Abstract
The aim of this work is to develop a self-nanoemulsifying drug delivery system (SNEDDS) for Hesperidin (HES) and Rutin (RUT) to improve their biopharmaceutical properties. The wound healing potential of HES-RUT-SNEDDS was compared to those of pure HES suspension (HES-s), empty SNEDDS (E-SNEDDS), and standard Fusidic Acid via topical application. To produce various HES-RUT-loaded SNEDDS, aqueous phase titration was used to select cinnamon oil, Labrasol and Tween 80 (surfactants), Transcutol (co-surfactant) from a diverse pool of surfactants, oils and co-surfactants. The thermodynamic stability of HES-RUT-loaded SNEDDS was assessed by examining the globule size, surface morphology, zeta potential, polydispersity index (PDI), and percent (%) transmittance. The improved physicochemical properties of the optimized HES-RUT-SNEDDS (S-N4) formulation included particle size, zeta potential, and % transmittance. Smooth and spherical particles were discovered using Atomic Force Microscopy (AFM). These improved SNEDDS formulations demonstrated enhanced solubility and skin permeation. When compared to HES-s, E-SNEDDS, and standard fusidic acid, the optimized HES-RUT-SNEDDS demonstrated significant wound healing activity following topical application. HES-RUT-SNEDDS is a promising approach for enhancing the wound-healing potential of HES and RUT through topical administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound is a physical injury caused by internal or external skin tearing, breaking, or fractures. Poor wound healing can significantly lower an individual’s quality of life and may result in immobility and functional disability. Wound healing is an intricate cascade of cellular and biochemical events, which preserves the structural features of tissues while supporting injured tissues in regaining their normal physiological integrity. This entails constant interactions between cells and the matrix, enabling a range of associated events like inflammation, proliferation, and remodeling of damaged tissues [1, 2].
Different drugs can be administered through penetration or orally to heal wounds, however, systemic drug delivery can pose severe side effects and quickly deactivate the loaded drug, rendering it worthless [3]. Additionally, topically administered drugs have not been very helpful in promoting wound healing due to skin barriers, the drug’s physicochemical properties including, insufficient skin permeability, decreased solubility, instability due to hostile conditions in the wounded areas, and undesirable side effects [4]. Therefore, the development of an innovative topical drug delivery system is required for efficient wound healing.
Flavonoids are one the most contemplated groups among various phytochemicals, widely known for their anti-microbial, antioxidant, anti-inflammatory, antiulcerogenic, antiviral, anticancer and immune-modulatory activities [5]. HES has been found with numerous biological activities like antioxidant, anti-inflammatory, analgesic and anti-microbial [6]. However, the wound healing ability of HES has been revealed from both clinical trials and experimental animal models [7]. HES therapy accelerates angiogenesis and vasculogenesis by up-regulating the expression of vascular endothelial growth factor C (VEGF-c), angiopoietin-1 (Ang-1)/ tyrosine kinase with immunoglobulin and epidermal growth (Tie-2), transforming growth factor beta (TGF-β) and protein, SMAD-2/3 messenger ribonucleic acid (mRNA) to improve wound healing in progressive diabetic foot ulcers [8]. While its topical administration speeds up the healing of both natural and radiation-induced wounds. This healing effect was as a result of nuclear factor erythroid 2-related factor (NRF2) pathways being stimulated, scavenging of free radicals and radiation, and suppression of cyclooxygenase-2 (COX-II) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB) pathways [7]. HES has also been shown to provide many advantages for cutaneous functioning including wound healing and anti-inflammation [9].
RUT is another common flavonol glycoside, which has, numerous pharmacological actions, including cytoprotective and antioxidative properties, and healing of wounds. RUT exerts free radical scavenging action on oxidizing species like superoxide radicals, which is crucial for accelerating the healing process after injury [10]. Commercial antioxidative drugs for wound healing have been developed using these rutin-related capabilities. RUT substantially promoted fibroblast proliferation and collagen formation in vitro, according to studies [11].
Despite their remedial benefits, HES and RUT have several limitations encompassing poor water solubility, low permeability, which greatly restrict their therapeutic utility [12]. Various techniques have been employed to address the problems with solubility of HES and RUT including, polysaccharide hydrogel [13], and self-emulsifying drug delivery system (SEDDs) [14]. To validate HES and RUT’s efficacy, however, greater attention needs to be given to their solubility and bioavailability issues.
The emerging nano-drug delivery approaches based on nanomedicine have demonstrated significant potential for enhancing the pharmacological profile of different bioactive phytoconstituents. More recently, SNEDDS have been developed for topical administration to enhance the pharmacological properties of various plant-derived compounds [15]. SNEDDS are nano-emulsion pre-concentrates in which the drug is incorporated into an oil phase, using a surfactant and a co-surfactant that can produce incredibly small nanoscale droplets or nano-emulsions when slightly agitated in aqueous medium. These systems outperform other colloidal drug delivery systems intended for topical administration in wound healing, due to their simple manufacturing technique, low processing cost, thermodynamic stability and nanoscale droplets [3]. Additionally, SNEDDS may deliver the drugs deeper into skin tissues in large quantities, resulting in faster and better wound healing [16]. In view of the above discussion, this study is aimed to produce novel HES-RUT-SNEDDS for topical application to ensure efficient wound healing in an animal model.
