Abstract
In the present work, an effective, ecofriendly and novel photocatalytic composite of Ni-ZnO/g-C3N4 (NZG) has been successfully prepared via a hydrothermal method. The crystalline structure and phase purity of the produced g-C3N4, Ni-ZnO, and Ni-ZnO/g-C3N4 nanocomposite were ascertained using XRD analysis. Both the g-C3N4, and Ni-ZnO retained peaks showed minor changes that suggested component interaction. Determine the functional groups and validate the composite’s development using FTIR analysis of the combined g-C3N4, and Ni-ZnO characteristics, along with the addition of new Zn-O and C-N stretching bands. The optical properties and bandgap energy were observed for g-C3N4, Ni-ZnO, and Ni-ZnO/g-C3N4 nanocomposite were 360, 380, and 470 nm−1 with 2.74, 2.94 and 2.86 eV. The homogeneous distribution of Ni-ZnO nanoparticles on g-C3N4, sheets, with strong contact at the interface and consistent elemental composition, was shown by using TEM analysis to investigate the morphological and elemental composition. The photocatalytic degradation efficiency was investigated against RB5 dyes. The Ni-ZnO/g-C3N4 nanocomposite showed excellent than pure Ni-ZnO and g-C3N4 catalyst reached 89.7% degradation for RB5 after 120 min under UV light. Due to this enhanced stability, in addition to the improved electron hole separations and synergistic photocatalytic mechanism between Ni-ZnO and g-C3N4 is an excellent photocatalytic activity against waste water management. The positive control chloramphenicol was showed the inhibition zone was against Sterptococcus aureus and Enterococcus faecalis for 16 nm and 18 nm, for negative control there was no zone of inhibition, and the Ni-ZnO/g-C3N4 NCs showed the maximum zone of inhibition was observed in Enterococcus faecalis for 21 nm at and 100 µg/mL respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution of the environment, especially of the water supply, has a negative impact in life on Earth. Numerous pollutants have been found in the water reservoirs, including biotic (pathogenic microorganisms and viruses) and chemical (industrial effluent and herbicides) contamination [1,2,3].Common water contaminants include colors, pharmaceuticals, cosmetics, fertilizers, herbicides, and industrial outflow. Among these are dyes, which are hazardous chemicals that are used extensively in a variety of sectors, including paper, cosmetics, leather, plastic, printing, textiles, and labs [4,5,6]. In terms of biology, it is probable that the water includes a range of pathogens, including bacteria, fungus, viruses, microalgae, helminths, and protozoa. These pathogens can cause waterborne illnesses in humans, including cholera, dysentery, polio, and typhoid. Unfortunately, low-income residents of rural and isolated locations typically have less access to better sources of drinking water, which increases their risk of contracting diseases from harmful germs. For research and engineering applications, it is therefore imperative to provide a low-cost, effective method for water disinfection and microbiological control. Since then, pollution and infections caused by bacteria have posed a threat to human health [7]. While there are a number of methods for treating contaminated water, visible-light-based photocatalysis is thought to be a green strategy and has attracted attention all over the world because of its limitless solar energy [8,9,10]. In an environmentally benign and sustainable manner with no greenhouse gas emissions in the process, photocatalysis technology has outstanding potentials for the total elimination of organic and other biochemical contaminants [11]. It has been discovered that the nanostructure semiconductor metal oxide photocatalysts may readily breakdown a wide variety of organic and biological contaminants when exposed to UV-visible light. Since the photocatalysis is a process which involves the breaks down or disintegration of different colors, organic waste, and biological species like hazardous fungus and viruses by making use of the UV, sun or visible light in a sustainable manner [12,13,14]. Numerous efficient chemical, physical, biological, and hybrid removal and treatment methods for water polluted with different colorants have been developed by researchers over the past few decades [15]. The most powerful oxidizing species for water treatment and industrial effluents, hydroxyl radicals (⋅ OH), are produced during photocatalytic reactions in electrochemical oxidation [16]. Advanced oxidation processes (AOPs) have been clearly demonstrated as a promising alternative to conventional wastewater treatments because they result in a significant reduction in operational costs [17,18,19]. AOPs can be broadly defined as aqueous phase oxidation methods based primarily on hydroxyl radicals, HO•, in the mechanism leading to the destruction of the pollutant compounds. The hydroxyl radical can be generated photochemically, and it has high effectiveness for the oxidation of organic matter. The heterogeneous photocatalytic process is one of the most important AOPs. This process is based on the oxidation of polluting compounds found in air or water by means of a reaction occurring on a semiconductor catalytic surface activated by light with a specific wavelength [20, 21] RB5 is commonly used in food products, textiles, and the water tracing industry. It is a dangerous substance that can lead to many illnesses, particularly when ingested by people or animals. According to Ghafuri et al. (2018) [22], RB has been linked to a number of disorders, including irritation of the skin and eyes, developmental toxicity, carcinogenicity, neurotoxicity, and