Abstract
The microwave-assisted combustion process (MCP) was adapted to prepared Zinc doped Co3O4 spinel nanoparticles. Scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), diffuse reflectance spectroscopy (DRS), energy dispersive X-ray analysis (EDX), X-ray diffraction (XRD), and vibrating sample magnetometer (VSM) techniques were used to investigate the structural, optical, morphological, magnetic, and catalytic properties. The cubic spinel structure was obtained without impurities in the X-ray diffraction (XRD) patterns of undoped Co3O4 and Zn2+ doped Co3O4 (x = 0.1 and 0.3) respectively. However, as the Zn2+ concentration increased, at x = 0.5, a new hexagonal phase appeared in addition to the cubic phase, with mean crystallite size of the cubic spinel structure extending from 48.6 to 25.5 nm. It is found that Zn2+ doping in Co3O4 matrix can induce a negative shift in the flat-band potential (VFB) and increases the isoelectric point. The Co–O stretching mode of the cubic spinel Co3O4 structure is responsible for the occurrence of FT-IR bands at about 662 and 573 cm−1. Kubelka–Munk (K–M) method is utilized to deduce the direct band gap and decline in the band gap values (1.87–1.72 eV) observed with rise in Zn2+ content. TG–DTA analysis confirms the weight loss and exothermic transitions. Scanning and transmission electron microscopy were used to study the morphology and the images depicted with intragranular pores, fused grains with different grain boundaries and homogeneous distributions. The transition from paramagnetic to super-paramagnetic behavior was most likely caused by the exchange of Zn and Co ions, as well as the phase composition of ZnO (hexagonal phase) and Co3O4 (cubic phase). The as-fabricated Zn2+ doped Co3O4 nanoparticles were evaluated for the catalytic activity tests carried out in a batch reactor operating under atmospheric conditions. The high doping concentration (about, x = 0.5) sample exhibited excellent catalytic activity and it exhibited better conversion efficiency and selectivity of 97.3% and 95.3%, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanostructure materials and spinel nanoparticles have been widely studied in the past decades due to its attractive structural, electrical and magnetic characteristics. Magnetic nanoparticles are highly polarized with an electronic spin, resulting in 80% of high magneto-resistance at room temperature [1, 2]. Currently, the synthesis of electrochemical, magnetic, catalytic, optical, mechanical, and thermal properties of cobalt oxide formed excessive concentration due to its various technological applications, such as contain magnetism, solid-state sensors, lithium-ion batteries, heterogeneous catalysts, gas sensors, energy storage, solar energy absorbers, particularly in super capacitors, because of its higher surface area, and electrochemical devices [3,4,5].
Co3O4 is a widely used magnetic material that has piqued the interest of researchers in electrolysis, which is shown to exhibit remarkable photo-electrochemical properties due to an excellent stability (in alkaline media) during oxygen redox processes and provides promising redox potential abilities [6,7,8]. Co3O4 and/or Co-based nano-composite materials an enhancement agent for magnetic resonance imaging or correspondingly offers stimulating benefits on several engineering and medicine (drug delivery application) [6, 8]. Co3O4 is a transition (binary) metal oxide with cubic normal spinel crystal structure and one of the important and attractive p-type semiconductors with anti-ferromagnetic material with Neel temperature (TN) of ∼30 K [3, 9, 10]. The anti-ferromagnetic ordering of CO3O4 is caused by super-transfer interactions, which may occur via two positive path ways, for example (i) Co2+–O–Co3+–O–Co2+, (ii) Co2+–O–Co2+[3]. The cobalt oxide has an essential narrow band gap of 0.74 eV, which is far narrower than the commonly agreed band gap values ranging from 1.5 to 2.5 eV, and this band gap value is derived from a simple dipole prohibited d–d transition between tetrahedral-site Co2+cations [7, 11]. Cobalt oxides are formed in a variety of oxidation states, which makes the materials attractive. The composite might occur in the form of the rock salt structure of cobalt (II) oxide (CoO), Alternatively, spinel cobalt (II, III) oxide (Co2O3 and Co3O4), fabricated by a process of mixed oxidation (Co2+ and Co3+) state [8]. It is well known that magnetic Co2+ ions occupy the tetrahedral sites as Co3O4 crystallizes in the cubic natural spinel form, while the non-magnetic Co3+ ions occupy the octahedral sites [5]. Cobalt oxide nanoparticles as one of the fascinating magnetic properties, it has greater attention owing to its uses in supercapacitor [12], lithium-ion battery [13], gas sensor [14], heterogeneous catalyst [15], and so on. This has been widely analyzed as steady catalysts for oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) because of its lower price [16]. The oxides are common in nature, and only CoO and Co3O4 are stable, with Co3O4 having the greatest steadiness of all of the cobalt oxides.