Experimental
Materials and Methods
HES was procured from Sigma (Germany) and RUT monohydrate was purchased from (Daejung Chemicals & Metals CO., LTD, Korea). Merck Darmstadt (Germany) supplied oleic acid, olive oil, soya bean oil, cinnamon oil, nutmeg oil, Tween 80, labrasol, Tween 20, transcutol, polyethylene glycol 400 (PEG 400), and propylene glycol (PPG). Cremophor EL and captex 300 were supplied by Shanghai Macklin Biochemical China; the remaining solvents utilized in this investigation were of analytical grade.
Drug Solubility Study
To choose the most relevant constituents from a wide range of ingredients, exhibiting sufficient solubility for the chosen drug, the solubility of HES and RUT in various vehicles (surfactants, oils, and co-surfactants) was evaluated. For this purpose, 1 mL of each selected vehicle was mixed with the excess drug and the mixture was vortexed for 15 min prior to shaking for 24 h at room temperature with the help of a shaker. The mixture was then subjected to centrifugation at 12,000 rpm for 15 min. The supernatant was dissolved in 2 mL methanol and evaluated. UV-visible spectrophotometer (UV-240, Shimadzu, Kyoto, Japan) was used to measure the amount of drug solubilized in each vehicle at 287 nm (for HES) and 356 nm (for RUT), respectively. According to linear working plots, obtained as a result of UV-visible spectra, R2 value of 0.9907 was true for HES while in case of RUT, the R2 value was 0.9921. The limit of detection (LOD) value for HES plot was 0.1 mg/ mL while that for RUT plot was 0.04 mg/ mL. The corresponding limit of quantification (LOQ) value was 0.3 mg/ mL for HES while in case of RUT it was 0.12 mg/ mL.
Selection of Surfactant and Co-surfactant
The surfactant was selected on the basis of its emulsifying ability as described previously [17]. The count of inversions needed for the emulsification of the lipid phase in water was used to determine surfactant emulsification potential. Various surfactants were combined with equal amounts of the selected oil (100 mg) before being heated at 45–55 °C. After adding 30 mL of distilled water to the mixture, the count of inversions necessary for the emulsification of oil in aqueous medium was noted. The obtained mixture may be allowed to stand for 2 h before being tested for percent transmittance at 638 nm, using a UV-vis spectrophotometer with distilled water as a blank. In contrast, to improve the surfactants emulsifying ability was the main criteria for the selection of a co-surfactant. A suitable co-surfactant was chosen using a turbidimetric approach from a broad range of co-surfactants. Hence, the chosen surfactant was blended with different co-surfactants in a 2:1 ratio to produce mixtures named “S-mix”. To get homogeneous ingredients, the mixtures were subjected to heating at 45–50 °C before oil was added to the S-mix in a ratio of 1:1. To prepare nano-emulsions, every mixture solution (250 mg) can be emulsified in 30 mL of distilled water. The relative turbidity of the transparent nano-emulsions was evaluated and allowed to stand for 2 h before recording their % transmittance as described for surfactant testing.
Construction of Pseudo Ternary Phase Diagram
To optimize the described components for the SNEDDS formulation, a ternary phase diagram of olive oil, cinnamon oil, labrasol, Tween 20, PEG 400 and PEG 200 was constructed. Several mixtures of the phase diagram were developed by changing the concentrations of cinnamon oil, olive oil, labrasol, Tween 20, PEG 400, and PEG 200 in the ranges of 40 to 55%, 20 to 30%, and 15 to 20%, respectively, until the final volume of every single mixture reached 100%. Different formulations were prepared prior to the phase diagram development. The first was comprised of 55% oil, 15% co-surfactant and 30% surfactant. The oily phase was kept constant while preparing the additional compositions by lowering the surfactant concentration by 25% and raising the co-surfactant concentration to 100%. To determine the optimal SNEDDS formulation, an experimental design of 11 formulations was conducted by adjusting the concentrations of oils, surfactants and co-surfactants employing MODDE software (version 12.1). Following the nano-emulsification of 100 mg of each mixture in 10 mL of distilled water, a Zeta-Sizer was used to analyze the zeta potential, size, and size distribution. Zeta-Sizer (Zetasizer Nano ZS90 Malvern Instruments, Malvern, UK) was used to investigate the size, zeta potential and size distribution, following the nano-emulsification of 100 mg of each mixture in 10 mL of distilled water.
Preparation of HES-RUT-SNEDDS
Based on its smaller size, one formulation from the ternary phase diagram was chosen for the HES-RUT-SNEDDS preparation. To obtain drug-loaded HES-RUT-SNEDDS, formulations N2 (Tween 20 and labrasol: 30%, PEG 200 and PEG 400: 15%, olive oil and cinnamon oil: 55%) were chosen for drug loading. The formulations were supplemented with 32 mg RUT and 1.6 mg HES, which were then dissolved with the help of vortex mixing. The drug-incorporated SNEDDS were stored for 24 h, before dilution and size evaluation. The ability to produce the smallest particle size with the highest zeta potential value and spontaneous emulsification efficiency, HES-RUT-SNEDDS was selected as the drug-loaded formulation.
Characterization
Percent (%) Transmittance
A 10 µL sample of each SNEDDS formulation was mixed with a 10 mL of distilled water. The obtained nano-emulsion was examined for phase separation and drug precipitation utilizing distilled water as a blank and a measurement of the % transmittance at 638 nm.