chronic toxicity to people and animals. TiO2, ZnS, ZnO, WO3, and CdS are common semiconductor materials for photocatalytic degradation and UV light-induced broad applications. However, most of these agents have drawbacks, such as high cost, easy availability, stability, controlled efficiency, etc. [23, 24].There are several established techniques for creating nanoparticle, including solgel, hydrothermal, co precipitation, thermal decomposition, and electrochemical processes [25]. Due to its many benefits, including cheap cost, easy to use equipment, environmental friendliness, and gentle preparation conditions, the hydrothermal approach has drawn the attention of several researchers [26]. There are several photocatalytsts like TiO2, ZnO, SnO2, CuO, among them the zinc oxide (ZnO) is a noteworthy semiconductor that has garnered a lot of interest. High photosensitivity, biocompatibility, and chemical stability are shown by ZnO’s environmentally benign behavior and n-type semiconductor nature [27, 28]. Doped nanomaterials have a significant band-gap (Ebg) and are non-insulating in their non-stoichiometric state. Since it is non-stoichiometric, mostly due to oxygen vacancies, it is conducting. The extremely low formation energy of zinc interstitials and oxygen vacancies in ZnO causes these defects to occur quickly, which is why Ni-ZnO NPs have an experimentally enhanced conductivity [29, 30]. Graphitic carbon nitride (g-C3N4) has garnered significant interest due to its high absorption efficiency of visible light and its high activity. Because of the strong covalent link between the carbon and nitrogen atoms in the conjugated layer structure, it has excellent chemical and thermal stability and only comprises the elements that are abundant on Earth: carbon and nitrogen [16]. Furthermore, as noted by Zheng et al. (2012) and Ismael et al. (2019) [31, 32], its moderate bandgap energy of 2.7 eV (460) nm allows it to capture visible light and have an appropriate conduction bond (CB) and valence bond (VB) edge location for both water reduction and oxidation. The most practical ways to enhance virgin g-C3N4’s photocatalytic activity are to build a heterostructure with metal ions, semiconductors, graphene, carbon nanotubes, and polymers. Because of its increased specific surface area and pore size, the K doped g-C3N4 photocatalyst demonstrated good photocatalytic degradation of tetracycline under visible light [33,34,35]. Transition metal doping of semiconductors (ZnO, g-C3N4 or ZnO/g-C3N4) as co-catalysts has shown effective in improving visible light absorption and preventing electron-hole pair recombination. In contrast to costly metals such as Au, Pt, and Ag, inexpensive Ni ions possess exceptional electron conduction capabilities, making them a versatile component in photocatalyst design. This is particularly useful in increasing absorption in the visible spectrum [36,37,38]. In contrast to ZnO or g-C3N4, Ni/ZnO and Ni/g-C3N4 have been shown to exhibit better antibacterial and photocatalytic effectiveness [39,40,41]. To the best of our knowledge, no one has yet disclosed how Ni/ZnO/g-C3N4 photocatalysts are made. Combining the beneficial effects of Ni doping with g-C3N4 and ZnO, two effective photocatalysts, will be intriguing since it might result in a unique composite of Ni/ZnO/g-C3N4. Here, we demonstrate the simple chemical hydrothermal technique for creating a heterostructured Ni/ZnO/g-C3N4 (NiZG) photocatalyst that exhibits superior photocatalytic and antibacterial capabilities when exposed to ultraviolet light.
Interestingly, the composite outperformed ZnO, Ni/ZnO, g-C3N4, and Ni-ZnO/ g-C3N4 in terms of bactericidal and catalytic activity. The simultaneous integration of g-C3N4 and Ni doping into the ZnO matrix resulted in increased visible light absorption, interference with e-h pair recombination, and better charge conduction. The Ni-ZnO/ g-C3N4 nanocomposite combines the different properties of graphitic carbon nitride (g-C3N4) and nickel-doped zinc oxide (Ni-ZnO). Ni-ZnO has excellent photocatalytic properties, whereas g-C3N4 offers stability, a high surface area, and visible light absorption. Combining these materials is expected to boost photocatalytic effectiveness due to mutually beneficial interactions. Through research and experimentation, we want to demonstrate that the unique Ni-ZnO/g- g-C3N4 nanocomposite is a very effective material for environmental remediation, offering a dual approach to tackling pollution and microbial contamination.
Materials and Methods
Chemicals and Materials
All chemicals were analytical grade (A.R.) and used as received without further purification. Melamine (C3H6N6), Nickel nitrate (Ni(NO3)2·6H2O) 98%, Zinc nitrate (Zn(NO3)2·6H2O) 98%, Sodium hydroxide (NaOH) 97% and hydrogen peroxide (H2O2) 13%, RB5 dye were purchased by SRL Pvt, Ltd., India, used directly in the experiments without any additional cleaning.
Preparation of Ni-ZnO
In a characteristic synthesis of Ni-ZnO, 0.5 mmol of Nickel nitrate was dissolved in 100 ml of (deionized water) DI water. Simultaneously, 1 mmol of Zinc nitrate was dissolved into another 100 ml of DI water. The Zinc nitrate solution was added to the Nickel solution while continuously stirring and the NaOH solution until a pH 9 was achieved. Afterwards, the combined solution was moved into a stainless-steel autoclave lined with Teflon. This autoclave was then positioned in an oven at a temperature of 160 °C for 20 h. After that, the resulting samples were collected and washed multiple times using ethanol and DI water. The resultant consequence was dried at 100 °C for 10 h; finally got Ni-ZnONanoparticle [42].