Various methods have been used to create magnetic cobalt oxide nanoparticles such as sol–gel [17], chemical vapor deposition [18], sonochemical [19], spin coating [20], spray pyrolysis [21], solid-state thermal decomposition method [22], mechano-chemical reaction [23], precipitation [24], hydrothermal [25] are some of the general synthesizes methods used for CO3O4. These methods, however, either take time or require costly instruments. In such situations, the synthetic route with microwave assisted is an appropriate approach; the narrow size distribution and high purity are the additional advantages [10].
However, there are few studies on the synthesis of ZnO and Co3O4 using MCP. This work emphases on the preparation of Zn2+ doped Co3O4 using MCP. The outcome of Zn2+ doping on Co3O4using microwave effect for secondary (ZnO at x = 0.5) appearance is explored. Zn2+ is doped with Co3O4 nanoparticles owing to its good conductivity, thermo chemical stability and low manufacture price. ZnO is an inherently n-type semiconductor with a 3.37 eV optical band gap and a high exciton binding energy of 60 meV [9].
The present work aims to exploit the preparation methodology of Co3O4 and doping Zn2+ in CO3O4 nanoparticles and various physical characterization techniques are employed to study the structural, optical, thermal, surface morphology and magnetic characteristics are studied in detail and elaborated in the subsequent sections. The as-prepared undoped cobalt oxide and zinc doped cobalt oxide nanoparticles have also been assessed for the glycerol oxidation to discover their possible catalytic application.
Experimental
Materials
The chemicals such as cobalt nitrate, zinc nitrate and L-alanine of analytical grade (99.9%), obtained from SD Fine- chemicals, India. The chemicals were used directly without performing any extra purification. Double distilled water is utilized during the process of sample preparation.
Synthesis of Zn2+ Doped Co3O4 Nanoparticles
Appropriate amounts of cobalt nitrate, zinc nitrate (precursors-oxidizers), and L-alanine (fuel) were utilized in the microwave combustion technique to create spinel nanoparticles. The fuel-to-oxidizer (F/O) ratio retained at 1 as per the principle of propellant chemistry [2]. The standard procedure, extracted homogeneous solution (precursors), was dissolved in de-ionized water and stirred for 45 min at 300 K. In a microwave (800 W), which operates at 2.45 GHz, the homogeneous solution was introduced for a short period of 15 min. The precursor solution was dehydrated and combusted after reaching the threshold temperature and the black fluffy powder as obtained was calculated at 550 °C for 2.5 h. The attained samples were processed at a stoichiometric concentration, Co1-xZnxO4 with x = 0, 0.1, 0.3 and 0.5 were labeled as (a), (b), (c) and (d), respectively.
Characterizations
The thermogravimetric behavior of the as-prepared powders was examined using an Exstar TG/DTA 7200 instrument from SII Nanotech at a heating rate of 5 °C min−1 in a nitrogen atmosphere. X-ray diffraction (Rigaku Model Smartlab 3 kW X-ray diffractometer) in 2θ scale 10–80° fitted with Cu K radiation = 1.5406 Å was used to define the structural properties of the as-prepared powders. Thermo Scientific make NICOLET iS10 is utilized to perform FTIR studies and to determine function classes. In order to study optical characteristics Evolution 300 UV–visible spectrophotometer of Thermo Scientific make is used at RT. HITACHI S4800 equipped with EDS of HORIBA EMAX make was employed to perform morphological studies and to confirm elemental composition. A PMC Micro Mag 3900 type VSM equipped with a 1 T magnet is used to study magnetization curves at RT.