Evaluation of Droplet Size, Polydispersity Index and Zeta Potential
To determine the droplet size, zeta potential and PDI of the formulated SNEDDS, a zeta sizer was employed. 10 µL of SNEDDS were reconstructed in 10 mL of distilled water to produce nano-emulsion and their globule size, zeta potential, and PDI parameters were evaluated. All characterization procedures were accomplished in triplicate.
Analysis of Thermodynamic Stability
Heating-cooling Cycle
To evaluate the stability of the chosen formulation, 3 heating and cooling cycles were conducted. HES-RUT-SNEDDS was subjected to 4 ºC for 24 h, then 45 ºC for the next 24 h. Three cycles were completed in total. After each cycle, any possible separation of phases and drug precipitation within the formulation were observed.
Freeze-thaw Cycle
Following an incubation period of 24 h, for each temperature, 3 freeze-thaw cycles were carried between − 20 ºC and 25 ºC. For each cycle, any drug precipitation or phase separation that experienced a stress condition was recorded.
Centrifugation
To investigate the effect of centrifugation-induced stress against SNEDDS formulation, a reconstituted 100 mg of HES-RUT-SNEDDS solution in 10 mL of distilled water was centrifuged at 12,000 rpm for 15 min. The nano-emulsions that resulted were then examined for the presence of phase separation or drug precipitation.
Robustness to Dilution
The stability of HES-RUT-SNEDDS was evaluated under more diluted conditions. In this procedure, distilled water and buffer solutions with pH values of 4.6, 6.8, and 1.2, at ratios of 250, 500 and 1000 were used to dilute the SNEDDS formulation. The finally prepared nano-emulsions were noted for physical changes such as precipitation and coalescence.
FTIR Analysis
An FTIR Spectrophotometer (IR-470, Shimadzu, Kyoto, Japan) was employed to evaluate HES, RUT and HES-RUT-SNEDDS for the potential interactions between the ingredients used in the SNEDDS formulation and the loading drug. Solid samples were analyzed using the KBr disc method, while liquid SNEDDS was directly examined in the FTIR Spectrophotometer.
Atomic Force Microscopy (AFM)
To characterize the morphology of the designed SNEDDS as well as HES and RUT-loaded SNEDDS preparation, atomic force microscopy (AFM, 5500, Agilent, Santa Clara, USA) was employed. When the SNEDDS had been sufficiently diluted, a drop of sample was placed on a mica slide, dried under air at 25 °C, and then evaluated for AFM imaging with the help of a microscope, using non-contact mode.
Analysis of Wound Healing Process
The animal study was conducted on Wistar rats (strain, female albino weight: 200–250 g) for the evaluation of HES-RUT-SNEDDS formulation efficacy in wound healing activity. The animals were kept in strictly controlled conditions of temperature and humidity within the animal house. Animals were allowed to use standard food and water ad libitum throughout the study and were housed in a 12-hour light-dark cycle. The animal study was strictly adhered to the ethical standards established for the use of laboratory animals (1979). We employed a rat model of wound excision for the evaluation of wound healing process. Animals were placed in 5 groups (n = 6) at random. The animals were anesthetized with ether, depilated and then wounded. The dorsal areas of each anesthetized rat were epilated before soaking in 70% ethanol solution.
A full-thickness acute excision circle of 10 mm was made on the dorsal aspects of each animal. To relieve pain, a subcutaneous injection of lidocaine hydrochloride (2%) having 1: 80,000 epinephrine (4.4 mg/kg) was administered near the injured area as soon as the animal was wounded. Animals were placed in separate cages to avoid further injury from physical encounters. Excluding those left untreated, each animal’s wounded area was applied with 10 mg/kg of body weight from each assigned therapy. Then, a Vaseline Gauze dressing was applied to the wounded area, and it was replaced once every day. Group 1: Control group; untreated animals.
Group 2: Optimized SNEDDS without HES and RUT-loaded (E-SNEDDS) treated animals.
Group 3: Topically treated once daily with pure hesperidin suspension (HES-s).
Group 4: HES and RUT-loaded optimized SNEDDS (10 mg/kg body wt.) treated animals.
Group 5: Positive control; Fusidic acid (Fusidin; 10 mg/kg body wt.), treated animals.
Each animal’s wounded area was assessed and documented on days 0, 3, 7, 10, and 14. On day 14, all rats were sacrificed by ether overdose.
The animal related study was approved by Ethical Committee of Abdul Wali Khan University, Mardan, KPK, Pakistan, via Ethical Approval # EC/AWKUM/2023/40 dated February 02, 2023 with certification number (Ref. No./ AWKUM/2023).
Wound Measurement
The diameter of the wounded area was measured for each animal in all five groups with the help of a Vernier Caliper (0–150 nm). The percentage of wound contraction was measured by employing the following formula [18].
Statistical Analysis
All outcomes were presented as mean ± SEM. Statistical analysis was conducted with the help of two-way ANOVA followed by Bonferroni post-test. All of the analyses were performed using the Graph Pad Prism 5 software. P value less than 0.05 was considered statistically significant.
