of sugammadex. Thus, we demonstrated that sugammadex alone did not change the depth of general anesthesia with sevoflurane. In contrast to previous clinical studies investigating only low to moderate doses of sugammadex on the depth of anesthesia, we also included the dose recommended for reversal of intense neuromuscular block. As possible changes in depth of anesthesia are likely to be associated with hemodynamic changes, hemodynamic variables (BP and HR) were also monitored and recorded at the same time points with the entropy and BIS values.
Sugammadex alone is not given to patients, therefore the present study design is not applicable to clinical practice. Nevertheless, changes in entropy and BIS values, as well as changes in hemodynamics and hemoglobin saturation should be attributed to reasons other than sugammadex itself.
In conclusion, our results demonstrate that sugammadex alone does not affect the depth of anesthesia or patient hemodynamics in contrast to sugammadex given for reversal of the neuromuscular block produced by rocuronium or vecuronium.
References
Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex. Anesthesiology. 2008;109:816–24.
Aho AJ, Kamata K, Yli-Hankala A, Lyytikainen L-P, Kulkas A, Jantti V. Elevated BIS and Entropy values after sugammadex or neostigmine: an electroencephalographic or electromyographic phenomenon? Acta Anaesthesiol Scand. 2012;56:465–73.
Dahaba AA, Bornemann H, Hopfgartner E, Ohran M, Kocher K, Liebmann M, Wilfinger G, Metzler H. Effect of sugammadex or neostigmine neuromuscular block reversal on bispectral index monitoring of propofol/remifentanil anaesthesia. Br J Anaesth. 2012;108:602–6.
Sparr HJ, Vermeyen KM, Beaufort AM, Rietbergen H, Proost JH, Saldien V, Velik-Salchner C, Wierda JMKH. Early reversal of profound rocuronium-induced neuromuscular blockade by sugammadex in a randomized multicenter study efficacy, safety, and pharmacokinetics. Anesthesiology. 2007;106:935–43.
Forbes AR, Cohen NH, Eger EI. Pancuronium reduces halothane requirement in man. Anesth Analg. 1979;58:497–9.
Lanier WL, Iaizzo PA, Milde JH, Sharbrough FW. The cerebral and systemic effects of movement in response to a noxious stimulus in lightly anesthetized dogs. Possible modulation of cerebral function by muscle afferents. Anesthesiology. 1994;80:392–401.
Illman H, Antila H, Olkkola KT. Reversal of neuromuscular blockade by sugammadex does not affect EEG derived indices of depth of anesthesia. J Clin Monit Comput. 2010;24:371–6.
Dahl V, Pendeville PE, Hollmann MW, Heier T, Abels EA, Blobner M. Safety and efficacy of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in cardiac (heart failure) patients undergoing noncardiac surgery. Eur J Anaesthesiol. 2009;26:874–84.
Cammu G, Coart D, De Graeve K, Beelen R. Reversal of rocuronium-induced neuromuscular block with sugammadex in heart failure patients: a prospective observational study. Acta Anaesthesiol Belg. 2012;63:69–73.
Musizza B, Ribaric S. Monitoring the depth of anaesthesia. Sensors. 2010;10:10896–935.
Vasella FC, Frascarolo P, Spahn DR, Magnusson L. Antagonism of neuromuscular blockade but not muscle relaxation affects depth of anaesthesia. Br J Anaesth. 2005;94:742–7.
Ekman A, Fink R, Eriksson L, Brudin L, Sandin R, Sundman E. Neuromuscular block and the electroencephalogram during sevofurane anaesthesia. NeuroReport. 2007;18:1817–20.
Greif R, Greenwald S, Schweitzer E, Laciny S, Rajek A, Caldwell JE, Sessler DI. Muscle relaxation does not alter hypnotic level during propofol anesthesia. Anesth Analg. 2002;94:604–8.
Acknowledgments
The authors thank Mrs. Aikaterini Dimitriou for helping in the statistical analysis of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Registration: ClinTrials.gov NCT01301261.
Rights and permissions
About this article
Cite this article
Fassoulaki, A., Chondrogiannis, K. & Staikou, C. Sugammadex at both high and low doses does not affect the depth of anesthesia or hemodynamics: a randomized double blind trial. J Clin Monit Comput 31, 297–302 (2017). https://doi.org/10.1007/s10877-016-9844-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9844-6