Abstract
In this work, the effect of heat treatment in the synthesis of vanadium pentoxide (V2O5) on its physicochemical properties was studied. Furthermore, the effect of liquid and viscous electrolytes based on lithium perchlorate (LiClO4) on the performance of electrochromic devices (ECDs) was evaluated using heat-treated V2O5 as electrochromic film. V2O5 was synthesized using the chemical bath technique. The V2O5 without and with heat treatment at 400 °C was characterized using FT-IR, UV-Vis, XRD, XPS, FESEM, AFM, and cyclic voltammetry (CV) techniques. Better physicochemical behavior was shown by the thermally treated V2O5, which is why it was used in the ECDs. Single ECDs were characterized using optical transmittance spectra, kinetic curves, and electrochemical impedance spectroscopy. A broadening of the absorption band towards the red and better electrochemical properties were observed when a thermal treatment was applied to the amorphous V2O5. A charge storage capacity of 1061.42 mC/cm2 was obtained for the crystalline V2O5 film (with heat treatment) compared to the charge storage capacity of 303.38 mC/cm2 obtained for the amorphous V2O5 film. A better distribution and decrease in grain size was observed for V2O5 with heat treatment. The CV curves showed higher stability of crystalline V2O5 compared to amorphous V2O5, even after several cycles and at different scan rates. A higher flow of ions between the working electrode and the counter electrode was achieved with the use of liquid electrolyte in the ECD, allowing a more efficient ECD with greater change in transmittance in the near-infrared region, compared to the use of viscous electrolyte. This research work contributes to the improvement of the physicochemical properties of V2O5.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The physicochemical properties of V2O5 have been of great interest for the development of supercapacitors, gas sensors, batteries, photocatalysts, infrared light detectors and electrochromic devices [1, 2]. Specifically, its crystalline structure in the form of layers and its relationship with electrochromic performance has caused great interest in the scientific community [3]. Among the different types of vanadium oxides that exist, V2O5 is the most stable [4]. V2O5 has a direct band gap of 2.2 to 2.7 eV, exhibits multicolor electrochromic behavior, and the different classes of nanostructures in which it has been obtained have allowed it to be classified as a very promising material for the development of current technology [5,6,7].
The electrolyte and electrodes are the main components of an electrochromic device. In these components, oxidation-reduction (redox) reactions are carried out with the help of an external potential, which modifies the chemical structure of the electrochromic material and, consequently, produces a change in its color. These components must work together to achieve an electrochemically and thermally stable, durable, and efficient device [8]. The thermal and electrochemical stability, as well as the durability of these electrochromic devices, can be achieved by using a suitable electrolyte. Likewise, the efficiency of these devices is greatly influenced by the electrochromic material in question.
The electrochromic materials most used for the manufacture of electrochromic devices are transition metals such as V2O5, which is perhaps one of the most promising oxides to be applied in this type of technology. The laminated structure of V2O5 in the form of layers facilitates the intercalation of Li+ ions, allows operation at low voltages with efficient consumption of electrical energy and presents a variety of colors when oxidized or reduced [9, 10]. However, V2O5 has a low charge storage capacity, low electrical conductivity (10− 2-10− 3 S/cm), a low Li+ diffusion coefficient (10− 12-10− 13), volumetric changes during the test cycles and a low difference in transmittance between the colored and bleached states [11]. In this regard, efforts have been made to provide solutions to the problems presented by V2O5. These efforts have consisted of synthesizing V2O5 by different methods, manufacturing nanostructured films, and performing some type of surface treatment [10].
The type of electrolyte (liquid or viscous (gel)) allows some advantages and disadvantages in the development of electrochromic devices. It has been reported in the literature that the use of liquid electrolytes allows high ionic conductivities to promote reversible oxidation and reduction reactions, taking full advantage of the properties of the electrochromic material [12]. However, this type of electrolytes is not common in applications where large surface areas are required due to potential leakage, evaporation, and low structural stability [13]. One solution to the problem presented by liquid electrolytes is to increase their viscosity to increase mechanical resistance; electrolytes with a high degree of viscosity allow the manufacture of light, safe, thin, and handy electrochromic devices (ECDs) [8]. These higher viscosity electrolytes are gel-shaped and are called polymeric electrolytes (PEs).
In this work, the effect of two types of electrolytes (liquid and viscous) on the performance of electrochromic devices manufactured with V2O5 heat treated at 400 °C was studied. There are few works in the literature that report the performance of electrochromic devices using different types of electrolytes based on thermally treated V2O5 electrochromic film. Among the works found in the literature, Liu et al., [6] reported obtaining V2O5 nanorod films from V2O5 macropore films by thermal treatment. The heat-treated V2O5 nanorod films showed higher ion storage capacity than that exhibited by the unheated V2O5 macropore films. The heat-treated V2O5 nanorod films were used as ion storage and electrochromic layers to fabricate dual electrochromic devices using a liquid electrolyte. In another study, Lin et al., [10] observed an improvement in the electrochromic properties by obtaining an increase in the crystallinity of V2O5 through thermal treatment. This facilitated the intercalation of Li+ ions, increasing the charge storage capacity and promoting a greater difference in transmittance between the colored and bleached states.
In this work, V2O5 thin films obtained by chemical synthesis were thermally treated and characterized by different laboratory techniques to determine the effect of thermal treatment on their physicochemical properties. The results show a substantial increase in the charge storage capacity (ions) of more than three times the magnitude when applying thermal treatment to the V2O5 films, this behavior has not been reported in the literature. Likewise, two types of electrolytes based on LiClO4 were prepared, liquid and viscous (gel). These were used in conjunction with heat-treated V2O5 films to fabricate single ECDs. The effect of the type of electrolyte on the performance of the ECDs was studied. Furthermore, the mechanisms controlling the electrochromic process at the V2O5/PE interface were determined using an alternating current electrochemical (EIS) technique.
Materials and Methods
Materials
Vanadium (IV) oxide sulfate hydrate (VOSO4, 97%), sodium hydroxide (NaOH, 97%), poly(methyl-methacrylate) (PMMA, Mw = 120 000), lithium perchlorate (LiClO4, 95 + %), propylene carbonate (PC, 99%), ethylene carbonate (EC, 98%), tetrahydrofuran (THF, 99.9%) were provided by Aldrich. Methyl alcohol (CH3OH, 99.9%) and isopropyl alcohol (C3H8O, 99.9%) were provided by Fermont.
Synthesis of V2O5
V2O5 was synthesized using the chemical bath deposition method, following the procedure reported by Pande et al. [14]. An aqueous solution of vanadyl sulphate (VOSO4, 25 mL, 0.1 M) was prepared with NaOH (0.4 mL, 1 M) by heating at 60 °C with constant stirring for 3 h. The green precipitate obtained was washed with water, methyl, and isopropyl alcohol. Finally, the precipitate obtained was dried at a temperature of 60 °C for 24 h.
Preparation of Electrolytes
Two types of electrolyte were prepared to assemble the electrochromic devices: (1) viscous electrolyte (gel) composed of a mixture of PMMA, LiClO4, PC and EC, with 25, 5, 35 and 35 wt%, respectively, which were dissolved in 2 mL of THF; (2) liquid electrolyte composed of LiClO4, PC and EC, with 6.8, 46.6 and 46.6% wt, respectively.
Manufacturing of Electrochromic Devices
The V2O5 thin films were prepared by the spin-coating method as follows: the V2O5 powder was dispersed with isopropyl alcohol with a V2O5/C3H8O ratio (5 mg/8 mL), obtaining a transparent green solution. Then, the solution was added dropwise in small portion by spin coating on an indium tin oxide (ITO) glass for 2 min at a speed of 2000 rpm. To obtain multilayer substrates, 4 mL of total solution was deposited. Finally, the V2O5 multilayer substrates were heat treated at 400 °C for 1 h.