Results and Discussion
Drug Solubility Study
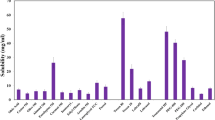
To identify a suitable surfactant, co-surfactant and oil for the formulation of HES-RUT-SNEDDS, a drug solubility analysis was performed. A greater solubilizing potency of oils, co-surfactants and surfactants is crucial for the drug packaging in SNEDDS formulation [19, 20]. The solubility of HES in various oils, surfactants, and co-surfactants is depicted in Fig. 1 (A & B). Olive oil exhibited the greater solubilizing capability for HES with a solubility of 3.2 mg/mL. Tween 20 and PEG 400 exhibited maximum solubilizing capabilities from the surfactants and co-surfactants, with 3.2 mg/mL and 4.0 mg/mL, respectively. Likewise, cinnamon oil possessed a greater solubilizing ability for RUT (70 mg/mL). Among the surfactants, labrasol and PEG 400 demonstrated solubilizing capacities of 13 mg/mL and 8 mg/mL, respectively, as depicted in Fig. 2 (A &B).
As discussed previously, HES and RUT exhibit hydrophobic characteristics, rendering them less soluble in water. However, they readily dissolve in a range of polar solvents, including methanol, acetonitrile, ethanol, and similar compounds [21]. While screened oils exhibited limited solubility in hydrocarbons as they are mainly composed of fatty acid esters which possess a greater proportion of hydrocarbon constituents. Consequently, the increased hydrocarbon content in screened oils hinders the solubility of HES and RUT in these substances. The exceptional solubility of cinnamon oil could be attributed to its polar nature. HES and RUT possess hydrogen bond donors that readily engage in hydrogen bonding and pi-pi interactions with polar media. The presence of cinnamaldehyde in cinnamon oil may be responsible for its capacity to solubilize RUT within acceptable limits, which renders the oil more polar than other lipids.
Selection of Surfactant and Co-surfactant
Emulsification is a key parameter to consider when choosing surfactants and co-surfactants to design stable SNEDDS. The hydrophilic-lipophilic balance (HLB), emulsion base viscoelasticity, and lipid-surfactant affinity are some of the important factors that determine a surfactant emulsifying capacity [22]. Oil-in-water nano-emulsions are formed when the HLB values of surfactants are greater than 10 [23]. All of the surfactants utilized in the current study were non-ionic in nature and demonstrated an HLB value greater than 10. Pharmaceutical formulations are commonly incorporated with non-ionic surfactants since they are less toxic than ionic surfactants [24]. Moreover, non-ionic surfactants are not very sensitive to variations in pH and ionic strength [25] and they are therefore more effective in oral delivery systems [26]. A specified oil phase was used to treat with different surfactants to achieve maximum compatibility of both components. The % transmittance values are shown in (Table 1). Except for labrasol, all of the tested surfactants were highly compatible with olive oil. They were able to produce nano-emulsions with transmittance values higher than 96%. The studied surfactants also displayed high compatibility with cinnamon oil. Their ability to produce nano-emulsions with transmittance values higher than 95% was successful. Based on the findings of drug solubility and % transmittance, Tween 20 and labrasol were chosen for the manufacture of SNEDDS. Co-surfactants are included in lipid-based formulations to improve the absorption and dispersibility of drug molecules [27]. PEG 400 and PEG 200 were among the co-surfactants tested in this study. Each of the co-surfactants was found to be effective because they improved the nano-emulsification of the selected surfactants Tween 20 and labrasol. When evaluated for emulsification with Tween 20 and labrasol, they revealed a transmittance of more than 95%. PEG 200 and PEG 400 were chosen as co-surfactants for the formulation and development of the intended SNEDDS preparation due to their drug solubility and surfactant emulsification properties.
Construction of Ternary Phase Diagram
A ternary phase diagram of oil, surfactant and co-surfactant was designed to locate the self-nanoemulsifying region and optimize the concentrations of selected components. By adjusting the concentrations of surfactant, oil and co-surfactant, different ternary mixtures were prepared. Following 100 times dilution with distilled water, all of the mixtures were evaluated for PDI, size and zeta potential determination. A reduction in droplet size occurs when the concentration of surfactant is increased while the concentration of co-surfactant is decreased. However, when the concentration dropped, the main constituent of SNEDDS (oil) had a direct impact on droplet size. The colored area on the ternary phase diagram indicates the self-nano emulsifying area. The change in color of each contour corresponds to its size, zeta potential and PDI. The largest area covered by the blue color specifies that multiple compositions can be constructed to produce droplet sizes in the 102 nm ranges, whereas more possible component combinations for achieving SNEDDS formulations with a zeta potential of -22 mV are depicted by the green color in the zeta-potential contour. According to the PDI contour, more compositions can be constructed to attain a PDI in the range of 0.3 as depicted in Fig. 3.
Table 2 is a reflection of value incorporated in Fig. 3 showing specific values of particle size, zeta potential and PDI.
Characterization
Droplet size, Polydispersity Index, Zeta Potential and Shape Determination
The particle size of SNEDDS is one of the key factors that greatly influence the loading, release and absorption of loading drugs. The releasing drug finds a large surface area due to the smallest droplet sizes. Also, SNEDDS absorption is affected by droplet size since smaller droplets are quickly absorbed, thereby increasing the oral bioavailability of the loaded medication [28, 29]. The particle size of E-SNEDDS was measured to be 93.47 ± 08 nm with a PDI of 0.368 ± 0.08. The droplet in the emulsions or suspensions influences the rate of drug release and other parameters like biodistribution, absorption and therapeutic efficiency. As the SNEDDS are based on forming emulsions with droplet size below 200 nm, hence they can improve the above qualities to a great extent as compared to normal systems. In our case the resuling droplet size is suitable and according to the size needed in case of SNEDDS. A PDI closer to zero shows a uniform particles distribution and is needed for consistent drug release while higher value close to 1 shows a broad size distribution which indicates instability and inconstant drug release. In our case these values are directed toward zero rather than 1 which indicate acceptable stability. Following HES and RUT loading, the particle size was increased to 182.67 ± 08 nm with a PDI of 0.372 ± 0.02, as shown in (Table 3) and Fig. 4 (A & B).