Preparation of g-C3N4 and NiZnO/g-C3N4nanocomposite
The g-C3N4 powder was acquired through the thermal polycondensation of melamine. In a standard procedure, 15 g of melamine was exposed to annealing at 520 °C for 5 h. After that, the resulting yellow-coloured g-C3N4 products were subsequently crushed with a mortar for subsequent applications. The NiZnO/g-C3N4 (NZG) nanocomposite was produced by a hydrothermal method. Initially, 1 g of g-C3N4 was dispersed in 30 mL of DI water and exposed to ultrasonic dispersion for 30 min simultaneously, 0.5 mmol of Nickel nitrate was dissolved in 50 ml of DI water, and then 1 mmol of Zinc nitrate was added to another 50 mL of distilled water. Subsequently, the Zinc nitrate and g-C3N4 solution were combined with the Nickel nitrate solution and added NaOH solution until a pH 9 was achieved continuously stirred for 2 h. The resultant mixture was moved into an autoclave and heated at 160 °C for 12 h, after which it was allowed to cool down to room temperature. The product underwent multiple cycles of centrifugation with ethanol and DI water. Then it was dried at 100 °C for 12 h in a hot air oven to yield the fine powder, resulting in the development of the NZG nanocomposite [43] (Fig. 1).
Characterization of NiZnO/g-C3N4
XRD is used to determine the crystal structure and phase purity of the nanoparticle. The identifying of the crystalline phases and size are through X-ray diffraction (XRD - Rigaku Smart lab diffractometer). Fourier Transmission Infra red Spectra (FTIR) is used to study the functional groups on the surface of the nanoparticle. Identifying the chemical bonds of NiZnO/g-C3N4 nanocompsite was used by Fourier Transmission Infra red Spectra model of VERTEX 70. It can provide the information about the interaction between Ni, ZnO and g-C3N4. The size, shape, and internal structure of NiZnO/g-C3N4 nanocompsite were determined by Transmission Electron Microscopy (TEM) Tecnai G2 instrument from FE1. Finally, the synthesized NiZnO/g-C3N4 nanocompsite optical and bandgap energy were analyzed used by UV-vis spectroscopy.
Photocatalytic Degradation of NiZnO/g-C3N4
The photocatalytic experiments were conducted by the synthesized materials including Ni-ZnO and g-C3N4 against RB5 dye in an aqueous medium under sunlight. The photocatalytic dye degradation was executed by dissolving 200 mg of synthesized samples in 200 mL of dye solution (10 mg L − 1). The dye catalysis was performed under solar radiation at ambient conditions (68–73 Klux). Initially, each catalyst was agitated for 30 min in the dark to equilibrate the system. Then, the sample suspensions were exposed to the sunlight to initiate the photocatalytic reaction. Next, 6 mL of each suspension was taken at specific intervals, centrifuged and then analyzed by scanning (400–800 nm) via a UV–vis spectrophotometer (UV-3600, Shimadzu). Similarly, the photocatalyst stability was monitored by recycling five times.
Antibacterial Activity
The antimicrobial propensity of the fabricated samples (Ni-ZnO, g-C3N4andNi-ZnO/g-C3N4 against Sterptococcus aureus and Enterococcus faecalis was assessed quantitatively using the disc diffusion method. Chloremphenicol and DMSO used as a positive and negative control. Briefly, onto sterile Petri plates containing Mueller hinton agar, pure culture of the bacteria (106 CFU/ml) was spread using a sterile cotton swab. Different concentrations (75, 100, 125 µg/ml) of nanoparticle suspension was inserted using the diffusion method, and the plate were maintained at 4 ◦C for 20 min. Subsequently, the test plates were incubated for one day (24 h) at 37 ± 2 ◦C, for which zone of inhibition was calculated.
Results
XRD Analysis
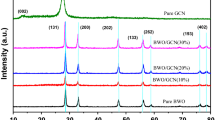
The XRD patterns of the prepared g-C3N4, Ni-ZnO and Ni-ZnO/g-C3N4 are shown in Fig. 2. Two distinct peaks can be seen in the g-C3N4 XRD pattern. One peak is located at 27.4° and is indexed as a (002) crystal plane, which indicates that aromatic systems are interlayer stacked. The primary peak’s presence at 27.4° supports g-C3N4 nanosheet creation [44, 45]. The principal XRD peaks of zinc oxide (ZnO) can be found at 31.61◦, 34.28◦, 36.10◦, 47.4◦, 56.45◦, 62.72◦, 67.8◦, 68.93◦, 75.3◦ and 79.5◦. These correspond to the (100), (002), (101), (102), (110), (103), (112), (201), (222) and (202) crystal faces, and they are in good agreement with the standard diffraction patterns of the wurtzite hexagonal structure of ZnO (JCPDS Card No. 01-089-0511) [46]. ZnO peaks are often seen at 2θ values of 31.8°, 34.4°, 36.3°, 47.5°, 56.6°, 62.9°, and 68.0°, corresponding to the (100), (002), (101), (102), (110), (103), and (112) planes [47]. The distinctive peaks of g-C3N4 are often seen at 2θ = 13.1° and 27.3°, which correspond to the (100) and (002) planes, respectively. The (002) peak represents the interlayer stacking of conjugated aromatic systems in g-C3N4 [48]. In XRD patterns of 3% Ni/ZnO and NiZG-70 samples, Ni doping into ZnO did not add or disappear a diffraction peak, confirming that Ni doping has not changed the wurtzite structure [49]. No further peaks consistent with any other contaminants were discovered in the Ni-ZnO/g-C3N4combination, which displays the identical XRD spectra as those of Ni-ZnO and g-C3N4. The XRD study of the Ni-ZnO/ g-C3N4 nanocomposite demonstrates that a composite material was successfully formed, keeping the crystalline structures of ZnO and g-C3N4, with Ni either doping or dispersion inside the ZnO lattice.