The electrolyte prepared in Sect. 2.3, liquid or gel, was applied to one of the electrodes (V2O5 film/ITO) and then another electrode (bare ITO) was placed on top of the electrolyte. Subsequently, the edges of both electrodes were sealed with silicone to prevent electrolyte leaks. The single electrochromic device configuration was glass/ITO/V2O5/electrolyte/ITO/glass.
Characterization
The FT-IR spectra of V2O5 were obtained on a Perkin Elmer spectrometer (in ATR mode), working in a range of 4000 –400 cm− 1. UV-Vis analysis of V2O5 was performed on a GENESYS™ 10 S UV–Vis spectrophotometer, working in a wavelength range of 190–1100 nm. For UV-Vis analysis, the films were previously prepared using the spin-coating technique following the procedure described in Sect. 2.3 (using Corning glass substrates). The XRD patterns of the V2O5 powders were obtained using a Bruker diffractometer (D8 ADCANCE eco), with Cu-Kα radiation, operated at 40 kV, 25 mA, in the 2θ range of 10–70°. XPS analysis was obtained on a XPS Perkin-Elmer Phi-5100 with a non-monochromatic magnesium anode and an emission of Kα of 254 KeV. The morphology of V2O5 powders was performed on a field emission scanning electron microscope, FESEM (JSM-7800 F). Cyclic voltammetry (CV) measurements were performed using a BAS Epsilon 100 B/W potentiostat/galvanostat connected to a three-electrode cell. The three-electrode cell consisted of a V2O5-film/ITO (working electrode), a platinum mesh (counter electrode), and a home-made Ag/Ag+ [(0.01 M AgNO3) (0.1 M tetrabutylammonium perchlorate (TBAp) in acetonitrile as the filling solution)] as reference electrode; liquid electrolyte (transport ions) was used. The thickness and roughness of the V2O5 thin films were measured by AFM (easyscan2 from Nanosurf) in contact mode, the silicon cantilever had a length of 450 μm with a force constant of 0.2 N/m. The transmittance spectra of the manufactured ECDs were measured in the wavelength range of 350–1100 nm using a Oceanoptics Spectrometer (S2000) and a light source (DH2000 BAL, 200–2000 nm). Optical transmission spectra of the ECDs were obtained using an optical array consisting of a solid state laser (532 nm, POUT = 20 mW), a Thorlabs photodiode sensor (S120VC, 200–1100 nm), and a Hewlett Packard power supply (E3610A). Electrochemical impedance spectroscopy (EIS) measurements were carried out using a two-electrode electrochemical cell: V2O5-film/ITO (working electrode) and a bare ITO (counter electrode). Measurements were performed at room temperature and controlled by a Gill AC potentiostat/galvanostat with an AC potential amplitude of 10 mV, a frequency range of 0.1 Hz to 30 kHz, and 100 readings per decade.
Results and Discussion
Effect of Heat Treatment on Vanadium Oxide
FTIR Analysis
Figure 1 shows the FT-IR spectra of V2O5 before (a) and after (b) heat treatment at 400 °C. Figure 1a shows a band at 960 cm− 1assigned to the unshared V = O stretching vibration, a band at 800 cm− 1 assigned to the bonded oxygen vibrations that are shared by two vanadium atoms V–O–V, a band at 517 cm− 1 assigned to the stretching mode of oxygen, which is shared between three vanadium atoms [15]. The peak at 1627 cm− 1 corresponding to the C = O stretching vibration and the peaks at 3565 and 3266 cm− 1 corresponding to OH groups confirm the presence of organic residues (solvents used in the reaction and washing of the product) in the sample [16]. In the spectrum of the heat-treated V2O5 (Fig. 1b), the peaks observed at 3565, 3266 and 1627 cm− 1 in the untreated V2O5 disappeared due to the loss of solvent impurities in the sample. In heat-treated V2O5, the structure of orthorhombic crystals of V2O5 is evident with the peak referring to the vanadyl mode (double bond of oxygen and vanadium, V = O) at 1020 cm− 1 (generally, the V = O peak in crystalline V2O5 is in the range of 1017–1021 cm− 1) [16]. According to Fang et al. [15] the band at 960 cm− 1 of V2O5 before heat treatment changed to 1020 cm− 1 after heat treatment, indicating that the bond length of V = O is shortened after annealing. Two other peaks were observed at 825 and 590 cm− 1, which are attributed to the asymmetric (νas V-O-V) and symmetric (νs V-O-V) stretching of the V-O-V bond, respectively [17]. According to Fu et al. [18] the peak at 825 cm− 1 can also be attributed to the coupled vibration between the V = O and V-O-V bonds. At the peak of 420 cm− 1 a shoulder was observed at around 490 cm− 1 which, according to Przesniak-Welenc et al., corresponds to the bending vibration of the V-O-V bond [16]. This illustrates that vanadium ions with lower valence states existing in the sample without heat treatment have been oxidized to V2O5 [15].
FTIR spectra were deconvoluted in the Origin program using the Lorentz-function. Figure 2 shows the FTIR absorption spectra of V2O5 without and with thermal treatment (solid black line) in the range of 400 to 1100 cm− 1 (area of peaks related to V2O5), and its deconvolution is shown (colored areas) to identify the IR modes. Through the deconvolution of the FTIR spectra, it is observed that the thermal treatment completely modified the FTIR spectrum of V2O5. It was confirmed that with the thermal treatment the V2O5 completely changed to the orthorhombic crystalline phase, due to the absence of the 960 cm− 1 peak present in the V2O5 without thermal treatment. According with Fang et al. [15] the band at 960 cm− 1 of the V2O5 before heat treatment shifted to 1020 cm− 1 after heat treatment, indicating that the bond length of V = O becomes shorter after annealing.
UV-VIS Analysis
The UV-Vis results of V2O5 without and with heat treatment are shown in Fig. 3. V2O5 without heat treatment (Fig. 3a) showed strong absorption in the UV region, with a maximum value at 290 nm, and a shoulder at the visible region, with a maximum value at approximately 400 nm. Yalagala et al. obtained similar results for V2O5 nanosheets [5]. On the other hand, the V2O5 with heat treatment (Fig. 3b) showed a wide absorption band between the UV and visible regions, presenting two absorption maxima at 290 and 375 nm, and a shoulder with a maximum value at approximately at 470 nm. It is worth mentioning that the V2O5 film without heat treatment showed light green coloration, while the film with heat treatment showed a light yellow coloration. The color change observed in heat-treated V2O5 films has been attributed to the effect of heat treatment, which causes an increase in the crystallinity of V2O5, and therefore a change in its crystalline structure [19]. Furthermore, it is well known that vanadium oxides have a wide variety of crystal structures, this diversity comes from their wide range of oxidation states, from V+ 2 a V+ 5 [20]. According to Chang et al. this color change due to heat treatment leads to the formation of different surface morphologies that trap or scatter light [21]. According to Le et al. the fully stoichiometric phase of pure V2O5 exhibits orange color in optical coloration, the non-stoichiometric phase exhibits different colors, such as pale yellow, yellow-brown, dark green, and grayish black [22]. The heat-treated V2O5 phase is probably more stoichiometric than the untreated V2O5 phase.
The band gap values of the V2O5 films were calculated using the values of the beginnings of the absorption bands; the results are shown in Fig. 4. The heat-treated V2O5 film showed a lower band gap value (1.7 eV) compared to the untreated film (2.5 eV). The lower band gap value observed in the heat-treated V2O5 film is due to the broadening of the absorption band towards the red (Fig. 3) with respect to the film without heat treatment [23]. It has been reported that the optical properties of V2O5 micro-nanostructures are strongly affected by many factors, such as morphology, phase transition, and external treatment. Most reports describe a band gap between 2.0 and 2.7 eV for V2O5 [24]. However, there are reports describing smaller band gaps of V2O5, similar to what we obtained with heat treatment, around 1.7 eV [25,26,27]. Raj et al. [25] found that the optical bandgap value of V2O5 films deposited on glass and Si substrates was in the range of 2.5–3.49 and 1.72–2.45 eV, respectively. They determined that V2O5 films deposited on glass substrate showed an amorphous nature, while those deposited on Si substrate showed a crystalline nature. Probably in our case the samples without heat treatment have an amorphous nature and the heat-treated ones are crystalline.