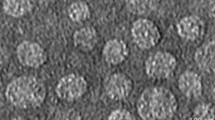
When HES and RUT were encapsulated, the PDI also increased slightly. Another crucial feature that affects the physical stability of the SNEEDS droplets is their zeta potential. The nano-emulsion droplets remain suspended due to their higher zeta potential (positive or negative) [26]. The zeta potentials of E-SNEDDS and HES-RUT-SNEDDS were determined to be -21.9 ± 0.50 and − 26.7 ± 1.30 mV, respectively, as shown in Fig. 5 (A & B). Both of these values especially in case of HES-RUT-SNEDDS confirm higher the physical stability. The negative charge observed on the surfaces of both SNEDDS formulations can be linked to the presence of hydroxyl groups within the structure of the co-surfactant. The zeta potential of the formulations provides evidence of their ability to resist droplet aggregation, which can be attributed to the repulsive forces triggered by the presence of droplets with similar charges. The surface morphologies of E-SNEDDS and drug-loaded SNEDDS formulations were assessed using AFM. As depicted in Fig. 6, the droplets were found with spherical shape.
Thermodynamic Stability
The thermodynamic stabilities of emulsions and nano-emulsions could be used to distinguish them from one another. Because SNEDDS spontaneously turns into nano-emulsions upon dilution, the formulation should be robust to creaming and precipitation of all kinds.
During prolonged storage, the drug frequently precipitates and starts to form crystals; these crystals grow with time and begin to settle at the bottom surface of the vessel [30]. Based on this information, the long-term stability of both HES-RUT-SNEDDS formulations was tested against different stress conditions such as temperature stress (i.e., heating-cooling cycle, freeze-thaw cycle) and centrifugal stress. The findings of the study revealed that the developed SNEDDS formulations (HES-RUT-SNEDDS) exhibited greater thermodynamic stability and these were not proven to any of the above instability issues.
Robustness to Dilution
The SNEDDS formulations are pre-concentrates that produce oil in water (o/w) nano-emulsion upon dilution when slightly agitated. These systems are expected to experience phase separation when exposed to the gastrointestinal (GI) tract’s unlimited dilution due to the precipitation of impregnated lipophilic drugs as a result of their poor aqueous solubility. Moreover, these formulations are subject to frequent pH changes as they move along the GI tract, changing from an acidic (stomach) to an alkaline (intestine) environment that may cause the drug to precipitate by its pH-dependent solubility [31]. As a result, the optimum SNEDDS formulations should therefore be resistant to phase separation and drug precipitation at higher dilutions. Based on these findings, the SNEDDS formulation was examined for stability in distilled water and buffer at pH 6.8, 4.6, and 1.2. It was found that neither of the SNEDDS showed any sign of phase separation or drug precipitation, demonstrating that the prepared formulations were resistant to dilution.
FTIR Spectroscopy
FTIR spectroscopy is widely regarded as the most robust technique for the characterization of possible interactions between drugs and substances. As illustrated in Fig. 7, the FTIR spectrum of RUT exhibited discernible peaks attributed to the existence of different functional groups, including 3433 and 3329 cm− 1 (corresponding to O-H stretching), 2905 cm− 1 (associated with CH2 stretching), 1658 cm− 1 (indicative of C = O groups), 1601 cm− 1 (representing C = C stretching for the aromatic system), and 1364 cm− 1 (indicating C-O-H vibrations [32]. The FTIR spectrum of HES exhibited prominent peaks originating from different functional groups, including 3423, 2920, 1647, 1601, and 1060 cm− 1. These peaks can be ascribed to the vibrational modes associated with O-H stretching, C-H stretching, C = O stretching, C = C stretching, and C-O stretching, respectively. The observed peaks were consistent with the data reported in the literature [33]. In the HES-RUT-SNEDDS formulation, the absorption frequency associated with O-H stretching was observed to shift to 3376 cm− 1. Conversely, the absorption peaks corresponding to C-H stretching, C = O stretching, and aromatic C = C stretching were found to occur at the same positions.
Wound Healing Assessment
The photographs of the wounded area contraction of the test animals are shown in Fig. 8. The control groups included untreated, optimized nano-emulsions without HES and RUT-loaded, pure hesperidin suspension, optimized HES-RUT-SNEDDS, and fusidic acid, a commercially available antibiotic (positive control). Figure 9 presents the results of a comparison of the optimized HES-RUT-SNEDDS, pure HES-s, and standard fusidic (Fusidin; positive control) on the contraction of the rat wounded area. These findings demonstrated that test samples, including optimized HES-RUT-SNEDDS, HES-s, and fusidic acid-treated rats improved wound contraction by day 14. In comparison with the control group, the optimized HES-RUT-SNEDDS and pure HES-s significantly accelerated the contraction of the wounded area from day 7 to day 14 (P < 0.05). In addition, as depicted in Fig. 9, fusidic acid and HES and RUT-loaded SNEDDS significantly improved the process of wound healing contraction from days 7 to 14 in comparison to the control group (P < 0.001). Because of their nanoscale particle, greater penetration, and long-lasting releasing action, HES-RUT-SNEDDS exhibited a similar effect at each stage of wound healing as fusidic acid. The complementary properties of cinnamon oils might have accelerated the healing process. The results of epithelization and wound contraction for HES-RUT-SNEDDS were very similar to those for fusidic acid.