Catalytic Activity
The catalytic conversion of glycerol was carried out in a batch reactor operating under atmospheric conditions using a two-necked round bottom flask (250 ml) equipped with reflux condenser and temperature controller. The starting mixture containing zinc doped cobalt oxide catalysts (50 mg), glycerol (C3H8O3) as reactant (0.05 mol), hydrogen peroxide (H2O2) as oxidant (0.05 mol) and dimethyl sulfoxide (DMSO) as solvent, was placed in a two necked round bottom flask equipped with a water condenser. The temperature was maintained at 80 ºC for 390 min (6.5 h) and then cooled down to 304 Kelvin. The oxidized products were analyzed by gas chromatography (GC) with flame ionization detector, using a MAYURA 1100. The main by-products were confirmed by gas chromatography mass spectrometer (GC–MS) using Agilent Technologies Model 7890A.
Results and Discussion
Structural Analysis
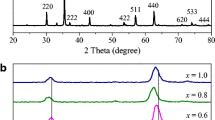
The development of X-ray diffraction (XRD) patterns as a result of Zn2+ doping material is seen in Fig. S1. Pure Co3O4 and Zn0.1Co0.9O4, Zn0.3Co0.7O4 samples show typical peaks of a single phase with a cubic structure and a space group \(Fd\overline{3} m\), in accordance with JCPDS card No: 43-1003). No other peaks have been noticed; this implies that microwave combustion method, followed by 2.5-h calcination at 550 °C, is adequate to prepare a single phase Co3O4 nanoparticles. The characteristics peaks at about 2θ values 19.0°, 31.2°, 36.8°, 38.5°, 44.8°, 59.3°, 65.2° are (111), (220), (311), (222), (400), (511), and (440) crystallographic planes, correspondingly. However, with increase in Zn2+concentration x = 0.5, the new secondary ZnO phase (space group P63mc) with hexagonal structure (the characteristics peaks at 2θ ≈ 32.1° (100), 34.8° (002), 36.6° (101), 57.0° (110), 63.3° (103), 68.2° (112)) looks, in accordance with JCPDS No. 75–1526. It was found that higher doping concentration x = 0.5 (Zn0.5Co0.5O4), a new secondary ZnO hexagonal process occurs. As Co2+ ions are replaced by Zn2+ ions, two phase-systems with cubic (Co3O4) and hexagonal (ZnO) structures form, suggesting the forming of a composite. The amplitude of the peak corresponding to the hexagonal step is observed to decrease as Zn2+ concentration increases.
The Debye–Scherrer formula was used to calculate the average crystallite size (D) of Zn2+ doped Co3O4 [26].
where, λ is the wavelength of an X-ray, β is the full width at half maximum (FWHM) and θ is the Bragg diffraction angle. The reported results show that the average crystallite size of undoped cobalt oxide is 48.6 nm, while for Zn2+ doped samples it has been detected slightly smaller range 42.9–25.5 nm as shown in Table 1. Increased substitutions of Zn2+ concentrations resulted in a haphazard decrease in the crystallite size of Co3O4 nanoparticles.
The XRD patterns were used to quantify the lattice parameters of a Zn.2+ doped Co3O4 method using Eq. (2)
where h, k, and l are the miller indices, a is the lattice parameter, and d is the inter-atomic spacing. Table 1 depicts the cubic phase's lattice parameter and unit cell number. However, lattice constant an increases with increasing x value. The diffraction peaks (311) shift toward a lower angle as the concentration of Zn2+ increases. The shift in peak position between the samples with a Zn2+ doping level from x = 0 to 0.5 is Δθ = 0.11°, indicating that the lattice constant increased from 8.0515 to 8.0632 Å. This variation can be attributed to the greater ionic radius Zn2+ (rZn2+ = 0.074 nm at tetrahedral coordination) ions strengthened the lattice parameter compared to the comparatively smaller ionic radius Co2+ (rCo2+ = 0.072 nm at tetrahedral coordination) ions [27].
In addition, the micro-strain (e), lattice strain (ε) and dislocation density (δ) are also calculated using Eqs. (3), (4) and (5) respectively. [28]
Table 1 summarizes the comprehensive structural parameters for the (311) peak, demonstrating that as doping concentration increases, the crystallite size decreases while the micro-strain, lattice strain, and dislocation density increase, owing to doping greater ionic radius (Zn) into lesser ionic radius (Co).