X-Ray Analysis
X-ray analysis was carried out to determine the amorphous or crystalline structure of the samples. The XRD results of the V2O5 powders without and with heat treatment are shown in Fig. 5. The diffraction pattern of V2O5 without thermal treatment (Fig. 5a) showed broad diffraction peaks with low intensity and absence of several peaks characteristic of the reflection planes of the crystalline phase of V2O5. However, the heat-treated V2O5 sample showed a crystalline nature (Fig. 5b), the diffraction peaks correspond to the Shcherbinaite crystalline phase (α-V2O5, diffraction chart No. 01-072-0598 with an orthorhombic crystalline system) with cell constants a = 11.5030 Å, b = 4.3690 Å and c = 3.5570 Å. These results are consistent with those obtained by FTIR. The absence of peaks related to other vanadium oxides with different oxidation states or polymorphs of V2O5 indicates that by performing a thermal treatment at 400 °C for a period of 1 h, a pure and uniform V2O5 phase was obtained. Compared to the heat-treated V2O5, the peaks shown by the V2O5 without heat treatment (Fig. 5a) were of lower intensity and wider, however, the position of the peaks did not show any change (except for a slight shift of the peak located at 50.4° (without heat treatment) to 51.1° (with heat treatment)), likewise, there were no additional peaks. This suggests that V2O5 without heat treatment is in a mostly amorphous state. These results corroborate what was indicated in the previous section, that V2O5 with an energy gap of 2.5 eV corresponds to the amorphous state and with 1.7 eV to the crystalline state. Kim et al. [28] obtained similar diffraction patterns for amorphous and crystalline V2O5, they observed the amorphous state of V2O5 by mechanically grinding the crystalline V2O5, decreasing the particle size, but increasing its amorphous character. In our case we obtained crystalline V2O5 from amorphous V2O5 by applying a thermal treatment.
XRD patterns of V2O5 were deconvolved by the Origin program using the Lorentz-function. In the deconvolution of V2O5 without thermal treatment, the maxima of the broad peaks at 12.4°, 15.1°, 18.2°, 26°, 30.1°, 34.6°, 46.9°, 50.4° and 60.9° were identified, showing the mostly amorphous nature of the V2O5. The assumption was that the narrow (sharp) peaks corresponded to crystalline domains, whereas the broad peaks corresponded to the amorphous contribution. The crystallinity of each sample was calculated as follows: Crystallinity (%) = (Ac/At) x 100, where, Ac and At represent the area of the crystalline domain and the area of the complete domain (crystalline + amorphous), respectively. The results of % crystallinity were 48.8 and 87% for V2O5 without and with heat treatment, respectively.
The crystal size was calculated using Scherrer equation (D = Kλ/(βcosθ)), where λ (1.5406 A˚ ) is the X-ray wavelength; β is the full width at half maximum (FWHM) intensity, θ is the Bragg diffraction angle, and K = 0.9 is the particle shape factor or Scherrer constant [29]. The most prominent crystalline peak in the XRD patterns (2θ = 26°) corresponding to (101) plane of α-V2O5 with an orthorhombic crystalline system, was chosen for the crystallite size calculation. The crystallite size for V2O5 without heat treatment was 5.8 nm, for heat-treated V2O5 the crystallite size increases at 56.5 nm. S. Sutrave et al. [30] and Mouratis et al. [31], reported lower values of crystal size for V2O5, 28 and 22 nm, respectively. The heat treatment increased the crystallinity in V2O5 because the V-ions will react with the excess oxygen, causing the vacancies to decrease. Hongying Liu et al. [32] reported the annealing treatment controlled the concentration of oxygen vacancies into a V2O5 nanorod, significantly promoted the diffusion kinetics of Li+, obtained V2O5 films whit good ion-storage capacities, simultaneously possess good electrochromic properties, independently of the lithium-ion diffusion distance, good electrolyte permeability, and low charge transfer resistance. Through electrochromic characterization we obtained similar results.
XPS Analysis
Figure 6 show the deconvoluted spectra of V2O5 without and with heat treatment, the photoelectron spectra of the two samples corresponding to the O1s and V2p core levels indicate the exclusive presence of vanadium oxide. For V2O5 with heat treatment: the peak at ~ 530 eV relates to the BE of the O-V bonds, and the peak at ~ 532 eV is ascribable to surface contamination mostly from C bonds (C–O, C = O and C–(OH)), an average BE for the V5+ component of the V2p3/2 signal is found at ~ 517.1 eV, the sample did not show V4+ component [33]. The results confirm that vanadium oxide without heat treatment corresponds only to V2O5.
When the sample is subjected to heat treatment, the average BE of the V2p3/2 signal is found at ~ 515.9. In the literature, a shift of the V5+ component of the V2p3/2 signal towards lower BE with the thermal treatment of V2O5 has been reported [34]. In our case, because the X-ray pattern perfectly matched that of V2O5, and also because of the wide variety of binding energy assignments in the literature, we concluded that we obtained V2O5.
FESEM Analysis
The surface morphology of materials plays a crucial role in the properties and performance of ECDs. For the application of electrochromic devices, one of the main objectives is to obtain a surface morphology capable of interacting homogeneously with the electrolyte. The morphology of the V2O5 powder was characterized and analyzed by FESEM, Fig. 7. The V2O5 without thermal treatment (Fig. 7a-b) shows a morphology in the form of lumps, with a variety of sizes, some more compacted than others. On the other hand, Fig. 7c-d shows the effect of thermal treatment on V2O5. A change in morphology is observed, showing more defined lumps with a more homogeneous size. Likewise, in the higher magnification images (Fig. 7b and d) a greater definition of the particles of the thermally treated V2O5 can be observed. This indicates that increasing the temperature to 400 ºC activates surface diffusion, causing the formation of crystalline grains [35], Fig. 7c. Increasing the temperature in the amorphous V2O5 (Fig. 7a) allowed its structure to change to a completely crystalline state (Fig. 7b). Using the ImageJ program, an average particle diameter of 301 nm was determined for the V2O5 morphology without heat treatment, as well as an average particle diameter of 177 nm for the V2O5 morphology with heat treatment, the histograms are shown in the insets of Fig. 7b and d, respectively. The decrease in particle size determined by FESEM analysis could be due to the dehydration process during heat treatment at 400 °C that causes a structural collapse after the removal of the organic linker units [36].
AFM Analysis
The surface morphology, roughness and thickness of the V2O5 films without and with heat treatment determined by AFM are shown in Fig. 8; Table 1. The V2O5 without heat treatment showed a granular morphology with the presence of agglomerates. The effect of the thermal treatment caused a change in the morphology of V2O5, the morphology was more homogeneous, the grain size was reduced, and the agglomerates were smaller. Likewise, the effect of the thermal treatment caused a decrease in the roughness and thickness of the V2O5 film, Table 1.