FTIR Analysis
The material’s elemental constituents, chemisorbed species, and presence of different functional groups were all determined using an FTIR spectrum. Ni-ZnO, g-C3N4, and Ni-ZnO/g-C3N4are discovered to include a variety of functional groups and chemisorbed species, as evidenced by the several series of peak ranges detected in FTIR spectra, which spectral range from 400 to 4000 cm−1(Fig. 3). The peaks at 2855 and 2955 cm−1 are caused by the C–H group’s symmetric and asymmetric properties. The presence of CO2 molecules in the air was the cause of the absorption peak detected at 2340 cm−1 [50]. Because nickel is present in the ZnO lattice structure, the appearance of the weak absorption band at around 870 cm−1can be attributed to the Ni–O vibrational frequency [51]. The g-C3N4 FTIR spectrum often has multiple distinctive peaks. The peaks at 1200–1650 cm^-1 represent the stretching vibrations of C-N and C = N heterocycles in the g-C3N4 structure [52]. The FTIR spectrum shows large absorption bands about 3000–3500 cm−1, indicating adsorbed water or hydroxyl groups on the composite surface. These hydroxyl groups can play an important role in photocatalytic reactions by promoting the production of reactive oxygen species [53]. According to Thirugnanam et al. (2013) [54], the peak at 3424 cm−1 can be ascribed to water (H2O) molecules and −OH (hydroxyl) groups that have been absorbed by the surface. The C-N stretching vibration is responsible for the peak at 1640 cm, while the aromatic C-N stretching vibrations are responsible for the other peaks at 1242, 1320, and 1403 cm−1, and the out-of-plane bending is responsible for the band at 828 cm−1. Heterocyclic C–N vibrations. Moreover, the Zn–O stretching vibrations can be connected to the band seen at 522 cm−1for the ZnO and g-C3N4/ZnO samples [55].This might involve the sharing of valence electrons in chemical bonds, which improves the composite and creates a hybrid structure. It can be principally deduced for the successful production of Ni-ZnO/g-C3N4composite from the FTIR analysis, which offers information about the matching of Ni-ZnO, g-C3N4, and Ni-ZnO/g-C3N4peaks with that of the composite.
Optical Properties
UV-Visible absorption spectroscopy was effectively used to examine the optical characteristics of pure Ni-ZnO, g-C3N4, and Ni-ZnO/g-C3N4nanocomposite. UV-Vis absorption spectra between 350 and 600 nm, as seen in Fig. 4a g-C3N4 and Ni-ZnOnanoparticle having absorption peaks in the range of 380 and 370 nm were used in the UV-vis absorption analysis of these samples. The addition of Ni-ZnO to g-C3N4 results in a composite with a higher absorption capacity; this is demonstrated by the absorption edges moving 360 nm toward longer wavelengths in comparison to g-C3N4. The interactions between g-C3N4 and Ni-ZnO in the composite are responsible for this modification. Similar to other publications, this one shows that pure g-C3N4has an absorption edge of about 380 nm in the UV-Visible range [56].Through the use of UV–Vis diffuse reflectance spectroscopy, the optical characteristics were determined. The absorption edge of the Ni-ZnO at 386 nm, and the absorption edge begin to blue shift (move towards lower wavelengths) as the adding ofg-C3N4. This further suggests that the band gap (Eg) grows, which will be caused by defects or strain. The bandgap energy of the prepared catalyst Ni-ZnO, g-C3N4, and Ni-ZnO/g-C3N4were 2.85, 2.74 and 2.76 (Fig. 4b) [57]. As a π-conjugative material with a moderate energy band gap, graphitic carbon nitride (g-C3N4) primarily functions as a free metal catalyst for oxidation [58], hydrogen storage and water 8 reduction. The strong chemical bonding surfaces between Ni-ZnO, g-C3N4, and Ni-ZnO/g-C3N4 that are implied by this finding, along with the energy region shifting from higher to lower, make this nanocomposite an appropriate material for photocatalysis.
Morphological Analysis
TEM analysis was used to examine the sample’s morphological features; the findings are displayed in Fig. 5a–c. The TEM picture of the Ni-ZnO/g-C3N4samplesshows that the nanocompsite’s morphology is hexagonal in shape and overlaps with a sheet-like structure. With curled edges where the sheets are folded and efoxilated into sheet arrangement, the g-C3N4exhibits morphology akin to a two-dimensional agglomerated lamellar sheet. The tristriazine units that make up the sheet layer are joined by planar amine groups and comprise graphic planes with the conjugated aromatic system. In a similar vein, the TEM pictures of ZnO show a cylindrical and hexagonal nanorod, which is consistent with the hexagonal phase seen in the XRD [59]. TEM pictures of Ni-ZnO nanoparticles often show quasi-spherical or hexagonal nanoparticles, which represent ZnO’s inherent wurtzite structure. The particle size typically ranges between 10 and 50 nm, depending on the production technique and doping concentration [60, 61].
Furthermore, the TEM picture of the g-C3N4/ZnO composite demonstrated that the nanorods are embedded in the sheet-like morphology, supporting the preservation of the g-C3N4compound’s sheet-like layered structure even in the composite. Additionally, the composite’s sheet-like shape suggests that there are more surface areas available, which in turn promotes increased photocatalytic activity. The lattice fringes and the lattice spacing of Ni-ZnO/g-C3N4 nanocomposite was 0.31 nm, which agreed to the (111) planes of Ni-ZnO particle (Fig. 5d).