Zeta Potential
Zeta potential measurements were used to investigate the surface changes of the materials. Figure 1 depicts the variation of zeta potential with pH in aqueous suspensions. The zeta potential results show that the particle surface charge of undoped and Zn2+ doped Co3O4 nanoparticles in ethanol medium ranged from + 19 to + 25 mV and − 16 to − 21 mV, respectively. These findings showed that the as-prepared nanoparticles were relatively stable in an aqueous suspension. Because of the high zeta potential, the undoped and Zn2+ doped Co3O4 material suspensions were more stable in alkaline medium. In general, nanoparticles with zeta potentials around 30 mV (− ve or + ve) demonstrated excellent colloidal stability. In contrast, the isoelectric point of the pure and Zn2+ doped Co3O4 nanoparticles increases from 5.3 to 6.1, preferring glycerol oxidation [29,30,31].
Optical Properties
The UV-DRS study is performed on the zinc doped cobalt oxide samples to determine the optical characteristics in the wavelength range of 200–800 nm. In the evaluation of the optical energy band gap the diffuse reflection research plays a major role. It is extracted utilizing the modified Tau’c relation [32] as given by Eq. (6):
where n = 1/2 and 2 for direct and indirect transitions, respectively, as a result, direct and indirect band gaps are created. The Kubelka–Munk (K–M) function [F(R)] is commonly used to transform diffuse reflectance into the corresponding absorption coefficient, as seen in Eq. (7), and is primarily utilized for investigating powder samples:
A graph is drawn between [F(R)hv]2 and h, and the linear regions of these graphs are extrapolated to [F(R)h]2 = 0 to yield the direct band difference values as shown in Fig. S2. The estimated band gap values of Zn2+ doped structure is noted to be 1.87, 1.82, 1.78, and 1.72 eV (Table 1) for x = 0, 0.1, 0.3, and 0.5 respectively. However, increasing the Zn2+ doping causes a decline in the band gap value; i.e., 1.87–1.72 eV for un-doped to 50% Zn2+ addition. The contraction in band gap with doping of Zn2+ ions is seen owing to the creation of sub-bands amid the energy band gap. Further, combination of these sub bands with the conduction band results in the formation of continuous band, thereby lowering the band gap value [33].
FTIR Analysis
Fig. S3 depicts the FTIR spectra of zinc doped cobalt oxide logged in the 4000–400 cm−1 region. The FT-IR spectral analysis was carried out to observe the development of nanostructure and the existence of surface functional groups. The bands associated with higher wavenumbers are mapped with H, O, and C bonds. Wide band at 3444 cm−1 is linked to the O–H longitudinal stretching vibration of adsorbed water molecules. The band at 2914 cm −1 are aligned with the C–H symmetric and asymmetric stretching sensations. Further, existence of a band at 1624 cm−1 confirms the occurrence of (H–O–H) bond vibration. Consequently, owing to the adopted combustion path of material synthesis, a weak band is seen at 1380 cm−1, which corresponds to the remaining nitrogen groups. The symmetric stretching of CO32− ion is allotted to the weak band at 1040 cm−1. The fingerprint stretching vibrational modes of Co–O bonds is confirmed by observation by two distinct, sharp bands at 662 and 573 cm−1, which ensures the formation of the cubic Co3O4 nanoparticles [34]. However, the increasing Zn2+ doping content at x = 0.5, the presence of band at 479 cm−1 corresponds to the stretching vibrational mode of hexagonal ZnO bond [35], which proved the high crystallinity of the sample as evidenced from XRD (x = 0.5) analysis.
TG/DTA Analysis
Thermogravimetric analysis was performed on the zinc doped cobalt oxide nanoparticles. The TG/DTA curves of the zinc doped system of the samples are given in Fig. S4. The initial stage of weight loss was noted to be 0.3, 0.1, 0.15 and 0.3% (0 ≤ x ≤ 0.5) between 50 and 218 °C, and further dehydration of H2O molecules which is present on the particle surface is observed due to the existence an endothermic peak in the DTA thermogram. The next stage of weight loss was observed to be 0.12, 0.1, 0.4 and 0.31% (0 ≤ x ≤ 0.5) occurring between 242 and 400 °C is due to the decay of the organic compound, removal of hydroxyl group and volatile components existing in the prepared sample. No weight loss was noticed after 400 °C, this may be due to institutional reorganization. As a result, calcination has been carried out at the temperature above 400 °C, which indicates the thermal stability of zinc doped cobalt oxide nanoparticles [36, 37].