Cyclic Voltammetry (CV)
CV measurements were carried out to study the electrochemical properties of V2O5 films with and without heat treatment, using a liquid electrolyte. Figure 9a shows the CV curves of the V2O5 film without heat treatment at different scanning rates (10, 20 and 100 mV s− 1). The shape of the voltammograms for the V2O5 film without heat treatment was similar to the voltammograms reported by Wu et al. [37] for the amorphous V2O5. In Fig. 9a, a linear increase in oxidation current was observed with increasing scan rate, suggesting that the kinetics of interfacial faradic redox reactions and electron and ion transport rates were fast enough with respect to at scanning rates [38, 39]. All voltammograms contain two pairs of redox peaks; however, at 10 mV s− 1 the redox peaks are better defined. The two redox couples for amorphous V2O5 (0.15/0.40 V and − 0.33/-0.07 V in the cathodic/anodic sweep for 10 mV/s) correspond to the vanadium valence changes of V+ 5 to V+ 4, and V+ 4 to V+ 3, respectively [40].
It is observed that the oxidation peaks tend to coalesce with increasing potential scan rates. Furthermore, a shift of the oxidation and reduction peaks was observed with increasing scanning rate, which could be attributed to the internal resistance of the electrode according to Khan et al. [39]. Liu et al. [6] observed this same behavior by increasing the scanning speed from 1 mV/s to 100 mV/s, a shift of the reduction peaks to lower potentials and a shift of the oxidation peaks to higher potentials; this is because, at low scanning speeds, time is not a limiting factor and the entire active surface is available to carry out the redox reactions. However, at high speeds and due to time limitations, diffusion limits the movement of Li+ and only the external active surface is used to carry out the redox reactions. This allows polarization of the electrodes, i.e. changes in the redox peaks. On the other hand, the redox peaks exhibited by this V2O5 film without heat treatment were not sharp or noticeable as in the case of the V2O5 film with heat treatment (Fig. 9b), suggesting that V2O5 is mainly in semicrystalline or amorphous form [6] as observed in XRD.
The heat-treated V2O5 film (Fig. 9b) also showed an increase in current with increasing of scanning rate. Likewise, a shift of the oxidation and reduction peaks was observed, like the V2O5 film without thermal treatment. However, the current values for the heat-treated film were five times higher than the values for the untreated film. This could be because an increase in the crystallinity of V2O5 allows a considerable increase in ionic transport and consequently increases the current. The above indicates that the V2O5 film with heat treatment has great potential to be applied in ECDs, because it shows greater charge storage (larger cycle area) than the V2O5 film without heat treatment. Furthermore, the V2O5 film with thermal treatment showed 4 pairs of redox peaks, the peaks being sharper and better defined compared to the film without treatment, indicating a higher crystallinity of the V2O5. Likewise, the voltammograms show greater stability of V2O5 than those corresponding to V2O5 without thermal treatment. V2O5 has been reported to be the most stable among all types of vanadium oxides [4]. With a decrease in scan rate, oxidation peaks move to lower potentials and reduction peaks move to higher potentials. At a scan rate of 20 mV/s− 1 (Fig. 9b), 4 cathodic peaks were observed at -0.05 V, -0.25 V, -0.53 V and − 0.90 V, corresponding to phase transitions α/ε, ε/δ, δ/γ, γ/ω, respectively: their anodic peaks corresponding at 0.17, -0.01, − 0.34, and − 0.60 V can be attributed to reverse phase transitions (ε/α, δ/ε, γ/δ, ω/γ) [41]. Liu et al. [41] also observed the 4 cathodic peaks, however, they only observed 2 anodic peaks. In our case, the four anodic peaks were defined. It is worth mentioning that for the scanning speeds of 100 and 50 mV/s, the minimum of the lowest potential reduction peak could not be observed, because it was located at a lower potential than the test.
To adequately characterize the insertion and extraction processes of Li+ ions from V2O5 without and with thermal treatment, the Randles-Sevcik equation was used [42]. According with the literature, the most reversible character is established with a linear dependence on the Randles-Sevcik graphs [43]. Figure 10 shows the correlations between the maximum peak currents ip and the square root of the scan rate, v1/2 (Randles-Sevcik plots). For the construction of Randles-Sevcik plots, voltammograms at different scanning speeds were used for the calculation. The redox couple between the ε and α phase transitions (anodic peak of 0.4 and cathodic peak of -0.22) for the thermally treated V2O5 (Fig. 9b), and the redox couple corresponding to the valence changes V+ 5/V+ 4 (0.15/0.40 V in the cathodic/anodic sweep) for V2O5 without heat treatment (Fig. 9a) were chosen for calculation. According to Tong et al. [42], the straight line fit of the maxima of the anodic and cathodic peaks, shows that the oxidation/reduction processes are controlled by the diffusion of ions from the electrolyte to the electrode surface. In Fig. 10b, the heat-treated V2O5 showed a linear relationship of cathodic and anodic peaks, demonstrating that the oxidation/reduction processes of the V2O5 layers are reversible and controlled by the diffusion of Li+ ions from the electrolyte to the electrode surface. However, in the case of V2O5 without thermal treatment (Fig. 10a), the redox couple behavior fits less to the linear behavior, indicating that V2O5 without thermal treatment is less reversible and has low diffusion of Li+ ions.
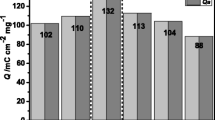
Figure 11 shows the CV plots for the V2O5 films without and with heat treatment at a scan rate of 100 mV s− 1. In the case of the thermally treated V2O5 film, color changes (blue↔green↔yellow) were observed at different potential values. However, in the case of the V2O5 film without heat treatment, the color changes were practically imperceptible to the human eye. Similar results were obtained by Shimizu et al., who observed a greater color change (blue↔green↔yellow) for a crystalline V2O5 film compared to an amorphous V2O5 film, which presented an absence of electrochromism and maintained a gray-green color during potential sweep cycles [44]. The electrical charge stored and discharged in the V2O5 films with and without thermal treatment was calculated from the integration of the area under the curve in the anodic and cathodic branches of the corresponding voltammograms (first redox cycle), the results are presented in the Table 2. It is important to mention that due to the almost rectangular shape of the voltammogram of the V2O5 film without thermal treatment, it could be used for the development of capacitors or supercapacitors. Otherwise, the voltammogram of the thermally treated V2O5 film deviates from the rectangular shape, because faradaic reactions have a greater influence on the process. All of this may also explain the color changes observed.
Liu et al. [6], when synthesizing by the V2O5 gel method with thermal treatment at 350 °C for 6 h, obtained an ion storage of 98.77 mC cm− 2, a lower value than that obtained in this work. Lin et al. [10], also observed the increase in cycle area with heat treatment, however, the increase was not as drastic as in our case. Likewise, Lin et al. [45] observed an increase in the charge storage capacity of the V2O5 film with an increase in film thickness, obtaining 29.18, 42.58 and 54.71 mC/cm2 for thicknesses of 98.9, 192.4 and 288.2 nm, respectively. In our case we obtained a charge storage capacity of 1061.42 mC/cm2 for a thickness of 137 nm, a value much higher than that reported in the literature. On the other hand, Asiabar et al. [46] studied the effect of thermal treatment at a temperature of 450 °C in different atmospheres, obtaining a charge storage capacity of 23.98, 57.54, 42.98 and 36.62 mC/cm2 in atmospheres of air, nitrogen, argon and oxygen, respectively. Values lower than those obtained in this work, where a heat treatment was carried out at 400 °C under an air atmosphere.
Effect of Electrolyte Type on ECDS Performance
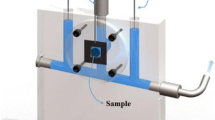
Single electrochromic devices were fabricated using the arrangement shown in Fig. 12, which consists of commercial ITO with thermally treated V2O5 film deposition as the working electrode, commercial ITO as the counter electrode, and the electrolyte (liquid or gel) as the ionic conductor. To determine the electrochromic properties of these devices, UV-Vis spectroscopy, optical kinetic transmittance, and electrochemical impedance spectroscopy (EIS) measurements were performed.