Images demonstrating the effects of different treatments including, the control group, pure HES-s, HES-RUT-SNEDDS, and standard antibiotic fusidic acid (Fusidin, positive control), on the wound contraction of the healing aspects in comparison with control after 0, 3, 7, 10, and 14 days of inducing wounding
The results for HES-RUT-loaded SNEDDS were found to be fairly significant (P < 0.001) when compared to respective E-SNEDDS and pure formulations. These results indicated that the optimized HES-RUT-SNEDDS demonstrated an outstanding wound healing activity following topical administration in comparison with both E-SNEDDS and pure HES-s. The optimized SNEDDS’s nano-range droplet size and the addition of solubilizers and penetration enhancers such as Labrasol, Transcutol, and Tween 80 enabled HES-RUT-SNEDDS to promote wound contraction in test animals.
Nevertheless, the healing ability of HES and RUT is strongly supported by previous studies. Multiple attempts have been made to manufacture HES and RUT in different nanoformulations to improve their therapeutic efficacy in wound healing [34, 35]. SNEDDS formulations, however, outperformed their plan drug in terms of wound healing properties [36]. Likewise, in this study, the co-delivery system (HES-RUT-SNEDDS) exhibited a synergistic healing effect (e.g., 100% wound healing), allowing for complete wound contraction in the designed wound excision rat model. The ability to produce wound contraction with combined HES and RUT-loaded SNEDDS formulation was successfully achieved in contrast to HES-s, E-SNEDDS and standard fusidic acid. The resulting healing outcomes of HES-RUT-SNEDDS are most likely driven by a particular rise in epithelial cell proliferation [37]. The main outcome of the current investigation was that HES-RUT-SNEDDS, when applied topically, significantly and more quickly improved the wound healing effects in the devised wound excision rat model. Our results also demonstrated that the rate of wound contraction in the devised animal model was greatly improved with the topical application of HES-RUT-SNEDDS, administered once daily for 14 days.
Based on previously published data, the injured tissues were repaired and kept normal functioning. Inflammation, angiogenesis, contraction, and tissue reformation are among the various phases of the healing process in a wounded area. The healing process requires one or several mechanisms to be completed [38, 39]. Following wounding, the optimized HES-RUT-SNEDDS was noted to decrease exudates and edema in the treated animals on every fourth day. After careful analysis, it was discovered that all of the wound healing outcomes were significantly superior in comparison with control and E-SNEDDS-treated animals, and comparable with those who received usual doses of fusidic acid (Figs. 8 and 9).
The basic purpose of this investigation was to prepare a combined SNEDDS formulation of HES and RUT, to enhance their wound healing properties. Lipid-based nano-formulations are among the best treatment strategies for achieving greater potency of RUT and HES-like phyto constituents following poor water solubility [40]. Transdermal drug delivery systems using SNEDDS have been the subject of various reports [41]. The main advantage of this approach is the highest stability of the hydrolysable drug molecule. After an occlusive topical application, it was frequently noticed that SNEDDS changed into a nano-emulsion when it was combined with an aqueous phase of skin, leading to the identification of a supersaturated system. Hence, a higher driving force was attained for the transdermal drug delivery system [41,42,43].
Herein, a novel HES-RUT-SNEDDS, an isotropic, transparent, and clear drug delivery system comprising oil, surfactant, and co-surfactant, was developed and optimized. It will yield ultra-fine nano-emulsion with little agitation and aqueous medium dilution, frequently smaller than 100 nm in size [41, 44]. Here, HES and RUT-loaded SNEDDS have been employed for the first time as nanocarriers for the transdermal administration of the HES and RUT in a combined formulation. HES-RUT-SNEDDS was applied topically because it can be spread exclusively on the skin surface. HES and RUT combinative formulation was able to reach the nanosize range, improving skin penetration and dissolution, since they could easily be mixed with transepidermal water loss.
Therefore, HES-RUT-loaded SNEDDS could be used to improve the dissolution and solubility rate of both loaded compounds as well as reduce the adverse effects related to oral drug delivery [45]. Upon dilution, SNEDDS formulations improved the aqueous phase, decreased the drug’s solubility, and thereby promoted skin penetration. It has resulted in an in-situ supersaturated nano-emulsion system that increases the drug’s thermodynamic activity and serves as a strong driving force for transdermal delivery [45, 46]. The addition of surfactants to the formulation of HES-RUT-SNEDDS spectacularly increased the transdermal distribution of HES and RUT in comparison to E-SNEDDS. The incorporation of Transcutol, Labrasol and Tween 80 dramatically enhanced the transdermal distribution of HES and RUT. The superior wound healing effect of HES-RUT-SNEDDS could be attributed to the increased skin penetration or delivery of both HES and RUT from SNEDDS formulation.