SEM Study
The surface morphology of the as prepared Zn2+ doped cobalt oxide is depicted in Fig. S5, combustion route entails the outflow of water molecules and volatile gases (O2, N2, and Co2), resulting in the development of a simple nanostructure with varying degrees of porosity. Upon visual inspection the obtained images confirmed the formation of the porous nanosized crystallized grains. Further the presence of fused grains with discrete grain boundaries served as origin point for pore walls and several small-sized isolated grains with pores. Energy dispersive X-ray (Fig. S6) analysis has been used to determine the elemental conformation of the Zn2+ doped Co3O4nanoparticles and the existence of Co/Zn and O elements were verified, and the respective fraction of elemental composition is shown in the inset table of Fig. S6.
TEM Study
Figure 2a, b illustrates transmission electron microscopy (TEM) images of undoped and Zn2+ (x = 0.3) doped Co3O4 nanoparticles, respectively. The as-prepared nanoparticles possess spherical morphology with some degrees of agglomeration. The average grain size particles in the Zn2+ (x = 0.3) doped Co3O4 nanoparticles clearly decreases when compared to the pure Co3O4 nanoparticles (51 to 36 nm), which is consistent with the size estimated from the Debye–Scherrer equation from XRD. Figure 2c is selected area diffraction (SAD) pattern from the particles confirming the presence of the Zn2+ doped Co3O4 nanoparticles. The rings are broadened considerably, indicating the nano-crystalline nature of the particles.
Magnetic Properties
The magnetization (M) vs field (H) hysteresis plot for zinc doped cobalt oxide system is shown in Fig. 3 it shows para and super-paramagnetic behavior (Table 2) at room temperature. Under the impact of magnetic field, the magnetization of the materials begins to saturate, and para to super-paramagnetic activity becomes dominant. The cobalt oxide nanoparticles were normal spinel structure, as cobalt (II) divalent and cobalt (III) trivalent metal ions occupy the tetrahedral and octahedral sites, respectively. The undoped Co3O4 exhibited paramagnetic behavior at room temperature, and it undergoes the weak exchange interaction between anti-ferromagnetism and ferromagnetism for surface effect below the Neel temperature at approximately 40 K. The interaction of antiferromagnetic strength is weak, that employed in unit cell all the spin-up Co2+ ion has tetrahedral coordination with nearest neighbor atom Co2+ ion with the spin down configuration [38, 39]. The corresponding undoped Co3O4 spinal nanoparticle is observed the saturation magnetization values (Ms) 5.8079 memu g−1. Although bulk cobalt oxide material is anti-ferromagnetic behavior, this change in magnetic property due to the finite size effects [40]. As increasing Zn2+ ion concentration from x = 0.1 to 0.5 are exhibited only super-paramagnetic behavior. These samples are found to non-saturate the magnetization event at the maximum applied field of 15 kOe. The undoped Co3O4 belong to paramagnetic materials and Zn2+ doped Co3O4 samples present weak ferromagnetic property as shown in Table 2 [41,42,43]. These samples corresponding to the saturation magnetization values (Ms) 99.695 to 1.8562 memu g−1. The coercivity (Hc) and remanence magnetization (Mr) was observed that it varies with increasing Zn2+ concentration, which ranges from 52.871 to 135.49 Oe and 53.299 to 1395.8 μemu g−1 (Table 2). These values remained to be dependent on crystallite size and shape of undoped and doped cobalt oxide nanoparticles [44].