Electrolyte Gel
Figure 13, shows the transmittance spectra of the colored and bleached states of the single ECD with a gel electrolyte (ECD-gel), in a wavelength range of 400–1000 nm, at a voltage of ±2 V. The device in the bleached state showed a weak yellow color, while in the colored state it showed a weak pale blue color, see the inset in Fig. 13. The V2O5 ECD-gel showed a maximum in the optical transmittance signal of 74.32% at a maximum wavelength of 675 nm. In the wavelength range of 400–620 nm, the transmittance signal is higher in the colored state. However, at longer wavelengths (620–1000 nm) the transmittance is higher in the bleached state. Furthermore, the spectral response of the ECD-gel showed a shift in wavelength from 532 to 486 nm for the bleached and colored state, respectively, corresponding to a maximum shift of 46 nm. According to the spectra in Fig. 13, a maximum optical contrast of 13.69% (in 400–620 nm range) and 3.81% (in 620–1000 nm range) was obtained at the wavelengths of 456 and 851 nm, respectively. The optical contrast is low compared to what is reported in the literature, probably due to the film thickness, which in our case was of the order of nm and in the literature thicknesses of microns are used [47, 48].
Figure 14 shows the optical transmission spectra representing the kinetics of the ECD-gel at 532 nm during repetitive electrochromic switching between + 2 and − 2 V. The kinetic response of the ECD-gel showed that after the first two cycles, the redox process reached stability (see Fig. 14a). However, once stability in the redox process was achieved, a minimal decrease in amplitude was observed after each measured cycle (see Fig. 14b). This small decrease in the transmission amplitude of each redox cycle with respect to time has been reported for single ECDs manufactured from V2O5 [49]. On the other hand, the noise observed in the graphs could be due to the difficulty that Li+ ions have in diffusing through the gel electrolyte to contribute to the redox process.
According to Fig. 14b (blue box), when a voltage of + 2 V is applied for a time of 60 s, the V2O5 is oxidized (bleached) increasing the output transmission signal up to values of 3.11 µW. While when a voltage of -2 V is applied for a time of 60 s, the V2O5 is reduced (colored), decreasing its output transmission signal to values of 2.98 µW. Considering that the incident laser power on the single device is 5.05 µW for a wavelength of 532 nm, we have a transmittance of 61.58% in the oxidized state and a transmittance of 59.01% in the reduced state.
Liquid Electrolyte
Figure 15 shows the transmittance spectra of single ECD with a liquid electrolyte (ECD-liquid), for the colored and bleached states in a wavelength range of 400–1000 nm at a voltage of ±2 V. The ECD-liquid showed a yellow color in the bleached state and a blue color in the colored state, as seen in the inset of Fig. 15. The colors observed with the ECD-liquid were more intense than those observed with the ECD-gel. The single liquid-ECD showed a maximum optical transmittance signal of 73.94% at a maximum wavelength of 700 nm. As in the previous case, in the wavelength range of 400 to 610 nm the transmittance signal is greater in the colored state, however at higher wavelengths the transmittance signal is greater in the bleached state. Furthermore, the spectral response of the ECD-liquid showed a shift in wavelength from 532 to 494 nm for the bleached and colored state respectively, corresponding to a maximum shift of 38 nm. According to Fig. 15, a maximum optical contrast of 9.68% (in 400–610 nm range) and 6.89% (in 610–1000 nm range) was obtained at the wavelengths of 471 nm and 852 nm, respectively.
Figure 16 shows the optical transmission kinetics of the ECD-liquid monitored at 532 nm during repetitive electrochromic switching between ±2 V. Unlike the gel electrolyte, good kinetic-optical stability was obtained with the liquid electrolyte from the beginning of the test. Furthermore, the liquid-ECD presented a very small decrease in the transmission signal in the redox cycles with respect to time, evidencing the good stability that the liquid electrolyte confers to the ECD based on thermally treated V2O5. On the other hand, the kinetic-optical graphs of the ECD-liquid presented lower noise compared to the ECD-gel, which could be due to the fact that Li+ ions diffuse more easily in the liquid electrolyte to participate in redox processes, such as it would have to be expected. It is important to mention that, in the ECD-liquid, the oxidation/bleaching (+ 2 V) and reduction/coloring (-2 V) processes are carried out in very similar times, indicating that the insertion/extraction processes of Li+ ions take the same work to perform.
According to Fig. 16 (orange box), when a voltage of + 2 V is applied for a time of 60 s, the V2O5 is oxidized (bleached) increasing the output transmission signal up to values of 3.32 µW, while when applies a voltage of -2 V for a time of 60 s, the V2O5 is reduced (colored), decreasing its output transmission signal to values of 3.03 µW. Considering that the incident laser power on the single device is 5.05 µW for a wavelength of 532 nm, the ECD-liquid presents a transmittance of 65.74% in the oxidized state and a transmittance of 60% in the reduced state.
In the literature, optical contrasts similar to those obtained in this work have been obtained in ECDs based on V2O5. Tong et al. [7] obtained an optical contrast of 9.6% at 460 nm, while a maximum value of 14.6% at 780 nm under ± 1.5 V, using the ITO/V2O5/liquid electrolyte/PEDOT/ITO configuration. Surca et al. [50] prepared a NiO1 − x/ormolyte/V-oxide film ECD and obtained an optical contrast of up to 9.2% at 634 nm. Lin et al. [10] obtained an optical contrast of 31% at 650 nm when the V2O5 film was annealed at 400 °C. This optical contrast was greater than the one obtained by us, probably due to the greater thickness of the film used, approximately double that of the one we used. Additional experimentation is necessary to increase the optical contrast of ECDs.
EIS Analysis
Electrochemical impedance spectroscopy (EIS) is a sensitive alternating current (AC) technique that provided a better understanding of the charge transfer characteristics at the V2O5 /electrolyte interface during the electrochromic process at a potential of 0 V. Figures 17 and 18 show Nyquist plots of ECDs made with two types of electrolytes (liquid or gel). The intersection of the graph on the X-axis at high frequencies (HF) represents the electrolyte ohmic resistance (Rs) [51]. For both electrolytes, a tendency to form a depressed semicircle at HF was observed (inset Figs). The diameter of this semicircle (X-axis intersection) observed in HF is assigned to the charge transfer resistance (Rct), which is related to the electrolyte-accessible surface area of the electrode during redox process [52]. In the case of the gel electrolyte, two straight lines with an angle greater than 45° with respect to the X-axis (real axis) were also observed, one at medium-low frequencies (MLF) and another quasi-vertical at low frequencies (LF). These two behaviors correspond to a Warburg impedance (W) and a limiting capacitance (LC), respectively [53]. The W represents the diffusive resistance and since the angle of the straight line with respect to the real axis is greater than 45°, the capacitive electrochemical behavior of the electrode is not controlled by the diffusion process [54]. The W is represented by the following equation [55]:
where ω is the frequency, Rw is the finite resistance at low frequencies, α is a time constant, meaning the diffusion time, and S is an exponent related with the roughness of the diffusion medium.
The straight line at LF is typical of intercalation electrodes in the form of thin films deposited on ITO, which is impermeable to Li+ ions [56]. In the case of the liquid electrolyte, the Warburg impedance was not observed and only the behavior of the limiting capacitance (quasi-vertical line) was observed. Figure 18 shows the equivalent circuits used to simulate the EIS data and the corresponding fit of the Nyquist plot (%Error < 0.4, ZVIEW software). In the circuits, Cdl is the double-layer capacitance and CPE is the constant phase element (CPE), it is used because of surface defects in non-ideal capacitors. The CPE is represented by the following equation [57]:
CPE =
where j is the imaginary number (j = \(\sqrt{-1}\)), ω is the angular frequency (ω = 2πf, f being the frequency) and Y0 and n are the characteristic parameters of the CPE (n = 1, capacitor, n = 0 resistor and n = -1 inductor). Table 3 shows the results obtained in the simulation of the EIS data.