Conclusion
The HES-RUT-loaded SNEDDS was synthesized and characterized through various advanced technique like AFM, DLS, Zeta potential and FTIR spectroscopy to verify its various types of improved parameters like size, charge on particles, interaction with drugs, solubility in best oil and so on as compared to standard drug fusidic acid, untreated, and E-SNEDDS groups. Further investigation demonstrated that the HES-RUT-loaded SNEDDS revealed effective wound healing properties in the current investigation. The optimized SNEDDS was characterized and tested on rat excision wounds, with comparisons to fusidic acid, untreated, and E-SNEDDS groups. HES-RUT-SNEDDS, like fusidic acid, significantly improved wound healing. Cinnamon oil combined with HES-RUT-SNEDDS also improved wound healing. The results of this study indicate that HES and RUT-loaded SNEDDS could be a successful wound healing strategy.
Data Availability
No datasets were generated or analysed during the current study.
References
Wallace HA, et al. Wound healing phases. 2017. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=H.A.+Wallace%2C+B.M.+Basehore%2C+P.M.+Zito%2C
Trott AT. Wounds and Lacerations: Emergency Care and Closure (Expert Consult-Online and Print). J. Health Sci. 2012. https://books.google.com.pk/books?hl=en&lr=&id=0DnsZgbaXqoC&oi=fnd&pg=PP2&dq
Akbik, D, Ghadiri, M, Chrzanowski, W, & Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014;116(1), 1–7. https://doi.org/10.1016/j.lfs.2014.08.016
Saghazadeh S, et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018;127:138–66. https://doi.org/10.1016/j.addr.2018.04.008
Saxena R, et al. Potential Pharmacological Health Benefits of Flavonoids. In The Flavonoids. 2024;101–129. https://www.taylorfrancis.com/chapters/edit/10.1201/9781003399964-9
Abuelsaad ASA, et al. Antimicrobial and immunomodulating activities of hesperidin and ellagic acid against diarrheic Aeromonas hydrophila in a murine model. Life Sci. 2013;93(20):714–722. https://doi.org/10.1016/j.lfs.2013.09.019
Jagetia GC, Rao KVNM. Topical application of hesperidin a citrus bioflavanone accelerates healing of full thickness dermal excision wounds in mice exposed to 6 Gy of whole body γ-radiation. Clin. Res. Dermatol. 2017;4(3):1–8. https://symbiosisonlinepublishing.com/dermatology/dermatology62.pdf
Li W, et al. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: Role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. EXCLI J. 2018;17:399. https://doi.org/10.17179/excli2018-1036
Man MQ, et al. Benefits of hesperidin for cutaneous functions. J. Evid.-Based Complement. Altern. Med. 2019;2019. https://doi.org/10.1155/2019/2676307
Prasad R, Prasad SB. A review on the chemistry and biological properties of Rutin, a promising nutraceutical agent. Asian J. Pharm. Pharmacol. 2019;5(S1):1–20. https://doi.org/10.31024/ajpp.2019.5.s1.1
Almeida JS. Formulações nanoestruturadas contendo rutina: desenvolvimento, atividade antioxidante in vitro e efeito sobre a cicatrização cutânea.2010. http://repositorio.ufsm.br/handle/1/5932
Costa R, et al. On the development of a cutaneous flavonoid delivery system: Advances and limitations. Antioxidants 2021;10(9):1376. https://doi.org/10.3390/antiox10091376
Tsirigotis-Maniecka M, et al. Polysaccharide hydrogel particles for enhanced delivery of hesperidin: Fabrication, characterization and in vitro evaluation. Colloids Surf. A: Physicochem. Eng. 2017;532:48–56. https://doi.org/10.1016/j.colsurfa.2017.07.001
Kumar PV, et al. Formulation design and evalution of rutin loaded selfemulsifying drug delivary system (SEDDs) using edible oil. Asian J Pharm Clin Res. 2012;5(1):76–8.
Kanwal T, et al. A novel cationic arginine-modified self-nanoemulsifying drug delivery system (SNEDDS) for improved anticancer and antioxidant activities of Naringin. J. Mol. Liq. 2023;391:123235. https://doi.org/10.1016/j.molliq.2023.123235https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s13205-019-1885-3
Van Staden D, et al. Development of topical/transdermal self-emulsifying drug delivery systems, not as simple as expected. Sci. Pharm. 2020;88(2):17. https://doi.org/10.3390/scipharm88020017
Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int. J. Pharm. 2007;329(1–2):166–72. https://doi.org/10.1016/j.ijpharm.2006.08.038
Koshak AE, et al. Wound healing activity of Opuntia ficus-indica fixed oil formulated in a self-nanoemulsifying formulation. Int. J. Nanomed. 2021:3889–905. https://doi.org/10.2147/IJN.S299696
Pouton CW. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 1997;25(1):47–58. https://doi.org/10.1016/S0169-409X(96)00490-5
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’drug delivery systems. Eur. J. Pharm. Sci. 2000;11:S93-8. https://doi.org/10.1016/S0928-0987(00)00167-6
Kurisawa M, et al. Enzymatic synthesis and antioxidant properties of poly (rutin). Biomacromolecules 2003;4(5):1394–9. https://doi.org/10.1021/bm034136b
Nepal PR, et al. Preparation and in vitro–in vivo evaluation of Witepsol® H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q10. Eur. J. Pharm. Sci. 2010;39(4):224–32. https://doi.org/10.1016/j.ejps.2009.12.004
Kommuru T, et al. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int. J. Pharm. 2001;212(2):233–46. https://doi.org/10.1016/S0378-5173(00)00614-1
Elnaggar YS, et al. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: design and optimization. Int. J. Pharm. 2009;380(1–2):133–41. https://doi.org/10.1016/j.ijpharm.2009.07.015
Constantinides PP. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 1995;12:1561–72. https://springerlink.bibliotecabuap.elogim.com/article/10.1023/A:1016268311867
Pouton CW, Porter CJ. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv. Drug Deliv. Rev. 2008;60(6):625–37. https://doi.org/10.1016/j.addr.2007.10.010
Porter CJ, et al. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008;60(6):673–91. https://doi.org/10.1016/j.addr.2007.10.014
Seo YG, et al. Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int. J. Pharm. 2013;452(1–2):412–20. https://doi.org/10.1016/j.ijpharm.2013.05.034
Liu Y, et al. Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2009;365(1–2):136–42. https://doi.org/10.1016/j.ijpharm.2008.08.009
Parmar N, et al. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Coll. Surf. B Biointerf. 2011;86(2):327–38. https://doi.org/10.1016/j.colsurfb.2011.04.016
Khan AW, et al. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22(4):552–61. https://doi.org/10.3109/10717544.2013.878003
Selvaraj K, et al. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Sci. 2013;5(3):743–9.