Catalytic Activity
Effect of Sample Composition
Zinc doped cobalt oxide nanoparticles have been used for the oxidation of glycerol in liquid phase batch reactor at atmospheric conditions. Increasing Zn2+ concentration (x = 0 to 0.5), the catalytic conversion of glycerol increased from 77.9% to 97.3% and selectivity of formic acid increased (as observed for GC–MS results shown in Fig. S7) from 68.8% to 95.3% respectively. This catalyst (x = 0.5) exhibits highest catalytic activity for the conversion of glycerol to formic acid as shown in Fig. 4. Thus, zinc (x = 0.5) doped cobalt oxide catalyst alone was selected for further studies.
Effect of Catalyst Loading
Figure 5 display the effect of catalyst loading on the conversion of glycerol and product selectivity using zinc (x = 0.5) doped cobalt oxide catalyst at 80 ºC for 6.5 h and the optimum reaction condition which maximized the production of formic acid from glycerol. The conversion and the selectivity’s of formic acid was the highest (97.3% and 95.3%) for 50 mg of catalyst loading. At high amount of catalyst, the conversion and the selectivity’s decrease (92.6% and 90.2%) which is due to the blocking of the pore on the catalyst surface, which in turn leads to drop in the reactive surface available to head the reaction [45].
Recycling Performance
The recyclability and recoverability of x = 0.5 sample of the nanoparticles were examined over five runs recycling test of glycerol oxidation as shown in Fig. S8. After the first reaction, the catalyst was filtered and cleaned with distilled water quite a few times, consequently it was dried at 180 ℃ in hot air oven for about 2 h and then it was examined for four successive runs under the same conditions. The catalytic transition of glycerol and selectivity of formic acid decreased from 97.3% to 77.7% and 95.3% to 74.9%, respectively. This may be due to the mass loss which has occurred during the catalyst washing and recovery procedures [46, 47].
Reaction Pathway
Scheme 1 shows the probable reaction mechanism for the catalytic oxidation of glycerol to formic acid. In the initial stage glycerol is oxidized to form either glyceraldehyde or dihydroxyacetone. On further losing an electron, glyceraldehyde forms glyceric and tartronic acid. Subsequently the glyceric acid experiences rapid C–C bond cleavage to form glycolic acid and formaldehyde, which on further oxidation to forms the formic acid [48, 49].
Conclusion
Microwave assisted combustion technique was used to successfully synthesize Zn2+ doped Co3O4 spinel nanoparticles. Undoped cobalt oxide and zinc doped cobalt oxide (x = 0.1 and 0.2) all exhibited a single-phase spinel with a cubic structure. Increase in doping (x ≥ 0.5), a new hexagonal phase was occurred with addition of cubic structure. Zn2+ doping into Co3O4 is observed to induce a negative shift in the flat-band potential and increase the isoelectric point. FT-IR study further confirms the bands at 662 and 573 cm−1, spinel cubic and hexagonal stretching modes, respectively. It is noted that with the increase of Zn2+ concentration, results in reduced band gap value. HR-TEM and SEM observations reveal that, the homogeneous distribution of Zn2+ in Co3O4 with high-quality lattice fringes. The existence of Zn, Co, and O was verified by EDX analysis. Depending on Zn2+ doping concentration, para-to-super-paramagnetic behavior is observed, combined with a significant difference in the magnetic parameters. Zn2+ doped Co3O4 spinel nanoparticles act as a suitable compound for the selective liquid phase conversion of glycerol to formic acid, exhibiting high conversion and selectivity more than 95%.
References
G. V. M. Williams, T. Prakash, J. Kennedy, Shen V. Chongc, and S. Rubanov (2018). J. Magn. Magn. Mater. 460, 229–233.
M. Sukumar, L. J. Kennedy, J. J. Vijaya, B. Al-najar, and M. Bououdina (2018). J. Magn. Magn. Mater. 465, 48–57.
H. B. Duvuru, S. K. Alla, S. K. Shaw, S. S. Meena, N. Gupta, B. B. V. S. Vara Prasad, M. M. Kothawale, M. K. Kumar, and N. K. Prasad (2019). Ceram. Int. 45, 16512–16520.
Ed Lester, Gabriele Aksomaityte, Jun Li, Sara Gomez, Jose Gonzalez-Gonzalez, Martyn Poliakoff (2012). Prog. Cryst. Growth Charact. Mater. 58, 3–13.