Photocatalytic Activity of RB Used by Ni-ZnO/g-C3N4
The photocatalytic performance of Ni-ZnO, g-C3N4, and Ni-ZnO/g-C3N4was assessed by photodegradation of an aqueous RB dye solution under UVA light irradiation (Fig. 6a). Figure 6 shows the degradation rates of the RB solution as measured by the C/C0 curves. In contrast to the Ni-ZnO and g-C3N4nanoparticle in isolation, the Ni-ZnO/g-C3N4combination exhibited superior photocatalytic activity (89.7%) (Table 1). In contrast, during the 120 min photocatalytic process, the degradation rates of pure Ni-ZnO and g-C3N4nanoparticle only reached 49.6% and 47.8%, respectively. Zinc oxide (ZnO) is a semiconductor that has a wide range of applications as a catalyst in chemical processes. Zinc oxide’s characteristics can be altered by adding nickel, which may increase the compound’s catalytic activity. It is hypothesized that g-C3N4contributes to the catalyst’s increased surface area.
Increased surface area is frequently linked to better catalytic activity. It allows for improved contact with reactants and more active areas for reactions to occur. Reactant molecules may have more adsorption sites thanks to g-C3N4, which would improve their interaction with the catalyst. Ni-ZnO and g-C3N4work together to provide synergistic effects that raise total catalytic activity. All of these findings point to the important roles that Ni and g-C3N4play in supporting the increased photocatalytic efficiency in the Ni-ZnO/g-C3N4.The RB dyes UV absorption spectra after being in contact with the Ni-ZnO/g-C3N4.NCs. The absorption intensity gradually decreases with an increase in irradiation duration. The RB dye is degrading, as shown by this drop in absorption intensity. Remarkably, the absorption peak almost disappears during a 120 min exposure to radiation, suggesting a significant decrease in the concentration of RB dye in the mixture. Figure 6c showed the photodegradation rate (C/C0) of RB dye removal using the bare Ni-ZnO, g-C3N4 and Ni-ZnO/g-C3N4NCs. During the 120-minute photocatalytic process, the photocatalytic efficiency of Ni-ZnO, g-C3N4 and Ni-ZnO/g-C3N4NCs for the degradation of RB dye was 49.6%, 47.8%, and 89.7%.
In contrast to the bare nanoparticle, the Ni-ZnO/g-C3N4NCs demonstrated a better photocatalyst for the RB dye degradation.
A pseudo-first-order kinetic model was fitted to the uniform photo-reaction kinetics depicted in Fig. 6b to help evaluate the precision of the current results. Equation illustrates how the pseudo-first-order kinetics offered by the Langmuir Hinshelwood model may effectively depict the photocatalytic degradation process.
Here, C0 is the initial concentration, Ct stands for the changing concentrations of IC at time interval t, and K represents the rate constant of the pseudo-first order.
As seen in Fig. 6b the rate constants (k) for the Ni-ZnO, g-C3N4 and Ni-ZnO/g-C3N4NCswere found to be 0.0991, 0.1142 and 0.2652 h−1.Notably, compared to the individual bare Ni-ZnO and g-C3N4catalysts, the reaction rate constant (k) of the Ni-ZnO/g-C3N4NCswere greater. The results indicate that the photocatalysts Ni-ZnO/g-C3N4Nsexhibit improved photocatalytic performance on the degradation of RB dye. The examined photocatalyst’s stability throughout the photodegradation reaction is one of its benefits. Figure 6, d demonstrates that there is no discernible decrease in the photocatalytic activity of Ni-ZnO/g-C3N4NC after five consecutive uses, each involving a 120-minute irradiation period. Thus, this may be regarded as an effective photocatalyst for the RB5 dye’s photodegradation. It is indicating that there is no obvious loss of photocatalytic activity after five cycles. Semiconductor materials like g-C3N4and Ni-ZnOcan absorb light, especially visible and ultraviolet light. These materials’ valence band electrons can be stimulated to the conduction band by light. In Ni-ZnO and g-C3N4, photogenerated electrons and holes separate in charge. To create charge carriers, electrons flow to the conduction band and holes to the valence band. Commonly utilized in many different applications, RB5 is a dye whose degradation is frequently the focus of environmental remediation procedures. Ni-ZnO and g-C3N4 electrons in the conduction band can engage in electron transfer processes with RB5 dye molecules. RB5 may be reduced by excited electrons, which can cause it to break down or change into less toxic metabolites. Organic contaminants linked to the dye can be broken down by oxidation processes involving holes in the valence band. In a composite catalyst, the presence of both Ni-ZnO and g-C3N4may have synergistic effects that might improve the electron-driven reactions’ efficiency g-C3N4could offer more active sites and encourage RB5 molecule adsorption.The photocatalytic process begins with the absorption of light by the semiconductor materials, namely Ni-ZnO and g-C3N4. Ni-ZnO absorbs UV light due to its bandgap energy, while g-C3N4 can absorb visible light. Upon absorption of photons, electrons in the valence band of the semiconductors are excited to the conduction band, creating electron-hole pairs.
Effects of the pH Levels on the RB5 dye Degradation
An acidic environment leads to the creation of more reactive sites, contributing to enhanced pollutant degradation. The effect of various pH values (2.0, 4.0, 6.0, 8.0, and 10.0) on reaction rates was studied (Fig. 7). At a pH of 4.0, the degradation efficiency of RB5 reached 89% within 120 min. This high degradation rate at pH 4.0 can be attributed to the release of metal oxides on the surfaces of nanoparticles in an acidic environment. In contrast, the removal of methylene blue was limited to pH levels of 2, 6, 8, and 10 due to the low concentration of H+, which is not conducive to effective removal.