The Rct values were 287.9 Ω and 354 Ω for ECDs with gel and liquid electrolyte, respectively. The Rct signifies the interfacial reaction between the electrochromic film or ion storage layer on the ITO coated with V2O5 films. The magnitude of Rct is proportional to the inserted/extracted number of Li+ ions in the V2O5. Thus, it is closely related to its electrochromic performance [58]. The Rct values for both electrolytes are similar, therefore, both electrolytes have a similar capacity to insert/extract Li+ ions at similar times into/from the V2O5 film. However, the gel electrolyte presented a resistive-capacitive behavior that could affect the electrochromic performance of the device. Rct values such as those reported in this work have been reported in the literature; ca. 200 Ω for a V2O5 xerogel film and ca. 578 Ω for a Ti-doped V2O5 thin compact film [42]. Li et al. reported orthorhombic and hydrated V2O5 films had Rct values of 79.3 Ω and 12.4 Ω, respectively [59]. Guo et al. obtained the Rct of 6.7 Ω for pristine V2O5 electrode [60]. It is worth mentioning that the Rct values will depend on several factors, one of which is the type of electrolyte and its composition. In this work, poly(methyl-methacrylate), lithium perchlorate, propylene carbonate and ethylene carbonate were used in different percentages and the gel electrolyte showed a resistive-capacitive behavior and the liquid electrolyte a more capacitive behavior. The impedance results are consistent with the results obtained in the direct current and optical tests, Li+ ions have a less tortuous path in the liquid electrolyte. Liu et al. manufactured a dual ECD with V2O5 deposited on the anode and cathode; V2O5 films were used as ion storage and electrochromic layers [61].
Figure 19 shows the Bode impedance modulus (|Z|) plots of the ECDs manufactured with liquid or gel electrolytes. In both cases of ECDs, the Bode plots show the same behavior: the |Z| increases with a decreasing frequency, with an increase observed at LF (below approximately 2 Hz). The |Z| is higher for the ECD with liquid electrolyte at 0.1 Hz, it reached 167 kΩ while the ECD with gel had 149 kΩ. In the ECD with liquid electrolyte, Li+ ions are doped into the V2O5 lattice, resulting in a decrease of the Li+ ions concentration in the electrolyte and an increase in |Z| compared to ECD with gel. The resistive behavior is predominant at LF (0.1 Hz) and the electrochromic reaction could be due to the intercalation mechanism [62].
The ionic conductivity of gel or liquid electrolytes was studied by εr and εi. The εr represents the stored charges of the material and also indicates the capacitive nature of the electrolytes, while εi stands for the energy loss to move the Li+ ions and align the dipoles when the polarity of the electric field is rapidly reversed [63]. Studies of εr and εi are necessary to understand the behavior and performance of gel and liquid electrolytes, and other electrolyte materials because they can provide very important information about the polarization effect at the electrode-electrolyte interface and help to better understand the conductivity trend. From the imaginary and real parts of the impedance (Z’’ and Z’, respectively), εr and εi were calculated using Eqs. (3) and (4) [64]:
where Z’’ is the imaginary part of the impedance, Z’ is the real part of the impedance, and ω = 2πf, where f is the frequency, Cο = εοA/t which is the vacuum capacitance of the measuring cell, where A and t denote the area and the separation length of the electrode, respectively. The quantity εο is the dielectric permittivity of the free space which is equal to 8.854 × 10−12 F.m− 1 [64].
The increase or decrease of ionic conductivity is also in accordance with the increase or decrease in εr and εi. Figure 20 shows the εr and εi vs. Log frequency for ECDs with gel or liquid electrolytes. The εr and εi values are high and show dispersive behavior at low- medium frequencies (below approximately 2 Hz for εr and 100 Hz for εi) due to the influence of polarization near the electrodes or accumulation of charge carriers [65]. The values of εr and εi were higher at almost all frequencies for the ECD with gel electrolyte, possibly because there are more charge carriers [66]. Furthermore, gel ECD may have allowed undissociated LiClO4 to convert to ions, so the charge stored in the electrolyte increased, resulting in an increase in εr values [67]. A decrease in εr and εi values was observed at higher frequencies; the values remained constant possibly due to the polarization effect generated by the accumulation of ions at the interface of the electrode and the electrolyte [64].
The complex dielectric modulus was used to study the conductivity relaxation behavior of gel and liquid electrolytes, as it has the capacity to undo the effects of electrode polarization to provide the corresponding electrical property [64]. From the complex dielectric constant, the real (Mʹ) and imaginary (Mʹʹ) modules were calculated using Eqs. (5 and 6) [68]:
Figure 21a and b show the Mʹ and Mʹʹ of the electric modulus vs. Log f for ECDs with gel or liquid electrolytes. It can be observed that both values of Mʹ and Mʹʹ increase towards high frequencies; this behavior has been reported in literature [64, 69]. The peak at higher frequencies in Mʹ indicates that the liquid electrolyte is a good ionic conductor [70]. Likewise, the small values of Mʹ and Mʹʹ at low frequencies are due to an acceleration in the migration of ions in polymeric electrolytes [64]. The values of Mʹ and Mʹʹ decreased towards lower frequencies because the polarization phenomena of the electrodes do not have a great influence on the electrochromic process. This tendency towards lower frequencies is also due to the good capacitance related to the electrode materials [64], V2O5 in this case.
The dielectric features of gel and liquid electrolytes were also analyzed using tan δ over a range of frequencies. This allowed the spatial relaxation process to be resolved. The tan δ values were calculated using Eq. (7) [64].
The tan δ vs. Log frequency for ECDs with gel or liquid electrolyte is shown in Fig. 22. An increase in tan δ values can be observed at high frequencies with a maximum value (relaxation peak) due to free Li+ ions from the LiClO4 that contributed to the relaxation process. The ECD with liquid electrolyte showed the highest tan δ values, indicating that the transport of Li+ ions was not blocked, resulting in a decrease in the concentration of mobile ions which subsequently leads to a decrease in the stored energy or tan δ [64].
Conclusions
V2O5 with an orthorhombic crystalline structure was successfully obtained from the thermal treatment of amorphous V2O5 synthesized by chemical bath. With thermal treatment of amorphous V2O5, it was possible to substantially increase the charge (ion) storage capacity by more than three times the magnitude, and reduce the bandgap from 2.5 to 1.7 eV. A better distribution and decrease in grain size was observed when applying the heat treatment to V2O5. The CV curves showed higher stability of crystalline V2O5 compared to amorphous V2O5, even after several cycles and at different scan rates. The single ECDs were manufactured based on thermally treated V2O5 using gel and liquid electrolytes, a maximum optical contrast of 13.7 and 9.7% was obtained in the visible region, respectively; however, in the wavelength range greater than 600 nm, the maximum optical contrast was 3.81 and 6.7%, respectively. The advantage of using a liquid electrolyte in the ECD was a shorter time to complete the colored state and better kinetic-optical stability, compared to the gel electrolyte. According to the EIS results, a resistive behavior was predominant in the ECDs and the electrochromic reaction could be due to the intercalation mechanism. Furthermore, the EIS results indicated that the liquid electrolyte is a good ionic conductor. Additional experiments are required to optimize the optical contrast of the ECD.
The increase in the charge storage capacity and the definition of the redox peaks in the cyclic voltammetry curves was due to obtaining a completely orthorhombic crystalline structure of V2O5, which brings with it interesting properties to be applied not only in the manufacturing of electrochromic devices but also in the manufacturing of batteries and supercapacitors.
Data Availability
No datasets were generated or analysed during the current study.
References
P. Hu, P. Hu, T.D. Vu, M. Li, S. Wang, Y. Ke, X. Zeng, L. Mai, Y. Long. Chemical Reviews. 123, 4353–4415 (2023). https://doi.org/10.1021/acs.chemrev.2c00546.