F. Manteghi, S. H. Kazemi, M. Peyvandipoor, and A. Asghari (2015). RSC Adv. https://doi.org/10.1039/C5RA09060A.
N. M. Dang, W.-W. Zhao, H. N. Shin-ichiYusa, and K. Nakashima (2015). New J Chem. https://doi.org/10.1039/c5nj00058k.
L. Qiao, H. Y. Xiao, H. M. Meyer, J. N. Sun, C. M. Rouleau, A. A. Puretzky, D. B. Geohegan, I. N. Ivanov, M. Yoon, W. J. Weber, and M. D. Biegalski (2013). J. Mater. Chem. C 1, 4628.
Damian C. Onwudiwe, Murendeni P. Ravele, and Elias E. Elemike (2020). Nano-Structures & Nano-Objects 23, 100470.
C. S. Jincy and P. Meena (2020). Inorg. Chem. Commun. 120, 108145.
A. S. Bhatt, D. K. Bhat, C.-w Tai, and M. S. Santosh (2011). Mater Chem. Phys. 125, 347–350.
R. Sukhin Saravan, M. Muthukumaran, M. Mubashera, M. Abinaya, P. VarunPrasath, R. Parthiban, F. Mohammad, W. C. Oh, and S. Sagadevan (2020). Opt Opt. https://doi.org/10.1016/j.ijleo.2020.164428.
H. Wang, L. Zhang, X. Tan, et al. (2011). J. Phys. Chem. C. 115, 17599–17605.
B. Yan, L. Chen, Y. Liu, et al. (2014). Cryst Eng Comm. 16, 10227–10234.
Y. Lü, W. Zhan, Y. He, et al. (2014). ACS Appl. Mater. Interfaces 6, 4186–4195.
L. Chen, J. Hu, R. Richards, S. Prikhodko, and S. Kodambaka (2011). Nanoscale 2, 1657–1660.
R. Gao, Z. Yang, L. Zheng, G. Lin, L. Liu, Y. Lee, H. \ Zhongbo, and X. Liu (2018). ACS Catal. 8 (3), 1955–1963.
A. M. El Sayed and S. El-Gamal (2015). J. Polym. Res. 22, 97.
L. Armelao, D. Barreca, S. Gross, and E. Tondello (2001). Surf. Sci. Spectra 8, 14.
R. V. Kumar, Y. Diamant, and A. Gedanken (2000). Chem. Mater. 12, 2301.
V. Patil, P. Joshi, M. Chougule, and S. Sen (2012). Soft Nanosci. Lett. 2, 1–7.
S. G. Victoria, A. M. E. Raj, and C. Ravidhas (2015). Mater. Chem. Phys. 162, 852–859.
M. Goudarzi, M. Bazarganipour, and M. Salavati-Niasari (2014). RSC Adv. 4, 46517.
X. Wang, X. Y. Chen, L. S. Gao, H. G. Zheng, Z. Zhang, and Y. T. Qian (2004). J. Phys. Chem. B 108, 16401.
S. A. Makhlouf, Z. H. Bakr, K. I. Aly, and M. S. Moustafa (2013). Superlat Microstruct 64, 107–117.
F. Moro, S. V. Y. Tang, F. Tuna, and E. Lester (2013). J. Magn. Magn. Mater. 348, 1–7.
M. Sukumar, L. John Kennedy, J. Judith Vijaya, B. Al-Najar, M. Bououdina, and G. Mudhana (2019). Vacuum 167, 407–415.
H. Bindu Duvuru, S. K. Alla, S. K. Shaw, S. S. Meena, N. Gupta, B. B. V. S. Vara Prasad, M. M. Kothawale, M. K. Kumar, and N. K. Prasad (2019). Ceram. Int 45, 16512–16520.
Ou. Kai, Shenwei Wang, Yu. Liyuan Bai, K. Z. Wang, and Lixin Yi (2019). Thin Solid Films 669, 247–252.
X. Li, Y. Qingjiang, Y. Cuiling, Y. Huang, R. Li, J. Wang, F. Guo, Y. Zhang, S. Gao, and L. Zhao (2015). J. Mater. Chem. A 3, 8076–8082.