Photocatalytic Mechanism of Ni-ZnO/g-C3N4 Nanocomposite
The effective phototcatalytic mechanism is once excited, the electrons (e-) and holes (h+) generated in the Ni-ZnO and g-C3N4 materials, respectively, become separated. This separation is facilitated by the different energy levels of the conduction and valence bands in the semiconductors.The photogenerated electrons in the conduction band of Ni-ZnO and the holes in the valence band of g-C3N4 migrate to the surface of the catalysts due to the concentration gradient. This migration process occurs within the materials.At the surface of the Ni-ZnO/g-C3N4nanocomposite, the photogenerated electrons reduce oxygen molecules (O2) to form superoxide radicals (O2•−), while the photogenerated holes oxidize water molecules (H2O) to form hydroxyl radicals (•OH) (Fig. 8).
The generated reactive oxygen species (ROS), including superoxide radicals and hydroxyl radicals, are highly reactive and can oxidize organic pollutants present in the surrounding environment. These ROS attack the organic molecules, breaking down their chemical bonds and ultimately leading to the degradation of pollutants into harmless substances such as carbon dioxide (CO2) and water (H2O).It’s important to note that charge carrier recombination, where electrons recombine with holes, can occur during the photocatalytic process. However, the introduction of Ni-ZnO/g-C3N4nanocomposite helps to suppress this recombination by providing additional active sites for charge transfer and prolonging the lifetime of the photogenerated charge carriers. Overall, the synergistic effect between Ni-ZnO and g-C3N4 in the nanocomposite enhances the photocatalytic activity, making them effective materials for environmental remediation and various other applications requiring photocatalysis.
Antibacterial Activity
Antibacterial activity of Ni-ZnO/g-C3N4NCs synthesized nanocomposite was studied against selected both gram positive and gram negative strains of bacteria by measuring their zone of inhibition (mm). The diameter of the zone of the inhibition for Ni-ZnO/g-C3N4NCs against Sterptococcus aureus and Enterococcus faecalis are about 14 nm and 21 nm for 50 µg/mL and 100 µg/mL respectively (Fig. 9). The results also indicate that the Enterococcus faecalis is extremely sensitive towards Ni-ZnO/g-C3N4NCs nanocomposite. When compared to gramnegative bacteria, the higher inhibition zone was observed against gram positive bacteria, which may be ascribed to the difference in cell wall composition of gram-positive and gram-negative bacteria.
The production of reactive oxygen species (ROS), which directly causes cell death by destroying vital cellular components including DNA and proteins, is the most potent antibacterial method (Fig. 10). More ROS have generally been reported to be produced by NPs with smaller crystallite sizes and greater specific surface areas. It is also proven that doping with Ni2+ reduced the size of the crystallites, increasing their surface area and enhancing their antibacterial activity. To demonstrate that the NZOs produced in this work generate more reactive oxygen species (ROS) than ZO [67]. Furthermore, new research suggests that upon direct cell contact, graphene and g-C3N4 nanosheets inactivate bacteria by causing membrane damage, which is mediated by physical disruption, charge transfer, and the production of reactive oxygen species. When compared to pure ZnOnanoparticle, the ZnO(0.47)Ni(0.03) nanocomposite, which has comparatively more oxygen vacancies and a smaller crystalline size, showed less bactericidal action against all bacterial species [68].This may be explained by the fact that the ZnO-doped Ni nanocomposite has less surface area due to the presence of low surface area nanorods. Among all the NiO-ZnOnanocomposite, Thambidurai et al. (2020) [47] found that the ZnO doped Ni nanocomposite exhibited better bactericidal activity than the pure ZnOnanoparticle due to its lowest crystalline size and greatest oxygen vacancies.
Conclusion
Based in this study, the novel combination of Ni-ZnO/g-C3N4 NCs was successfully synthesized by hydrothermal method. XRD, FTIR studies revealed that, the significance co ordination of Ni-ZnO with g-C3N4. TEM studies are confirmed that the hexagonal in shape of the nanocomposite. The creation of a heterojunction between Ni-ZnO with g-C3N4. NCs is responsible for the increased photocatalytic activity shown in Ni-ZnO/g-C3N4 NCs. The recombination of photogenerated electron-hole pairs is substantially suppressed by this heterojunction, which enhances photocatalytic efficacy. According to photocatalytic Ni-ZnO/g-C3N4 NCs synthesized via hydrothermal method its promising for use in the degradation of organic dye pollutantstoward RB dye degradation compared to pure g-C3N4 and Ni-ZnO particles.
Data Availability
No datasets were generated or analysed during the current study.
References
J.C. Sousa, A.R. Ribeiro, M.O. Barbosa, M.F.R. Pereira, S.M. Silva, J. Hazard. Mater. 344, 146–162 (2018).
L. Andrade, J. O’Dwyer, E. P. O’Neill, Hynds, Environ. Pollut. 236, 540–549 (2018).
M. Baghayeri, B. Mahdavi, Z.H.M. Abadi, S. Farhadi, Appl. Organomet. Chem. 32, e4057 (2018).
P. Movalli, O. Krone, D. Osborn, D. Pain, Bird Study. 65, S96–S109 (2018).
N. Kataria, V.K. Garg, Environ. Res. 172, 43–54 (2019).
M.L. da Costa, G. Pavoski, D.C.R. Espinosa, N.J.S. de Vasconcellos, W.L. da Silva, Water. Air. Soil. Pollut. 233:65, (2022).