Balakrishnan K, et al. Bioformulated hesperidin-loaded PLGA nanoparticles counteract the mitochondrial-mediated intrinsic apoptotic pathway in cancer cells. J. Inorg. Organomet. Polym. Mater. 2021;31:331–43. https://doi.org/10.1007/s10904-020-01746-9
Bagher Z, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J. Drug Deliv. Sci. Technol. 2020;55:101379. https://doi.org/10.1016/j.jddst.2019.101379
Chen LY, et al. Effects of rutin on wound healing in hyperglycemic rats. Antioxidants. 2020;9(11):1122. https://doi.org/10.3390/antiox9111122
Altamimi MA, et al. Development and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for curcumin transdermal delivery: An anti-inflammatory exposure. Drug Dev. Ind. Pharm. 2019;45(7):1073–8. https://doi.org/10.1080/03639045.2019.1593440
Sidhu GS, et al. Enhancement of wound healing by curcumin in animals. Wound Rep. Regen. 1998;6(2):167–77. https://doi.org/10.1046/j.1524-475X.1998.60211.x
Velnar T, et al. The wound healing process: an overview of the cellular and molecular mechanisms. Int. J. Med. Res. 2009;37(5):1528–42. https://doi.org/10.1177/147323000903700531
Grieb G, et al. Circulating fibrocytes—biology and mechanisms in wound healing and scar formation. Int. Rev. Cell Mol. Biol. 2011;291:1–19. https://doi.org/10.1016/B978-0-12-386035-4.00001-X
Ranjbar S, et al. Lipid-based delivery systems for flavonoids and flavonolignans: liposomes, nanoemul. Sol. Lip. Nanopart. Pharmaceu. 2023;14;15(7):1944. https://doi.org/10.3390/pharmaceutics15071944
Villar, AMS, et al. Design and optimization of self-nanoemulsifying drug delivery systems (SNEDDS) for enhanced dissolution of gemfibrozil. Int. J. Pharm. 2012;431(1–2):161–175.
Porter CJ, et al. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007;6(3):231–48. https://doi.org/10.1038/nrd2197
Singh B, et al. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit. Rev. Ther. Drug Carr. Syst. 2009;26(5). https://doi.org/10.1615/CritRevTherDrugCarrierSyst.v26.i5.10
Thomas N, et al. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J. Control Rel. 2012;160(1):25–32. https://doi.org/10.1016/j.jconrel.2012.02.027
Taha EI, et al. Fast ultra-fine self-nanoemulsifying drug delivery system for improving in vitro gastric dissolution of poor water soluble drug. Acta Pol. Pharm. 2015;72(1):171–8. https://doi.org/10.1016/j.molliq.2013.10.015
El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int. J. Pharm. 2008;355(1–2):285–92. https://doi.org/10.1016/j.ijpharm.2007.12.022
Acknowledgements
The authors are very thankful to ICCBS and AWKUM for providing the facilities for carrying out this research work. The authors are extremely thankful and cordially appreciate the financial assistance provided to researcher by King Saud University, Riyadh, SA through Research Support Project # RSP2024R79.
Funding
There was no funding for this manuscript.
Author information
Authors and Affiliations
Contributions
Ajmal Hayat: Methodology, Investigation, Formal analysis, Writing – original draft. Ismail Shah: Conceptualization, Formal analysis, Abdul Jabbar: Formal analysis, Data curation. Ayman Nafady: Funding acquisition, Resources, Final review. Aziz Balouch: Methodology, Characterization, Software. Muhammad Raza Shah: Supervision, Final review. Sayyed Ibrahim Shah: Characterization, Methodology. Razium Ali Somro: Resources, Final review. Sirajuddin: Correspondence, Final review, Submission.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
The animal study conducted in this work was ethically approved with the number: EC/AWKUM/2023/40 with Ref. No./Dean/AWKUM/2023/ dated 20-02-2023.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hayat, A., Shah, I., Jabbar, A. et al. Enhanced Wound Healing Activity in Animal Model via Developing and Designing of Self-nano Emulsifying Drug Delivery System (SNEDDS) for the Co-delivery of Hesperidin and Rutin. J Clust Sci (2024). https://doi.org/10.1007/s10876-024-02679-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10876-024-02679-w