M. Ahamed, M. Javed Akhtar, M. A. Majeed Khan, M. Z. Alaizeri, and H. Alhadlaq (2021). ACS Omega 6, 17353–17361.
Luiz C.A. Oliveira, Marcio F. Portilho, Adilson C. Silva, Hosane A. Taroco, Patterson P. Souza (2012), Appl. Catal. B., 117–118, 29–35.
M. Sundararajan, L. John, J. J. Kennedy, and U. A. Vijaya (2015). Spectrochim. Acta Part a Mol. Biomol. Spectrosc. 140, 421–430.
M. Sukumar, L. John Kennedy, J. Judith Vijaya, B. Al-Najar, and M. Bououdina (2018). Ceram. Int. 44, 18113–18122.
M. Sundararajan, J. Vidhya, R. Revathi, M. Sukumar, B. Arunadevi, R. Rajkumar, S. Ramachandran, M. Kamalakannan, Chandra Sekhar Dash, Jothi Ramalingam Rajabathar, and Selvaraj Aroykaraj, Inorg. Nano-Met., DOI: https://doi.org/10.1080/24701556.2021.2025400.
Mahmoud Nasrollahzadeh, BabakJaleh and AmenehJabbari (2014). RSC Adv., 4, 36713.
M. Sukumar, L. J. Kennedy, J. J. Vijaya, B. Al-Najar, and M. Bououdina (2018). New J. Chem. 42, 18128–18142.
S. Baskar, S. Yuvaraj, M. Sundararajan, and C. SekharDash (2020). J Supercond Nov Magn. https://doi.org/10.1007/s10948-020-05665-1.
Shan Fan, Wen Wang, Hua Ke, Yu. Jian-Cun Rao, and Zhou, (2016). RSC Adv. 6, 97055–97062.
Liangmiao Zhang, Xin Zhao, Wenjing Ma, Wu. Milin, Na. Qian, and Lu. Wencong (2013). CrystEngComm 15, 1389–1396.
F. Soofivand (2012). M. Salavati-Niasari 5, 64346–64353.
G. A. Babu, G. Ravi, T. Mahalingam, M. Navaneethan, M. Arivanandhan, and Y. Hayakawa (2014). J. Phys. Chem. C 118, 23335–23348.
M. Agila and S. Krithiga (2019). Int. j. eng. sci. manag. res. 2 (2), 475–482.
G. D. Tang, Z. Z. Li, L. Ma, W. H. Qi, L. Q. Wu, X. S. Ge, G. H. Wu, and F. X. Hu (2018). Phys. Rep. 758, 1–56.
S. Ramachandran, C. S. Dash, A. Tamilselvan, S. Kalpana, and M. Sundararajan (2020). J. Nanosci. Nanotechnol. 20, 2382–2388.
S. Thanasilp, J. W. Schwank, V. Meeyoo, S. Pengpanich, and M. Hunsom (2015). Chem. Eng. J. 275, 113–124.
M. Sukumar, L. J. Kennedy, J. J. Vijaya, B. Al-Najar, and M. Bououdina (2019). Mater. Sci. Semicond. Process. 100, 225–235.
M. Sukumar and L. John Kennedy (2019). J. Nanosci. Nanotechnol. 19, 826–832.
S. Vajíček, M. Štolcová, A. Kaszonyi, M. Mičušík, P. Alexy, P. Canton, G. Onyestyák, S. Harnos, F. Lónyi, and J. Valyon (2016). J. Ind. Eng. Chem. 39, 77–86.
G.-Y. Yang, Y.-H. Ke, H.-F. Ren, C.-L. Liu, R.-Z. Yang, and W.-S. Dong (2016). Chem. Eng. J. 283, 759–767.
Acknowledgements
The authors (R. Jothi Ramalingam and Hamad Al-Lohedan) are grateful to Deanship of Scientific research, King Saud University for financial support through vice Deanship of Research Chair, Research Chair of Surfactant.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dash, C.S., Sukumar, M., Ravi, V. et al. Effect of Zinc Doping on Structural, Optical, Magnetic, and Catalytic Behavior of Co3O4 Nanoparticles Synthesized by Microwave-Assisted Combustion Method. J Clust Sci 34, 2093–2101 (2023). https://doi.org/10.1007/s10876-022-02369-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02369-5