World Health Organization, Health topics: infectious diseases. https://www.who.int/topics/infectious_diseases/en/. R.D. Perry. (1997). J.D. Fetherston, Clin. Microbiol. Rev. 10, 35–66.
C. Yu, F. Cao, G. Li, R. Wei, J.C.M. Yu, R. Jin, Q. Fan, C. Wang, Sep.Purif. Technol. 120, 110–122 (2013).
L. Yang, W. Duan, H. Jiang, S. Luo, Y. Luo, Mater. Res. Bull. 70, 129–136 (2015).
M. Nazari, F. Golestani-Fard, R. Bayati, B. Eftekhari-Yekta, SuperlatticesMicrostruct. 80, 91–101 (2015).
H. Teymourinia, A.A. Alshamsi, A. Al-nayili, H.A. Alshamsi, R. Mohammadi, E. Sohouli, M. Gholami, Surfaces and Interfaces. 42,103412 (2023).
X.Zhang, A. Fujishima, M. Jin, A.V. Emeline, J. Murakami, J. Phys. Chem. B. 110 (50), 25142–25148(2006).
Y.P. Santos, E. Valença, R. Machado, M.A. Mac^edo, Mater. Sci. Semicond. Process. 86, 43–48(2018).
S.M. Tabatabaeinejad, Q. A. Yousif, H.A. Alshamsi, A. Al-Nayili, M.S. Niasari, Arab. J. Chem. 15, 6, 103826.
A. Al-nayili, H.A. Khayoon, H.A. Alshamsi, N.M. Cata Saady, Materials Today Sustainability. 24, 100512.
H. Teymourinia, A.A. Alshamsi, A. Al-nayili, E. Sohouli, M. Gholami, J. Ind. Eng. Chem. 125, 259–268 (2023).
S. Malato, P. Fernandez, M.I. Maldonado, J. Blanco, W. Gernjak, Catal. Today. 147, 1–5, 1–59, (2009).
W.L. da Silva, M. AzárioLansarin, J.Z.H. dos Santos, Water Science & Technology. 73.1, (2016).
L.N. Ribas, L.O. de Sousa Bulhões, W.L. da Silva, Water. Air. Soil. Pollut. 231, 191 (2020). https://doi.org/10.1007/s11270-020-04553-7
G.P. Chuy, P.C.L. Muraro, A.R. Viana, G. Pavoski, D.C.R. Espinosa, B.S. Vizzotto, W.L. da Silva, J. Inorg. Organomet. Polym. Mater. 32, 1213–1222 (2022).
W.L. da Silva, M. AzárioLansarin, J.H.Z. dos Santos, Z.N. Da Rocha, I.M. Pepe et al., Water. Air. Soil. Pollut. (2016) 227:242 DOI https://doi.org/10.1007/s11270-016-2932-x.
H. Ghafuri, M. Dehghani, A. Rashidizadeh, M. Rabbani, Optik. 179, 646–653 (2018).
F. Chen, Y. Cao, D. Jia, X. Niu, Ceram. Int. 39 (2), 1511–1517 (2013)
R.S. Sabry, Y.K. Al-Haidarie, M.A. Kudhier, J. Sol. Gel Sci. Technol. 78 (2), 299–306 (2016).
S.P.Rajendran, K. Sengodan, J. Nanosci. 1–7 (2017).
H.O.Chu, Q. Wang, Y.J. Shi, S. Geng, L.W. Guo, S. Zhou, D. Gibson, Y. Alajlani, C. Li, Nonferrous Met. Soc. China. (30), 191–9 (2020).
L.L. Yang, Q.X. Zhao, M. Willander, X.J. Liu, M. Fahlman, J.H. Yang, Appl. Surf. Sci. 256, 3592–3597, (2010).
S.Z. Kang, T. Wu, X. Li, J. Mu, Colloids Surf. A Physicochem. Eng. Asp. 369, 268–271 (2010).
E.S. Kumar, J. Chatterjee, N. Rama, N.D. Gupta, M.S.R. Rao, ACS Applied Materials and Interfaces. 3, 1974–79 (2011).
L. Huang, Z. Qin, G. Wang, M. Du, H. Ge, X. Li, Z. Wu, J. Wang, Research 49,4670–75 (2010).
Y. Zheng, J. Liu, J. Liang, M. Jaroniec, S.Z. Qiao, Energy Environ. Sci. 5, 6717–6731 (2012)..
M.Ismael, Y. Wu, Sustainable Energy Fuels. 3, 2907 (2019).
K. Maeda, X. Wang, Y. Nishihara, D. Lu, M. Antonietti, K. Domen, J. Phys.Chem. 113, 4940–4947 (2009).
W. Wang, P. Xu, M. Chen, G. Zeng, C. Zhang, C. Zhou, Y. Yang, D. Huang, C. Lai, M. Cheng, L. Hu, ACS Sustain. Chem. Eng. 6, 15503–15516 (2018).
Y.Xu, H. Xu, L. Wang, J. Yan, H. Li, Y. Song, L. Huang, G. Cai, Dalton Trans. 42, 7604–7613 (2013).
P. Wen, Y. Sun, H. Li, Z. Liang, H. Wu, J. Zhang, L. Jiang, Appl.Catal. 263, 118180 (2020).
S.P. Adhikari, H.R. Pant, J.H. Kim, H.J. Kim, C.H. Park, C.S. Kim, Colloids Surf. A 482, 477–484 (2015).
M.S.Abdel-Wahab, J. Jilani, I.S. Yahia, A.A. Al-Ghamdi, Superlattices Microstruct. 94 (2016) 108–118.