Z. Yu, J. Zheng, X. Jing, G. Hang, Q. Liu, W. Cai. Journal of Dispersion Science and Technology. 41, 2203–2210 (2020). https://doi.org/10.1080/01932691.2019.1656085.
H. Sun, W. Wang, Y. Xiong, Z. Jian, W. Chen. Chinese Chemical Letters. 109213 (2023). https://doi.org/10.1016/j.cclet.2023.109213
V. Mounasamy, G.K. Mani, S. Madanagurusamy. Microchimica Acta. 187, 1–29 (2020). https://doi.org/10.1007/s00604-020-4182-2
B. Yalagala, P. Sahatiya, C.S.R. Kolli, S. Khandelwal, V. Mattela, S. Badhulika, ACS Appl. Nano Mater. 2, 937–947 (2019). https://doi.org/10.1021/acsanm.8b02233
H. Liu, X. Liang, T. Jiang, Y. Zhang, S. Liu, X. Wang, X. Fan, X. Huai, Y. Fu, Z. Geng, D. Zhang, Solar Energy Materials and Solar Cells. 238, 111627 (2022). https://doi.org/10.1016/j.solmat.2022.111627
Z. Tong, N. Li, H. Lv, Y. Tian, H. Qu, X. Zhang, J. Zhao, Y. Li, Solar Energy Materials & Solar Cells. 146, 135–143 (2016). https://doi.org/10.1016/j.solmat.2015.11.008
S. Mondal, T. Yoshida, U. Rana, M.K. Bera, M. Higuchi, Solar Energy Materials and Solar Cells. 200, 110000 (2019). https://doi.org/10.1016/j.solmat.2019.110000
J. Kim, K.H. Lee, S. Lee, S. Park, H. Chen, S.K. Kim, S. Yim, W. Song, S.S. Lee, D.H. Yoon, S. Jeon, K.-S. An, Chemical Engineering Journal. 453, 139973 (2023). https://doi.org/10.1016/j.cej.2022.139973
T.-C. Lin, B.-J. Jheng, H.-M. Yen, W.-C. Huang, Materials. 15, 4598 (2022). https://doi.org/10.3390/ma15134598
P. Chen, G. Zheng, G. Guo, Z. Wang, J. Tang, S. Li, Z. Wen, S. Ji, J. Sun. Journal of Alloys and Compounds. 784, 574–583 (2019). https://doi.org/10.1016/j.jallcom.2018.12.373.
F. Baskoro, H.Q. Wong, H.-J. Yen. ACS Applied Energy Materials. 2, 3937–3971 (2019). https://doi.org/10.1021/acsaem.9b00295
S. Guan, W. Wang, J. Zheng, C. Xu, Electrochimica Acta. 354, 136702 (2020). https://doi.org/10.1016/j.electacta.2020.136702
S.A. Pande, B. Pandit, B.R. Sankapal, Materials and Design. 182, 107972 (2019). https://doi.org/10.1016/j.matdes.2019.107972
G.J. Fang, Z.L. Liu, Y. Wang, Y.H. Liu, K.L. Yao, Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 19, 887–892 (2001). https://doi.org/10.1116/1.1359533
M. Przesniak-Welenc, M. Lapinski, T. Lewandowski, B.L. Koscielska, W.W. Sadowski, Journal of Nanomaterials. 2015, 1–8 (2015). https://doi.org/10.1155/2015/418024
N. Shah, H. Nawaz, S.M. Abbas, A. Khesro, K. Ullah. Journal of Materials Research. 38, 2018–2029 (2023). https://doi.org/10.1557/s43578-023-00936-8
H. Fu, X. Jiang, X. Yang, A. Yu, D.S.G. Wang, Journal of Nanoparticle Research. 14, 871 (2012). https://doi.org/10.1007/s11051-012-0871-z
T.K. Le, P. Pham, C.-L. Dong, N. Bahlawane, D. Vernardou, I. Mjejri, A. Rougier, S.W. Kim. Journal of Materials Chemistry C. 10, 4019–4071 (2022). https://doi.org/10.1039/D1TC04872D.
V. Mounasamy, G.K. Mani, S. Madanagurusamy. Microchimica Acta. 187, 253 (2020). https://doi.org/10.1007/s00604-020-4182-2
C.-C. Chang, P.-W. Chi, P. Chandan, C.-K. Li, Materials. 12, 2475 (2019). https://doi.org/10.3390/ma12152475.
T.K. Le, P.V. Pham, C.L. Dong, N. Bahlawane, D. Vernardou, I. Mjejri, A. Rougier, S.W. Kim, Journal of Materials Chemistry C. 10, 4019–4071 (2022). https://doi.org/10.1039/D1TC04872D
V. Yelsani, N. Pothukanuri, U.B. Sontu, V. Yaragani, R.R.M. Venkata. Materials Science (MEDZIAGOTYRA). 25, 1 (2019). https://doi.org/10.5755/j01.ms.25.1.18492
T.K. Le, M. Kang, S.W. Kim, Ceramics International. 45, 15781–15798 (2019). https://doi.org/10.1016/j.ceramint.2019.05.339
P.D. Raj, S. Gupta, M. Sridharan, Ceram Int. 43, 9401–9407 (2017). https://doi.org/10.1016/j.ceramint.2017.04.110
T.K. Le, M. Kang, S.W. Kim. Ceramics International. 45, 15781–15798 (2019). https://doi.org/10.1016/j.ceramint.2019.05.339.
D.A. Cheema, M.O. Danial, M.B. Hanif, A.S. Alghamdi, M. Ramadan, A. Khaliq, A.F. Khan, T. Subhani, M. Motola. Coatings. 12, 1–11 (2022). https://doi.org/10.3390/coatings12040448
Y. Kim, S. Yu, J. Park, D. Yoon, A.M. Dayaghi, K.J. Kim, J.S. Ahn, J. Son, Journal of Materials Chemistry C. 6, 3451–3458 (2018). https://doi.org/10.1039/C7TC05876D
A. Kumar and P. P. Sahay. J Solgel Sci Technol, vol. 95, no. 1, pp. 34–51, Jul. 2020, doi: https://doi.org/10.1007/s10971-020-05298-9.
S. Sutrave et al. Applied Surface Science Advances, vol. 12, Dec. 2022, doi: https://doi.org/10.1016/j.apsadv.2022.100331
K. Mouratis et al. Materials, vol. 13, no. 17. MDPI AG, Sep. 01, 2020. doi: https://doi.org/10.3390/ma13173859
H. Liu et al. Solar Energy Materials and Solar Cells, vol. 238, May 2022, doi: https://doi.org/10.1016/j.solmat.2022.111627.
Ureña-Begara, F., Crunteanu, A. and Raskin, J.P. Applied Surface Science, 403, 717–727 (2017).
Mendialdua, J., Casanova, R. and Barbaux, Y.J.J.O.E.S., 1995. Journal of Electron Spectroscopy and Related Phenomena, 71, 249–261 (1995).