P. Pascariu, I.V. Tudose, M. Suchea, E. Koudoumas, N. Fifere, A. Airinei, Appl. Surf. Sci. 448, 481–488(2018).
P. Deng, J. Xiong, S. Lei, W. Wang, X. Ou, Y. Xu, Y, Xiao, B. Cheng, J. Mater. Chem. A. 7, 22385–22397 (2019).
S.Ma, S, Zhan, Y. Xia, P. Wang, Q. Hou, Q, Zhou, Catal. Today. 330,179–188 (2019).
I. Elhamdi, H. Souissi, O. Taktak, J. Elghoul, S. Kammoun, E. Dhahri, RSC Adv. 12, 13074–13086 (2022)
M.H. Qamar, S. Shahid, M. Javed, S. Iqbal, M. Sher, A. Bahadur, M. M. AL-Anazy, A. Laref, Colloids and Surfaces A: Physicochem.Eng. 614, 126176 (2021).
L.S. Lin, Z.X. Cong, J. Li, K.M. Ke, S.S.Guo, H.H. Yang, G.N. Chen, J. Mater. Chem. B. 2, 1031–1037 (2014).
S.C.Yan, S.B. Lv, Z.S. Li, Z.G. Zou, Dalton Trans. 39, 1488–1491 (2010).
J. Ashwini, T.R. Aswathy, A.B. Rahul, G.M. Thara, A.S. Nair, Catalysts. 11, 1507 (2021).
Z. L. Wang, J. Phys.: Condens. Matter, 16, (25) R829–R858, (2004).
X. Wang et al., Nature Materials, 8 (1), 76–80, (2009).
B. Pal, D. Sarkar, P.K. Giri, Appl. Surf. Sci. 356, 804–811(2015).
G.X. Tong, F.F. Du, Y. Liang, Q. Hu, R.N. Wu, J.G. Guan, X. Hu, J. Mater. Chem. B. 1, 454 (2013).
P.Gopal, N.A. Spaldin, Phys. Rev. B 74, 094418 (2006).
A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J. O. Muller, R. Schlogl, and J. M. Carlsson, J. Mater. Chem. 18, 4893–4908 (2008).
P. Sharma, V. S. Tripathi, and R. S. Yadav, Appl. Surf. Sci. 449, 188–198 (2018).
T.Thirugnanam, J. Nanomater. 1–7 (2013).
K.S. Babu, A.R. Reddy, Ch Sujatha, K. Venugopal Reddy, A.N. Mallika, J. Adv. Ceram. 2 (3), 260–265(2013).
R. Rajendran, O. Rojviroon, P. Arumugam, K. Natchimuthu, V. Umar Vasudevan, J.Kannupaiyan, R. Muangmora, P. Phouheuanhong, T. Rojviroon, J. Alloys. Compund. 976 (5), 173116 (2024).
C. Hariharan, Applied Catalysis A: General. 304, 55–61 (2006).
D.J.Jorge, M.T.S. Martin, A.S. Dhanoa, N. Rahman, J. Makwana, A. Tang, F. Sella, A.B. Corà, S. Firth, J.A. Darr, P.F. McMillan, The Journal of Physical Chemistry C. 117, 7178–7185 (2013).
J. Ishioka, K. Kogure, K. Ofuji, K. Kawaguchi, M.Jeem, T. Kato, T. Shibayama, S. Watanabe, AIP Adv. 7, 035220, (2017).
M. M. Rahman, M. Jamal, S. M. D. Tasnim, Physica E: Low-dimensional Systems and Nanostructures. 71, 135–140 (2015).
J. Zhou, L. Dai, J. Yang, ACS Appl. Mater. Interfaces. 11 (11), 11906–11915 (2019).
M. Gholami, M.S. Siboni, J. K. Yang, Korean J. Chem. Eng. 33, 812–822 (2016).
F. Xu, S.N. Sun, Y.X. Wang, F.L. Bian, H. Wang, Z. Fu, H.N. Cui, . Key Eng. Mater. 575, 306–309 (2014).
H. Huang, N. Huang, Z.H. Wang, G.Q. Xia, M. Chen, L.L. He, Z.F. Tong, C.G. Ren, J. Colloid. Interface. Sci. 502, 77–88 (2017).
J. Zhang, Y. Wang, S. Hu, Bull. Korean Chem. Soc. 36 (2015) 333–339.
Y. Zang, Y. Zuo, G. Li. J. Mater. Chem. A 2 (2014) 15774
A. Naskar, S. Lee, K. Kim, RSC Adv. 10, 1232 (2020).
O. Akhavan, E. Ghaderi, Toxicity of graphene and graphene oxide nanowalls against bacteria, ACS Nano. 4, 5731–5736 (2010).
Funding
The authors did not receive any funding.
Author information
Authors and Affiliations
Contributions
Kavitha - Visualization, Writing reviews; Jothimani Kannupaiyan- Draw a figures, Software, visualization; Ranjith Rajendran - Methodology, Investigation; Aswini Rangayasami - Writing original draft, review, editing.
Corresponding authors
Ethics declarations
Ethical Statement
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kavitha, T., Kannupaiyan, J., Rajendran, R. et al. Evaluate the Ni-ZnO/g-C3N4 Nanocomposite for Photocatalytic Degradation of Organic Defect Degradation and Antibacterial Activity. J Clust Sci 35, 2363–2375 (2024). https://doi.org/10.1007/s10876-024-02663-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-024-02663-4