T.K. Le, M. Kang, S.W. Kim. Materials Science in Semiconductor Processing. 94, 15–21 (2019). https://doi.org/10.1016/j.mssp.2019.01.026
Y.-S. Hsiao, C.-W. Chang-Jian, W.-L. Syu, S.-C. Yen, J.-H. Huang, H.-C. Weng, C.-Z. Lu, S.-C Hsu. Applied Surface Science. 542, 148498 (2021)
S. Wu, Y. Ding, L. Hu, X. Zhang, Y. Huang, S. Chen. Materials Letters. 277, 128268 (2020). https://doi.org/10.1016/j.matlet.2020.128268
X. Yu, B. Lu, Z. Xu, Adv Mater. 26, 1044–1051 (2014). https://doi.org/10.1002/adma.201304148
Z. Khan, B. Senthilkumar, S. Lim, R. Shanker, Y. Kim, H. Ko, Adv Mater Interfaces. 4, 1700059 (2017). https://doi.org/10.1002/admi.201700059
S. Deng, Z. Yuan, Z. Tie, C. Wang, L. Song, Z. Niu, Angew Chem Int Ed. 59, 22002–22006 (2020). https://doi.org/10.1002/anie.202010287
Y. Liu, M. Clark, Q. Zhang, D. Yu, D. Liu, J. Liu, G. Cao, Advanced energy materials. 1, 194–202 (2011). https://doi.org/10.1002/aenm.201000037
Z. Tong, H. Lv, X. Zhang, H. Yang, Y.T.N. Li, J. Zhao, Y. Li, Scientific reports. 5, 16864 (2015). https://doi.org/10.1038/srep16864
F.P. Gokdemir, O. Ozdemir, K. Kutlu, Electrochimica Acta. 121, 240–244 (2014). https://doi.org/10.1016/j.electacta.2013.12.164
Y. Shimizu, K. Nagase, N. Miura, N. Yamazoe, Solid State Ionics. 53–56, 490–495 (1992). https://doi.org/10.1016/0167-2738(92)90419-P
T.-C. Lin, B.-J. Jheng, W.-C. Huang, Energies. 14, 2065 (2021). https://doi.org/10.3390/en14082065
M.A. Asiabar, Z. Mohaghegh, F.E. Ghodsi, Applied Physics A. 124, 51 (2018). https://doi.org/10.1007/s00339-017-1476-2
R. Futsch, I. Mjejri, H. Rakotozafy, A. Rougier, Frontiers in Materials. 7, 1–10 (2020). https://doi.org/10.3389/fmats.2020.00078
S. Zhang, S. Chen, Y. Luo, B. Yan, Y. Gu, F. Yang, Y. Cao. Journal of Alloys and Compounds. 842, 155882 (2020). https://doi.org/10.1016/j.jallcom.2020.155882
W. Kang, C. Yan, X. Wang, C.Y. Foo, A.W.M. Tan, K.J.Z. Chee, P.S. Lee, Journal of Materials Chemistry C. 2, 4727 (2014). https://doi.org/10.1039/C4TC00158C
A.K. Surca, G. Dražić, M. Mihelčič, Solar Energy Materials and Solar Cells. 196, 185–199 (2019). https://doi.org/10.1016/j.solmat.2019.03.017
C.T. Prontera, N. Gallo, R. Giannuzzi, M. Pugliese, V. Primiceri, F. Mariano, A. Maggiore, G. Gigli, A. Sannino, L. Salvatore, V. Maiorano, Gels. 9, 310–344 (2023). https://doi.org/10.3390/gels9040310
M. Jamdegni1, A. Kaur, Journal of The Electrochemical Society, 166, H502-H509 (2019). DOI: https://doi.org/10.1149/2.0281912jes
M.N. Mustafa, M.A.A.M. Abdah, N.M. Saidi, W.P. Wong, Y.S. Tan, A. Numan, Y. Sulaiman, R. Walvekar, F.N.M. Azlan, M. Khalid, Journal of Power Sources. 595, 234079 (2024). https://doi.org/10.1016/j.jpowsour.2024.234079.
D. Ma, T. Yang, X. Feng, P. Wang, J. Huang, J. Wang, H. Li, Advanced Science. 11, 2307223 (2024). https://doi.org/10.1002/advs.202307223
V. Encinas-Sánchez, M.T. de Miguel, M.I. Lasanta, G. García-Martín, F.J. Pérez, Solar Energy Materials and Solar Cells. 191, 157–163 (2019). https://doi.org/10.1016/j.solmat.2018.11.007.
A. Florinda, B. Ferruccio, D. Franco, L. Airton, E. Masetti, Electrochimica Acta. 47, 2231–2238 (2002). https://doi.org/10.1016/S0013-4686(02)00061-0.
C. Byoung-Yong, Journal of Electrochemical Science and Technology. 11, 318–321 (2020). https://doi.org/10.33961/jecst.2020.00815
D. Mansour, O. Bouvard, A. Schüler, Energy Procedia. 122, 787–792 (2017). https://doi.org/10.1016/j.egypro.2017.07.397
S. Li, X. Li, Y. Li, B. Yan, X. Song, D. Li, Electrochimica Acta. 244, 77–85 (2017). https://doi.org/10.1016/j.electacta.2017.05.053
Y. Guo, J. Li, M. Chen, G. Gao, Journal of Power Sources. 273, 804–809 (2015). https://doi.org/10.1016/j.jpowsour.2014.09.118
Liu, Z., Wei, W., Xu, K., Wu, H., Su, T., Zhu, Y. and Wang, Z. Materials Research Express. 6, 1150a1 (2019). DOI https://doi.org/10.1088/2053-1591/ab4b87
M. Rozman, N. Cetin, U. Bren, M. Luksic, Electronics. 10, 1–11 (2021). https://doi.org/10.3390/electronics10202529
M. K. Jawad and A. M. A. Al-sammarraie, Indian Journal of Natural Sciences. 9, 14891–14899 (2018).
B. A. Abdulkadir, J. O. Dennis, M. F. B. Abd-Shukur, M. M. E. Nasef, F. Usman, International Journal of Electrochemical Science. 16, 2–15 (2021). https://doi.org/10.20964/2021.01.34
J. Chmelar, J. M. Dodda, P. Be, M. Toma, L. Kullova, and J. Kadlec, Journal of Materials Science. 50, 6477–6490 (2015). https://doi.org/10.1007/s10853-015-9206-7
S. B. Aziz, Iranian Polymer Journal. 22, 877–883 (2013). https://doi.org/10.1007/s13726-013-0186-7.
M. F. Shukur, R. Ithnin, H. A. Illias, and M. F. Z. Kadir, Optical Materials. 35, 1834–1841 (2013). https://doi.org/10.1016/j.optmat.2013.03.004
M. Coşkun, Ö. Polat, F. M. Coşkun, Z. Durmuş, M. Çağlar and A. Türüt, RSC Advances. 8, 4634–4648 (2018). https://doi.org/10.1039/C7RA13261A
A. Pawlicka, F.C. Tavares, D.S. Dorr, C.M. Cholant, F. Ely, M.J.L. Santos and C.O. Avellaneda, Electrochimica Acta. 305, 232–239 (2019). https://doi.org/10.1016/j.electacta.2019.03.055
V. Vibha, C. Meenal, A. Ekta, K. Anand, S. Sujata, A. Ashish, Journal of Materials Science: Materials in Electronics. 35, 345 (2024). https://doi.org/10.1007/s10854-024-12057-x
Acknowledgements
The authors thank to CIO and UNISON, for the facilities provided for the analysis XRD, FTIR, XPS, FESEM and AFM. M. Fuentes-Pérez thanks CONAHCYT-Mexico for the postdoctoral fellowship received to carry out a research stay at the Centro de Investigación en Ingeniería y Ciencias Aplicadas (CIICAp), UAEM through Agreement 11200/331/2023.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.F.P.: Conceptualization, Investigation, Validation, and Writing-Original draft preparation. J.C.U.: Investigation and Validation. U.L.S.: Investigation, original draft revision. D.H.M.: Investigation. S.G.C.: Investigation. M.E.N.: Conceptualization, Methodology, Project Administration, Resources, Supervision, and Writing-review & editing.
Corresponding authors
Ethics declarations
Ethics Approval Statement in the Manuscript
This declaration is “not applicable”.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fuentes-Pérez, M., Castrellón-Uribe, J., León-Silva, U. et al. Effects of Heat Treatment and Electrolyte Type on the Properties of Vanadium Pentoxide. J Clust Sci 35, 2571–2589 (2024). https://doi.org/10.1007/s10876-024-02653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-024-02653-6